Abstract
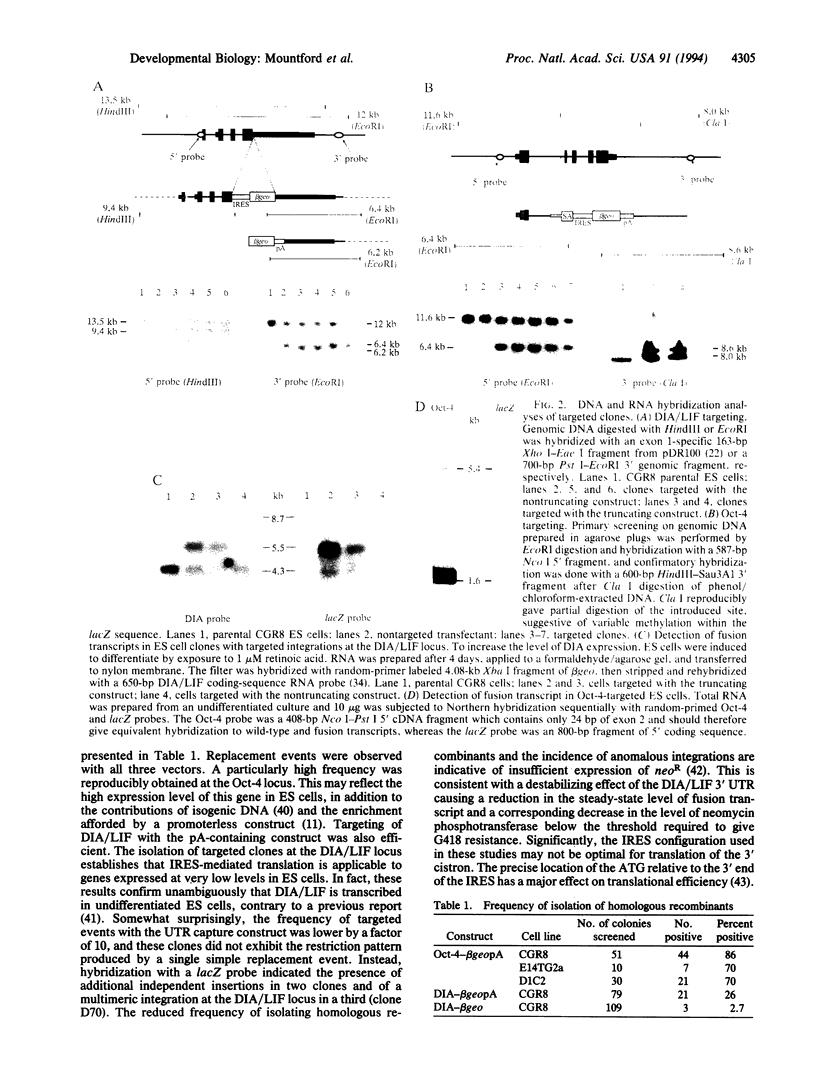
To investigate the activity of candidate regulatory molecules in mammalian embryogenesis, we have developed a general strategy for modifying and reporting resident chromosomal gene expression. The picornaviral internal ribosome-entry site was incorporated into gene targeting constructs to provide cap-independent translation of a selectable marker from fusion transcripts generated following homologous recombination. These promoterless constructs were highly efficient and have been used both to inactivate the stem-cell-specific transcription factor Oct-4 and to introduce a quantitative regulatory modification into the gene for a stem-cell maintenance factor, differentiation-inhibiting activity. In addition, the inclusion of a beta-galactosidase reporter gene in the constructs enabled accurate and sensitive detection of cellular sites of transcription. This has allowed visualization of putative "stem-cell niches" in which sources of elevated expression of differentiation-inhibiting activity were localized to the differentiated cells surrounding colonies of stem cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askew G. R., Doetschman T., Lingrel J. B. Site-directed point mutations in embryonic stem cells: a gene-targeting tag-and-exchange strategy. Mol Cell Biol. 1993 Jul;13(7):4115–4124. doi: 10.1128/mcb.13.7.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddington R. S., Morgernstern J., Land H., Hogan A. An in situ transgenic enzyme marker for the midgestation mouse embryo and the visualization of inner cell mass clones during early organogenesis. Development. 1989 May;106(1):37–46. doi: 10.1242/dev.106.1.37. [DOI] [PubMed] [Google Scholar]
- Bhatt H., Brunet L. J., Stewart C. L. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11408–11412. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. R. A physical map of the human pseudoautosomal region. EMBO J. 1988 Aug;7(8):2377–2385. doi: 10.1002/j.1460-2075.1988.tb03082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Conquet F., Brûlet P. Developmental expression of myeloid leukemia inhibitory factor gene in preimplantation blastocysts and in extraembryonic tissue of mouse embryos. Mol Cell Biol. 1990 Jul;10(7):3801–3805. doi: 10.1128/mcb.10.7.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escary J. L., Perreau J., Duménil D., Ezine S., Brûlet P. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993 May 27;363(6427):361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- Friedrich G., Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991 Sep;5(9):1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Ghattas I. R., Sanes J. R., Majors J. E. The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol. 1991 Dec;11(12):5848–5859. doi: 10.1128/mcb.11.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossler A., Joyner A. L., Rossant J., Skarnes W. C. Mouse embryonic stem cells and reporter constructs to detect developmentally regulated genes. Science. 1989 Apr 28;244(4903):463–465. doi: 10.1126/science.2497519. [DOI] [PubMed] [Google Scholar]
- Gu H., Zou Y. R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993 Jun 18;73(6):1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Hilton D. J., Gough N. M. Leukemia inhibitory factor: a biological perspective. J Cell Biochem. 1991 May;46(1):21–26. doi: 10.1002/jcb.240460105. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990 Sep;4(9):1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- Jasin M., Berg P. Homologous integration in mammalian cells without target gene selection. Genes Dev. 1988 Nov;2(11):1353–1363. doi: 10.1101/gad.2.11.1353. [DOI] [PubMed] [Google Scholar]
- Le Mouellic H., Lallemand Y., Brûlet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992 Apr 17;69(2):251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Deng C. X., Capecchi M. R. Introduction of a lacZ reporter gene into the mouse int-2 locus by homologous recombination. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7688–7692. doi: 10.1073/pnas.87.19.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Jang S. K., Paul A. V., Reuer Q., Wimmer E. Cardioviral internal ribosomal entry site is functional in a genetically engineered dicistronic poliovirus. Nature. 1992 Mar 19;356(6366):255–257. doi: 10.1038/356255a0. [DOI] [PubMed] [Google Scholar]
