Abstract
Importance
Patients often report greater visual difficulties at home than expected from vision testing in the clinic. Such discordance may be due to worse vision in the home than measured in clinic.
Objective
To compare vision measured between clinic and home and evaluate factors, including lighting, associated with these differences.
Design
Cross-sectional, between years 2005–2009.
Setting
Participants recruited from the Glaucoma and Comprehensive Eye Clinics at Washington University, St. Louis, MO underwent a clinic and home visit.
Participants
126 glaucoma and 49 non-glaucoma patients, ages 55–90 years, consecutively recruited and met inclusion criteria for this report; 166 eligible patients refused participation.
Exposure
Participants underwent a clinic and home visit randomized to order of completion. At each visit, masked and certified examiners measured binocular distance visual acuity (DVA) with a non-backlit chart, near visual acuity (NVA), contrast sensitivity (CS), CS with glare, and lighting.
Main Outcome Measure
Differences in vision between clinic and home.
Results
Mean scores for all vision tests were significantly better in the clinic than home for glaucoma and non-glaucoma patients (p<0.05, matched pair t-tests). For DVA, 29% of glaucoma participants read ≥2 lines better in clinic than home and 39% with advanced glaucoma read ≥3 lines better. For the entire sample, 21% of participants read ≥2 lines better in clinic than home for NVA and 49% read ≥2 triplets better in clinic for CS with glare. Lighting was the most significant factor associated with differences in vision between clinic and home for DVA, NVA, and CS with glare testing (p<0.05 multiple regression model). Median home lighting was 4.3 times and 2.8 times lower than clinic lighting in areas tested for DVA and NVA, respectively. Home lighting was below that recommended in ≥ 85% of participants.
Conclusions and Relevance
Vision measured in the clinic is generally better than vision measured at home, with differences mainly due to poor home lighting. Knowledge that vision discrepancies between patient report and clinical testing may be due to home lighting may initiate clinician-patient discussions to increase home lighting and improve vision of older adults in their homes.
Introduction
Clinicians often assume that vision measured in the clinic is equivalent to vision at home. Many patients, however, report visual difficulties greater than expected based on their vision testing in the clinic. Measurement disparities between clinical and non-clinical settings have been reported for blood pressure1,2 and cognitive function.3 Differences in vision measured in the clinic and home may also exist and partially explain the discord between patient report and clinical testing.
In a seminal study in 1978 comparing clinic and home visual acuity, Silver et al. reported poorer vision testing in the home than in clinic for 56 low vision patients, 4 of which had glaucoma.4 Increased home lighting improved visual acuity at home in the majority of these patients. These results, however, are not necessarily generalizable to glaucoma patients with mild or moderate visual impairment or older adults without ocular disease. The purpose of this report was to 1) compare vision between clinic and home and 2) evaluate factors, including lighting, associated with differences in vision between clinic and home in older adults with mild, moderate, and advanced glaucoma and no ocular disease.
Patients and Methods
Consecutive eligible patients, ages 55–90 years, with a clinical diagnosis of glaucoma and age range-matched (by decades) normal controls were recruited from their regularly scheduled eye appointments in the Glaucoma and Comprehensive Eye Clinics at Washington University School of Medicine between December 15, 2005 and July 7, 2009. A target sample size for this pilot study was 50 participants for each group of mild, moderate, and advanced glaucoma and 50 non-glaucoma participants. This sample size was selected to detect an effect size of 0.50 between normals and glaucoma groups with a power of 0.8 and two-sided alpha of 0.05. A glaucoma diagnosis was based on glaucomatous optic nerve cupping and reproducible visual field defects (i.e. three adjacent points depressed at p>5% with one point depressed at p<1%) in one or both eyes and included primary open angle, pigmentary dispersion, pseudoexfoliation, and chronic angle closure glaucoma. A non-glaucoma diagnosis was based on the absence of ocular pathology affecting the patient’s vision. Patients were excluded if they had neovascular, uveitic, or acute angle closure glaucoma, ocular hypertension, a glaucoma suspect status, nonglaucomatous ocular disease, visually significant cataracts (distance visual acuity ≤ 20/40 and ≥ grade 2 nuclear sclerosis), myopia > 6 diopters, current use of miotic glaucoma medications, incisional or laser eye surgery within 3 months of enrollment, severely impaired cognition (Short Blessed Test score >10), self-reported physical disability limiting function (e.g. stroke), unreliable visual field parameters (>20% fixation losses or >33% false negatives or false positives) resided in a nursing home, or for whom English was not their primary language.
