Key Clinical Message
It is important to be aware of the risk of abnormally invasive placenta in patients with a history of Asherman syndrome and uterine scarring. A prenatal diagnosis by ultrasonography is useful when planning of mode of delivery.
Keywords: abnormally invasive placenta, antenatal ultrasonography, Asherman syndrome, intrauterine adhesions, postpartum hemorrhage
Introduction
Asherman syndrome was first reported in 1894 by Heinrich Fritz 1. Asherman syndrome is also known as uterine atresia, amenorrhea traumatica, endometrial sclerosis, and intrauterine adhesions or synechiae 2. The prevalence of Asherman syndrome varies from 1.55 to 20% 3–6 according to population, mainly due to different diagnostic criteria, the number of abortions in the population, choice of management, awareness of clinicians, and incidence of infections (genital tuberculosis and puerperal infections) 4.
Asherman syndrome arises due to trauma of the endometrium and produces partial or complete obliteration of the uterine cavity or/and cervical canal 1,4,7,8. Trauma of the uterine cavity results in dysfunction of the endometrium which presents in conditions such as menstrual abnormalities, dysmenorrhea, infertility, and recurrent pregnancy loss 8.
Asherman syndrome can occur due to uterine and intrauterine surgery such as cesarean section, curettage, myomectomy involving the uterine cavity, endometrial ablation, and hysteroscopic removal of fibroids and polyps 4,7–11. However, intrauterine adhesions are also seen as a consequence of endometritis, congenital uterine abnormalities, and genetic predisposition 2. It is well-known that the endometrium is more susceptible to trauma in a pregnant uterus and the incidence of intrauterine adhesions following curettage for retained placental tissue is reported up to 40% 3 months after curettage 5. In patients who develop Asherman syndrome, curettage of a pregnant uterus has been shown as the cause in 64% of the cases. Hysteroscopic adhesiolysis can be an effective treatment of Asherman syndrome, with an overall conception rate of 40% following surgery 12.
Trauma of the endometrium can cause defects in the basal decidua (Nitabuchs layer) which in a gravid uterus can give rise to abnormal placentation. Placenta accreta arises when the anchoring villi of the placenta adheres to the surface of the myometrium 11,13.
Placenta accreta is associated with complications during delivery including life-threatening maternal hemorrhage, blood transfusions, and subsequent peripartum hysterectomy 9,10,14. Over the last decades an increase in incidence has been reported with a rise from 0.04% to 0.9%, possibly due to increased rate of uterine surgery 14–17.
We describe a case of placenta accreta in a patient with a history of Asherman syndrome due to uterine scarring after evacuation of retained tissue from a missed abortion.
Case Description
A 38-year-old woman, gravida 3, para 1 presented with a spontaneous pregnancy. Her previous surgical history included a cesarean section and a hysteroscopic removal of uterine synechiae secondary to Asherman syndrome.
One year prior to this pregnancy, the patient had a missed abortion at 7 weeks of gestational age. Medical induction with misoprostol was unsuccessful in two attempts. Due to retained tissue, a surgical evacuation of the uterus with dilatation and curettage was performed. In the following months, the patient experienced secondary amenorrhea, and the patient was referred for gynecologist due to suspicion of Asherman syndrome.
A transvaginal ultrasound at day 17 in menstrual cycle showed thick endometrium disrupted by defects, most likely due to strictures in the uterus.
Hysterosalpingography was attempted, but not completed due to the fact that it was not possible to inject contrast into the uterine cavity. The patient was scheduled for hysteroscopic surgery.
Hysteroscopic surgery revealed a wide adherence across the uterine cavity, just above the internal orifice. A small defect in the adherence was resected and incisions were made at position 3, 6, 9, and 12 according to myometrial scoring 18. The adhesion was removed and revealed a uterine cavity covered by endometrium in approximately 50% of the cavity (Figure1). The uterine scarring was classified as Asherman syndrome stadium II (ranging from I to III) according to The American Society for Reproductive Medicine classification 19. The patient received treatment with Progynova (Estradiol valerate) 2 mg twice daily in 4 weeks postoperatively to prevent recurrence of intrauterine adhesions.
Figure 1.

Hysteroscopic image. Uterine cavity with uterine scarring due to Asherman syndrome.
The patient attended postoperatively follow-up 4 weeks after surgery. A transvaginal ultrasound showed a uterus with visible endometrium along the whole cavity. A sonohysterography was performed to evaluate uterine cavity and tubal patency and revealed a normal cavity with flow of saline through both tubes.