- Nichols J., Evans E. P., Smith A. G. Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development. 1990 Dec;110(4):1341–1348. doi: 10.1242/dev.110.4.1341. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Pilipenko E. V., Gmyl A. P., Maslova S. V., Svitkin Y. V., Sinyakov A. N., Agol V. I. Prokaryotic-like cis elements in the cap-independent internal initiation of translation on picornavirus RNA. Cell. 1992 Jan 10;68(1):119–131. doi: 10.1016/0092-8674(92)90211-t. [DOI] [PubMed] [Google Scholar]
- Rathjen P. D., Nichols J., Toth S., Edwards D. R., Heath J. K., Smith A. G. Developmentally programmed induction of differentiation inhibiting activity and the control of stem cell populations. Genes Dev. 1990 Dec;4(12B):2308–2318. doi: 10.1101/gad.4.12b.2308. [DOI] [PubMed] [Google Scholar]
- Rathjen P. D., Toth S., Willis A., Heath J. K., Smith A. G. Differentiation inhibiting activity is produced in matrix-associated and diffusible forms that are generated by alternate promoter usage. Cell. 1990 Sep 21;62(6):1105–1114. doi: 10.1016/0092-8674(90)90387-t. [DOI] [PubMed] [Google Scholar]
- Robertson M., Chambers I., Rathjen P., Nichols J., Smith A. Expression of alternative forms of differentiation inhibiting activity (DIA/LIF) during murine embryogenesis and in neonatal and adult tissues. Dev Genet. 1993;14(3):165–173. doi: 10.1002/dvg.1020140303. [DOI] [PubMed] [Google Scholar]
- Rosner M. H., Vigano M. A., Ozato K., Timmons P. M., Poirier F., Rigby P. W., Staudt L. M. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990 Jun 21;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P. L., Robertson E. J., Goff S. P. Targeted gene disruption of the endogenous c-abl locus by homologous recombination with DNA encoding a selectable fusion protein. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3210–3214. doi: 10.1073/pnas.87.8.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Balling R., Hatzopoulos A. K., Suzuki N., Gruss P. Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO J. 1989 Sep;8(9):2551–2557. doi: 10.1002/j.1460-2075.1989.tb08393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Dressler G. R., Balling R., Rohdewohld H., Gruss P. Oct-4: a germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990 Jul;9(7):2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R. Octamania: the POU factors in murine development. Trends Genet. 1991 Oct;7(10):323–329. doi: 10.1016/0168-9525(91)90422-m. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Heath J. K., Donaldson D. D., Wong G. G., Moreau J., Stahl M., Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988 Dec 15;336(6200):688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Hooper M. L. Buffalo rat liver cells produce a diffusible activity which inhibits the differentiation of murine embryonal carcinoma and embryonic stem cells. Dev Biol. 1987 May;121(1):1–9. doi: 10.1016/0012-1606(87)90132-1. [DOI] [PubMed] [Google Scholar]
- Smith A. G. Mouse embryo stem cells: their identification, propagation and manipulation. Semin Cell Biol. 1992 Dec;3(6):385–399. doi: 10.1016/1043-4682(92)90010-s. [DOI] [PubMed] [Google Scholar]
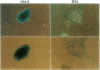
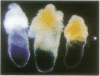
- Smith A. G., Nichols J., Robertson M., Rathjen P. D. Differentiation inhibiting activity (DIA/LIF) and mouse development. Dev Biol. 1992 Jun;151(2):339–351. doi: 10.1016/0012-1606(92)90174-f. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Kaspar P., Brunet L. J., Bhatt H., Gadi I., Köntgen F., Abbondanzo S. J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992 Sep 3;359(6390):76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Ure J. M., Fiering S., Smith A. G. A rapid and efficient method for freezing and recovering clones of embryonic stem cells. Trends Genet. 1992 Jan;8(1):6–6. doi: 10.1016/0168-9525(92)90004-n. [DOI] [PubMed] [Google Scholar]
- Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., Wagner E. F., Metcalf D., Nicola N. A., Gough N. M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988 Dec 15;336(6200):684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Wood S. A., Pascoe W. S., Schmidt C., Kemler R., Evans M. J., Allen N. D. Simple and efficient production of embryonic stem cell-embryo chimeras by coculture. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4582–4585. doi: 10.1073/pnas.90.10.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Maandag E. R., Berns A. Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5128–5132. doi: 10.1073/pnas.89.11.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]