Demographic and clinical data including cataract status, cup to disc ratio, central corneal thickness, visual field parameters, ocular and systemic medications, and co-morbidities were recorded. The study protocol was in accordance with the Declaration of Helsinki and was approved by the Human Research Protection Office at Washington University School of Medicine in St. Louis, Missouri. A written informed consent was obtained from all eligible participants prior to participating in the study.
Participants were scheduled for both a clinic and home visit; visits were randomized with respect to order of testing using a computer-generated, randomized, permuted block design with equal allocation to both testing orders. The clinic visit occurred in a clinic exam room and the home visit occurred in the participant’s primary home. Both clinic and home visits were attempted to be scheduled within 1–4 weeks to minimize changes in clinical status and between the hours of 9 am and 4 pm to minimize effects from outdoor lighting on clinical and functional testing. The time of day, visit duration, and weather (e.g. rainy, sunny) during the home visit were recorded.
Monocular visual field testing was conducted for glaucoma participants using the Humphrey Visual Field (HVF) Analyzer II (Carl Zeiss Meditec, Dublin, CA, USA) equipped with the SITA Standard algorithm. Participants with vision worse than 20/200 underwent a Goldmann visual field test. Visual field testing was not performed if a reliable visual field within 6 months of the first study visit was available. The visual field for each eye was classified into a glaucoma stage (0–5) using the Glaucoma Staging System.5 Reportedly, visual acuity in the better-seeing eye is a good predictor of visual disability6 thus the eye with the less severe glaucoma stage was used to further classify participants into mild (stages 0–1), moderate (stages 2–3), or advanced (stages 4–5) stage of glaucoma. Glaucoma classification of eight participants with Goldmann visual field testing in both eyes was determined by agreement of two clinicians (AB and SC).
Examiners
Over a four-year study period, examiners included 2 research coordinators and 19 graduate students in the Program in Occupational Therapy at Washington University, St. Louis, MO. Prior to data collection, examiners completed a 6-week course on test administration, lighting evaluation, safety training for home visits, and a certification for vision testing. Inter-grader reliability between examiners for vision testing was high. For years 2005–2007, the intra class correlation coefficients (ICCs) ranged from 0.91 to 0.94 (Shrout-Fleiss reliability: single score). For years 2007–2009, the mean absolute percent difference between examiners ranged from 0.23% to 1.64%. The mean absolute percent difference was calculated because the variance between subjects and between graders was so low that it violated the assumptions of the ICC. Examiners also completed a practice clinic and home visit on older adult volunteers monitored by one of the authors (AB, MP, or MG). Each study visit was conducted by 1–2 examiners. Examiners conducting the first and second visits were not necessarily the same. All examiners were masked to the participant diagnosis and used a scripted interview.
Clinical Measures of Vision
At clinic and home visits, participants underwent binocular testing of distance visual acuity (DVA), near visual acuity (NVA), contrast sensitivity (CS), and contrast sensitivity with glare testing (CS with glare). All vision testing used strict forced-choice testing procedures. The order of vision testing (i.e. NVA, DVA, CS, CS with glare) was identical for both clinic and home visits and occurred at approximately the same time within each visit. In the home, NVA testing occurred where the participant routinely performed near tasks (e.g. read books, paid bills) while DVA, CS, and CS with glare testing were performed in the room most utilized by the participant (e.g. living room). Alternate chart versions were used between clinic and home visits for each vision measure. Participants were tested with their habitual correction at both visits.
Distance visual acuity (DVA)
Binocular DVA was measured using non-illuminated ETDRS charts (Precision Vision, CAT. NO 2110) at 3.2 meter testing distance as previously described.7 Testing occurred at 1.6 meters for 2 glaucoma participants who were unable to read any letters at 3.2 meters and 8 glaucoma participants whose home space precluded testing at 3.2 meters. Distance visual acuity was scored as the number of letters correctly identified, adjusting for testing distance.8 Letters correctly identified were also converted to lines correctly seen using the classification: 3–7 letters =1 line, 8–12 letters = 2 lines and ≥13 letters = ≥ 3 lines.9,10 Prior studies have reported a clinically meaningful change in visual function with a ≥2 line change in visual acuity.10,11 Therefore, we used a ≥2 line difference between clinic and home as the threshold for a clinically significant difference.