The present pregnancy was conceived 2 months after follow-up. The patient attended first trimester screening at 12 weeks of gestation. A singleton fetus with normal nuchal translucency thickness and risk assessment was found. A routine scan was performed at 22 weeks of gestation and showed no sign of congenital abnormalities. Placenta was seen on the posterior uterine wall, low inserted approx. 18 mm from internal ostium (Figure2). The myometrium underlying placenta had normal appearance. The anterior uterine wall was found thin behind the bladder, measuring 4 mm.
Figure 2.
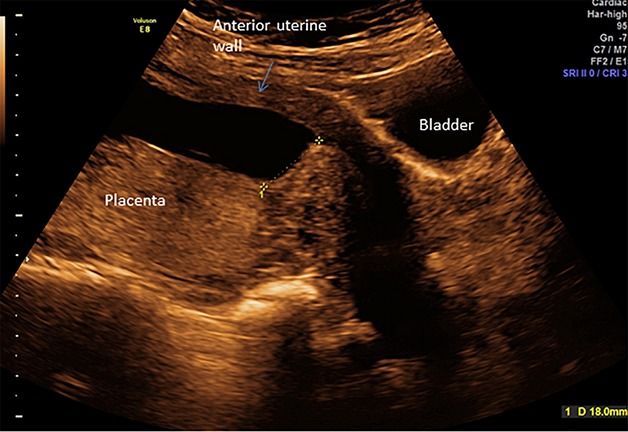
Transabdominal ultrasound scan at 22 weeks of gestation. Placenta on posterior wall located 18 mm from internal ostium.
The patient was monitored with ultrasonography every 3–4 weeks due to the previous history of Asherman syndrome.
The patient underwent an ultrasound scan at 35 weeks of gestation where the uterine wall was found thin at the anterior uterine wall (site of previous cesarean scar) with a myometrium of 2 mm (Figure3). Due to risk of uterine rupture during vaginal delivery, the patient was planned for cesarean section. The woman had future childbearing wishes, and was informed that excessive bleeding at delivery might lead to extensive uterine surgery and possibly hysterectomy.
Figure 3.
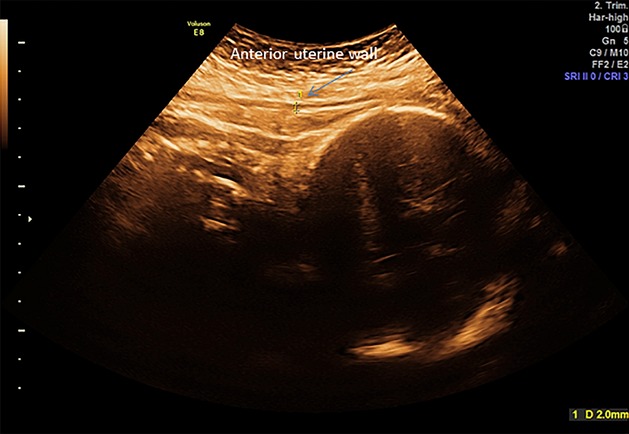
Transabdominal ultrasound scan at 35 weeks of gestation. Anterior uterine wall is measuring 2 mm at the site of previous cesarean section.
A cesarean section was performed at 38 weeks of gestation. During surgery the lower anterior uterine wall was found very thin, however, without dehiscence. Placenta was found partly abnormally adherent on the posterior uterine wall with the appearance of a placenta accreta. Manual removal of placenta was performed and bleeding was measured to approximately 1 liter intraoperatively. A Bakri balloon was inserted and the bleeding subsided. There was no need for uterine sutures or hysterectomy. The Bakri ballon was removed 24 h after the cesarean section and the woman was discharged in good condition 2 days postoperatively.
Discussion
Asherman syndrome is seen in women with previous trauma of the endometrium. The syndrome is well-known, however, evidence-based guidelines on prevention and management are lacking.
Dilatation and curettage are known risk factors for development of Asherman syndrome. Curettage is often performed due to retained tissue after abortion or delivery and with the focus entirely on emptying the uterus, and not on the prognosis for future fertility. Therefore, curettage is often too coarse and even with preventive means, such as hormonal treatment or cobber IUD postoperatively, formation of intrauterine adhesions can still occur. Therefore, emptying of the uterine cavity after abortion or delivery should be gentle and performed under ultrasonic guidance or by hysteroscopy 20.
Following diagnose and treatment of Asherman syndrome it is important to prevent formation of new adhesions. Treatment with estrogen has shown good results in preventing reformation of adhesions following hysteroscopic surgery for Asherman syndrome. Use of peroral estrogen gives better fertility and menstrual outcome when given in combination with ancillary treatment (IUD, balloon or hyaluronic acid) 21.
A recent study by Roy et al. reported conception rates of 58% in mild Asherman, 30% in moderate Asherman, and 33.3% in severe cases of Asherman syndrome following hysteroscopic adhesiolysis. Furthermore, the live birth rate reported was 86.1%, the miscarriage rate 11.1% and the cumulative pregnancy rate showed that 97.2% of the patients conceived within 24 months postoperatively 12.