Near visual acuity (NVA)
Binocular NVA was measured using Lighthouse Near Visual Acuity card (Lighthouse, CAT No. C170) at the standard distance of 16.0 inches and at the participant’s preferred reading distance. For this report, NVA scores from the participant’s preferred reading distance only are analyzed. Included in the analysis were only patients tested for NVA at preferred distance in both the clinic and home. Sixty patients were tested in the clinic at standard distance only and are therefore excluded from analysis of NVA testing. NVA testing occurred under diffuse overhead lighting in the clinic and under customary lighting for near work (including increased task lighting) in the home. NVA was scored similarly to DVA.
Contrast Sensitivity (CS)
Binocular CS was measured using the Pelli-Robson contrast sensitivity chart (Clement Clarke International, Ref # 7002250) at 1 meter.12 CS was scored by the number of triplets correctly identified (with at least 2 of 3 letters correctly identified in a triplet) and converted to log10 contrast for analysis. We used a ≥2 triplet difference between clinic and home as the threshold for a clinically significant difference.
CS with Brightness Acuity Tester (BAT) on low and medium glare
Binocular CS with glare testing was measured using bilateral BATs (Mentor, Norwell, MA) in conjunction with a Pelli-Robson chart of a version different than that used for CS testing. Participants placed one BAT over each eye and adjusted their positions until binocular fusion was obtained. Participants were asked to identify as many triplets as possible with both BATS placed on low setting (300 footcandles) and subsequently on medium setting (2500 footcandles). The examiner routinely checked for binocular fusion throughout testing and assisted with stabilization of the BATs if the participant was unable to do so. Low and medium glare testing was scored similarly to CS. Contrast sensitivity with glare testing on medium setting is reported in this analysis.
Lighting
A digital light meter (Extech Easy View, EA30) was used to measure incident light levels at the upper right and lower left corner of each vision chart at clinic and home visits. Vision testing in the clinic was conducted with lighting in the recommended range for vision testing of 200–550 lux. Data for vision tests were excluded if clinic lighting did not meet these requirements. At the home visit, lighting was measured in the location where NVA was tested and in the location where DVA, CS, and CS with glare testing occurred. Participants were tested in the home under habitual lighting conditions and, as stated previously, were allowed to use customary increased lighting for NVA testing. After completion of the first 10 participant visits, we improved our assessment of lighting by replacing a light meter measuring reflective light (Luna-Pro digital light meter, model number 4022) with the Extech light meter which measures incident light. For this reason, data from these 10 participants were excluded from analysis in this report.
Interviewer-administered questionnaires and performance-based measures
Self-reported questionnaires were administered by an examiner using large font-size cue cards with response options. Questionnaires pertinent to this report are explained in detail below.
Medical Index
A modified version of the Duke Medical Index was used to identify co-morbidities potentially affecting daily function and quality of life.13 The medical index includes arthritis, asthma, emphysema/bronchitis, high/low blood pressure, cardiac disease, circulatory disease, diabetes, anemia, stroke, neuromuscular disease, back pain, and cancer.
Hollingshead Index of Social Position
Education and occupation levels were coded using the scales from the Hollingshead Index of Social Position.14 Education level of the participant was classified on a scale of 1 (graduate professional training) to 7 (less than 7 years of school). We sub-classified patients with education levels 1–3 as “some college or more”. The occupation level of the head of the household was classified on a scale of 1 (e.g. major professional) to 7 (e.g. unskilled worker) with levels 1–3 sub-classified as “major or minor professionals”.
Short Blessed Test
The Short Blessed Test is a reliable and valid tool used to screen for dementia in community-dwelling and long-term care populations.15,16Scores range from 0 to 28 with scores >10 suggestive of cognitive impairment.17
Geriatric Depression Scale(GDS)
The Geriatric Depression Scale Short Form is a 15-item version of the 30-item screening test for depression.18,19 Scores range from 0–15 with scores ≥5 indicative of possible depression.
All data were entered in a double-data entry fashion with discrepancies manually checked and re-entered.
Statistical analysis
Descriptive statistics (mean, median, and standard deviation) are reported for vision measures (i.e. DVA, NVA, CS, CS with glare) and lighting levels assessed in the clinic and home. Analyses of lighting levels were conducted using a log transformation of lux. For the overall sample, vision scores and lighting were compared between clinic and home using matched pair t-tests. The effect of glaucoma severity on home lighting and on differences in vision scores between clinic and home (e.g. DVA in clinic minus DVA in home) were tested using analysis of variance (ANOVA). In addition, a planned comparison between non-glaucoma and the advanced glaucoma groups was performed.
For each vision measure (DVA, NVA, CS, and CS with glare), the difference in vision scores between clinic and home (e.g. DVA in clinic minus DVA in home) and the following factors were evaluated: age, gender, race, education, occupation, number of co-morbidities, glaucoma status, cataract status, Short Blessed Score, Geriatric Depression Scale score, and lighting differences between clinic and home. Factors correlated with differences in any of the vision scores (Pearson correlation with p ≤ 0.10) were included in multiple regression analyses with differences in vision scores as the dependent variable. Separate multiple regression analyses were performed for each vision test (DVA, NVA, CS, CS with glare). We performed similar analyses with the dependent variable dichotomized into ≥2 lines vs. <2 lines difference on vision testing between clinic and home (clinic minus home). A similar approach was used to analyze factors associated with home lighting in areas tested for DVA and NVA. Odds ratios to characterize the relationship between differences in lighting between clinic and home and differences in vision between clinic and home were calculated using a logistic regression model.
Statistical analyses were performed using SAS version 9.2; SAS Inc, Cary, North Carolina.
Results
Of 356 potentially eligible patients identified by chart review, 190 (53%) agreed to participate - 138 glaucoma and 52 non-glaucoma patients. Reasons for study refusal included lack of interest (n=62), health (n=39), scheduling (n=31), transportation difficulties (n=28), and refusal of home visit (n=6).
Participants were excluded from this report if 1. both clinic and home visits were not completed (n=3) 2. lighting was measured in reflective as opposed to incident levels (n=10) 3. lighting was not within the standard range for all clinic vision tests (n=2) or 4. binocular vision was no light perception (n=1). One participant was excluded for both criteria 1 and 2 above. Thus, data from 175 participants (92% of total 190 participants; 126 glaucoma and 49 non-glaucoma) were available for analysis. There were 58 glaucoma patients classified as mild, 49 as moderate, and 19 as advanced stage of glaucoma. There was a higher refusal rate of patients with advanced glaucoma due to unwillingness to complete a home assessment. Data were excluded from analysis if clinic lighting was not within 200–550 lux as required by protocol (n=20 for NVA, n=3 for CS, n=2 for CS with glare) or if habitual correction differed between clinic and home testing (n=8 for NVA, n=11 for DVA, n=10 for CS, n=10 for CS with glare).
Of the 175 participants, 89 (51%) completed the clinic visit first and 86 (49%) completed the home visit first. The mean number of days between clinic and home visits was 14.0 ± 20.6 days (median of 7.0 days). Fourteen percent of the total sample (n=24) had clinic and home visits scheduled >4 weeks apart. In this subset of participants, there was no difference by disease diagnosis (12% non-glaucoma vs. 14% glaucoma) or order of visits (11% clinic before home vs. 16% home before clinic). The mean visit duration was 0.9± 0.3 hours for the clinic visit and 1.5 ±0.4 hours for the home visit.
Baseline demographic characteristics of glaucoma and non-glaucoma participants in this report were similar with the exception of mean deviation on visual field tests (Table 1). For the overall sample, mean scores for DVA, NVA, CS, and CS with glare tests were statistically significantly better in the clinic than at home (p<0.05, Table 2). There was no overall linear trend by glaucoma severity for differences in vision scores between clinic and home for any of the vision tests (p>0.05). However, differences in vision scores between the clinic and home (clinic better than home) were significantly greater in the advanced glaucoma group compared to the non-glaucoma group for DVA testing (p=0.01) and significantly smaller in the advanced glaucoma group compared to the non-glaucoma group for CS testing (p=0.03).
Table 1.