Studies evaluating obstetric complications in pregnant women with previous Asherman syndrome are sparse. A number of cases have been reported but large observational studies are lacking. In 1982, Schenker and Margalioth 4 found an incidence of placenta accreta in 13–14% of patients with previous Asherman syndrome. Roy et al. 12 reported an incidence of postpartum hemorrhage due to adherent placenta in 12.5% of the 89 women who had undergone hysteroscopic adhesiolysis due to Asherman syndrome. These numbers present a remarkably low incidence of abnormal placentation in a group of women, who all had known trauma of the endometrium (i.e., 87.5% did not have an adherent placenta and subsequent postpartum hemorrhage).
This case report describes a patient with a placenta accreta on posterior uterine wall in a pregnancy following hysteroscopic removal of adhesions due to Asherman syndrome. The finding of an adherent placenta intraoperatively was unexpected, as the patient had been thoroughly examined with sonography during pregnancy.
Conventional 2 dimensional ultrasonography is currently the best screening tool for detecting placenta accreta with a sensitivity of 77–90.7%, a specificity of 96–98%, a positive predictive value of 65–93%, and a negative predictive value of 98% 22–24. Another diagnostic tool for detection of abnormal placentation is Magnetic Resonance Imaging (MRI). MRI has a sensitivity of 80–85% and a specificity of 65–100% 25. MRI can be used in conjunction with conventional ultrasonography 26, and can be helpful in some cases, especially if the placenta is located on the posterior uterine wall. Though it is important to notice, that for cases of placenta accreta, MRI is not a good prognostic tool for changing of surgical management 27. Retrospective studies have shown that women with an antenatal diagnosis of placenta accreta have less blood loss and requirement for blood transfusion than women in whom the abnormal placentation was diagnosed during cesarean section 28,29.
Any patient with a previous history of intrauterine surgery or Asherman syndrome should be thoroughly examined by a skilled sonographer for possible abnormal placentation. In case of any suspicion of abnormal placentation, the patient should be scheduled for planned cesarean section with a set-up of skilled clinicians due to risk of severe postpartum hemorrhage.
In conclusion, it is important to be aware of the risk of a placenta accreta in patients with a previous history of Asherman syndrome and uterine scarring. Antenatal diagnosis is important to counsel the pregnant woman and to plan mode of delivery.
Conflict of Interest
None declared.
References
- Asherman JG. Amenorrhoea traumatica (atretica) J. Obstet. Gynaecol. Br. Emp. 1948;55:23–30. doi: 10.1111/j.1471-0528.1948.tb07045.x. [DOI] [PubMed] [Google Scholar]
- March CM. Management of Asherman's syndrome. Reprod. Biomed. Online. 2011;23:63–76. doi: 10.1016/j.rbmo.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Dmowski WP. Greenblatt RB. Asherman's syndrome and risk of placenta accreta. Obstet. Gynecol. 1969;34:288–299. [PubMed] [Google Scholar]
- Schenker JG. Margalioth EJ. Intrauterine adhesions: an updated appraisal. Fertil. Steril. 1982;37:593–610. doi: 10.1016/s0015-0282(16)46268-0. [DOI] [PubMed] [Google Scholar]
- Westendorp IC, Ankum WM, Mol BW. Vonk J. Prevalence of Asherman's syndrome after secondary removal of placental remnants or a repeat curettage for incomplete abortion. Hum. Reprod. 1998;13:3347–3350. doi: 10.1093/humrep/13.12.3347. [DOI] [PubMed] [Google Scholar]
- Friedler S, Margalioth EJ, Kafka I. Yaffe H. Incidence of post-abortion intra-uterine adhesions evaluated by hysteroscopy–a prospective study. Hum. Reprod. 1993;8:442–444. doi: 10.1093/oxfordjournals.humrep.a138068. [DOI] [PubMed] [Google Scholar]
- March CM. Intrauterine adhesions. Obstet. Gynecol. Clin. North Am. 1995;22:491–505. [PubMed] [Google Scholar]
- March CM. Asherman's syndrome. Semin. Reprod. Med. 2011;29:83–94. doi: 10.1055/s-0031-1272470. [DOI] [PubMed] [Google Scholar]