Baseline Characteristics for Glaucoma and Non-glaucoma Participants
| Characteristic | Glaucoma (n=126) |
Non-glaucoma (n=49) |
|---|---|---|
| Mean ± SD or % | ||
| Age, years | 72.5 ± 7.7 | 70.7 ± 8.2 |
| Female | 57.1% | 61.2% |
| African American | 37.3% | 22.4% |
| Married | 52.4% | 59.2% |
| Some college or more | 69.0% | 69.4% |
| Major or minor professional | 54.9% | 70.8% |
| # systemic co-morbidities | 2.4 ±1.6 | 2.4 ±1.6 |
| Pseudophakic (at least 1 eye) | 59.5% | 46.9% |
| Spherical Equivalent, Diopters† | −0.36 | −0.64 |
| Visual field test MD, dB†* | −7.1 ± 8.1 | −2.0 ± 2.4 |
| Short Blessed test, score (0 best to 28 worst) | 3.1 ±4.0 | 2.7 ±4.1 |
MD = Mean Deviation; dB = decibel
In eye with less severe visual field loss
p<0.05, two-sample student’s t-test
Table 2.
Mean Vision Scores in Clinic and Home for Glaucoma and Non-glaucoma Participants
| Vision Measure | Glaucoma | Non-glaucoma | All | |||
|---|---|---|---|---|---|---|
| Clinic (n) | Home (n) | Clinic (n) | Home (n) | Clinic (n) | Home (n) | |
| Distance VA ± SD, mean letters | 53.4 ± 13.1* (119) |
48.9 ± 14.7 (119) |
58.8 ± 8.1* (45) |
56.1 ± 6.8 (45) |
54.9 ± 12.2* (164) |
50.9 ± 13.4 (164) |
| Near VA ± SD, mean letters | 58.7 ± 16.1 (67) |
57.0 ± 16.8 (67) |
66.1 ± 9.5 (36) |
64.1 ± 9.0 (36) |
61.3 ± 14.5* (103) |
59.5 ± 14.9 (103) |
| Contrast Sensitivity ± SD, mean log contrast | 1.50 ± 0.37* (117) |
1.46 ± 0.39 (117) |
1.78 ± 0.20* (45) |
1.70 ± 0.20 (45) |