- Bauer ST. Bonanno C. Abnormal placentation. Semin. Perinatol. 2009;33:88–96. doi: 10.1053/j.semperi.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Eshkoli T, Weintraub AY, Sergienko R. Sheiner E. Placenta accreta: risk factors, perinatal outcomes, and consequences for subsequent births. Am. J. Obstet. Gynecol. 2013;208:219.e1-7. doi: 10.1016/j.ajog.2012.12.037. [DOI] [PubMed] [Google Scholar]
- Tantbirojn P, Crum CP. Parast MM. Pathophysiology of placenta accreta: the role of decidua and extravillous trophoblast. Placenta. 2008;29:639–645. doi: 10.1016/j.placenta.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Roy KK, Baruah J, Sharma JB, Kumar S, Kachawa G. Singh N. Reproductive outcome following hysteroscopic adhesiolysis in patients with infertility due to Asherman's syndrome. Arch. Gynecol. Obstet. 2010;281:355–361. doi: 10.1007/s00404-009-1117-x. [DOI] [PubMed] [Google Scholar]
- Strickland S. Richards WG. Invasion of the trophoblasts. Cell. 1992;71:355–357. doi: 10.1016/0092-8674(92)90503-5. [DOI] [PubMed] [Google Scholar]
- Belfort MA. Placenta accreta. Am. J. Obstet. Gynecol. 2010;203:430–439. doi: 10.1016/j.ajog.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Hull AD. Resnik R. Placenta accreta and postpartum hemorrhage. Clin. Obstet. Gynecol. 2010;53:228–236. doi: 10.1097/GRF.0b013e3181ce6aef. [DOI] [PubMed] [Google Scholar]
- Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet. Gynecol. 2006;107:1226–1232. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- Esh-Broder E, Ariel I, Bashir N, Bdolah Y. Celnikier DH. Placenta accreta is associated with IVF pregnancies: a retrospective chart review. BJOG. 2011;118:1084–1089. doi: 10.1111/j.1471-0528.2011.02976.x. [DOI] [PubMed] [Google Scholar]
- Protopapas A, Shushan A. Magos A. Myometrial scoring: a new technique for the management of severe Asherman's syndrome. Fertil. Steril. 1998;69:860–864. doi: 10.1016/s0015-0282(98)00036-3. [DOI] [PubMed] [Google Scholar]
- The American Fertility Society. The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, mullerian anomalies and intrauterine adhesions. Fertil. Steril. 1988;49:944–955. doi: 10.1016/s0015-0282(16)59942-7. [DOI] [PubMed] [Google Scholar]
- Kjer JJ. Asherman syndrome in a Danish population. Acta Obstet. Gynecol. Scand. 2014;93:425–427. doi: 10.1111/aogs.12347. [DOI] [PubMed] [Google Scholar]
- Johary J, Xue M, Zhu X, Xu D. Velu PP. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J. Minim. Invasive Gynecol. 2014;21:44–54. doi: 10.1016/j.jmig.2013.07.018. [DOI] [PubMed] [Google Scholar]
- D'Antonio F, Iacovella C. Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2013;42:509–517. doi: 10.1002/uog.13194. [DOI] [PubMed] [Google Scholar]
- Warshak CR, Eskander R, Hull AD, Scioscia AL, Mattrey RF, Benirschke K, et al. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of placenta accreta. Obstet. Gynecol. 2006;108(3 Pt 1):573–581. doi: 10.1097/01.AOG.0000233155.62906.6d. [DOI] [PubMed] [Google Scholar]
- Comstock CH, Love JJ, Jr, Bronsteen RA, Lee W, Vettraino IM, Huang RR, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am. J. Obstet. Gynecol. 2004;190:1135–1140. doi: 10.1016/j.ajog.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Gielchinsky Y, Mankuta D, Rojansky N, Laufer N, Gielchinsky I. Ezra Y. Perinatal outcome of pregnancies complicated by placenta accreta. Obstet. Gynecol. 2004;104:527–530. doi: 10.1097/01.AOG.0000136084.92846.95. [DOI] [PubMed] [Google Scholar]
- Levine D, Hulka CA, Ludmir J, Li W. Edelman RR. Placenta accreta: evaluation with color Doppler US, power Doppler US, and MR imaging. Radiology. 1997;205:773–776. doi: 10.1148/radiology.205.3.9393534. [DOI] [PubMed] [Google Scholar]
- McLean LA, Heilbrun ME, Eller AG, Kennedy AM. Woodward PJ. Assessing the role of magnetic resonance imaging in the management of gravid patients at risk for placenta accreta. Acad. Radiol. 2011;18:1175–1180. doi: 10.1016/j.acra.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Warshak CR, Ramos GA, Eskander R, Benirschke K, Saenz CC, Kelly TF, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet. Gynecol. 2010;115:65–69. doi: 10.1097/AOG.0b013e3181c4f12a. [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Paavonen J, Loukovaara M. Stefanovic V. Antenatal diagnosis of placenta accreta leads to reduced blood loss. Acta Obstet. Gynecol. Scand. 2011;90:1140–6. doi: 10.1111/j.1600-0412.2011.01147.x. [DOI] [PubMed] [Google Scholar]