1.58 ± 0.36* (162) |
1.52 ± 0.36 (162) |
| Contrast Sensitivity with BAT medium glare ± SD, mean log contrast | 1.38 ± 0.41* (112) |
1.15 ± 0.40 (112) |
1.69 ± 0.21* (46) |
1.40 ± 0.30 (46) |
1.47 ± 0.39* (158) |
1.22 ± 0.39 (158) |
VA = Visual Acuity; SD = Standard Deviation; BAT = Brightness Acuity Tester.
Significant difference in vision scores between clinic and home, p<0.05, matched pair t-test
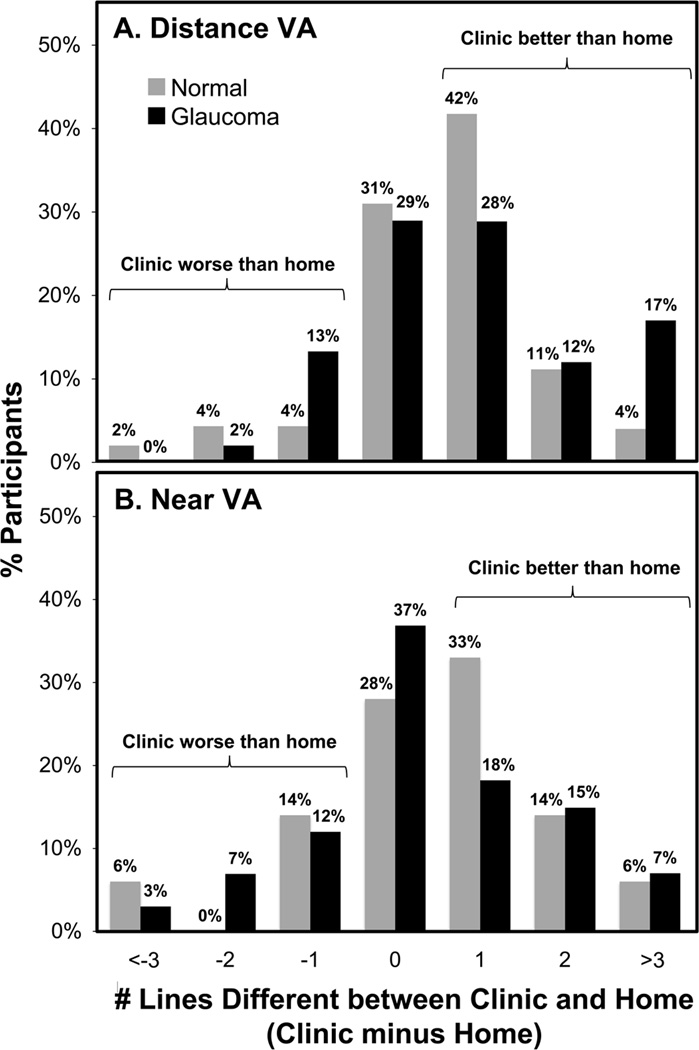
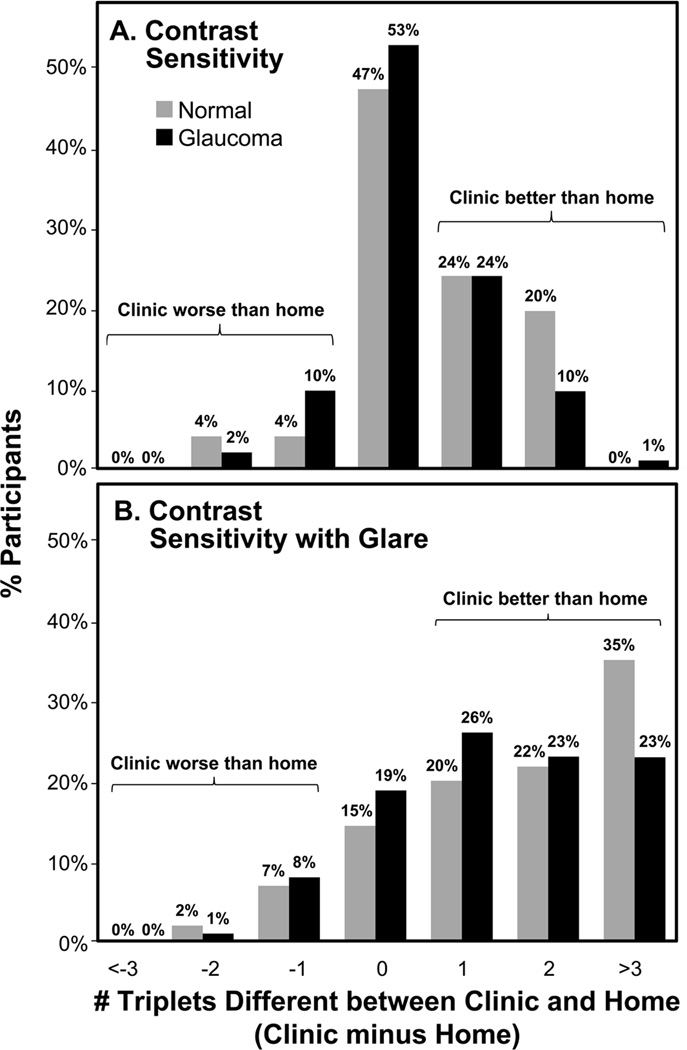
For DVA, 29% of glaucoma and 16% of non-glaucoma participants read ≥ 2 lines better in clinic than home (Figure 1A); 44% and 39% of the advanced glaucoma group read ≥ 2 lines and ≥ 3 lines better, respectively, in clinic than home. For NVA, 22% of glaucoma and 19% of non-glaucoma participants read ≥ 2 lines better in clinic than home (Figure 1B). For CS, 10% of glaucoma and 20% of non-glaucoma participants read ≥2 triplets better in clinic than home (Figure 2A). For CS with glare, 46% of glaucoma and 56% of non-glaucoma participants read ≥ 2 triplets better in clinic than home (Figure 2B).
Figure 1.
Differences in number of lines read correctly between clinic and home for Distance VA (A) and Near VA (B) testing for glaucoma and non-glaucoma participants. VA=Visual Acuity.
Figure 2.
Differences in number of triplets read correctly between clinic and home for CS (A) and CS with Glare (B) testing for glaucoma and non-glaucoma participants. CS=Contrast Sensitivity.
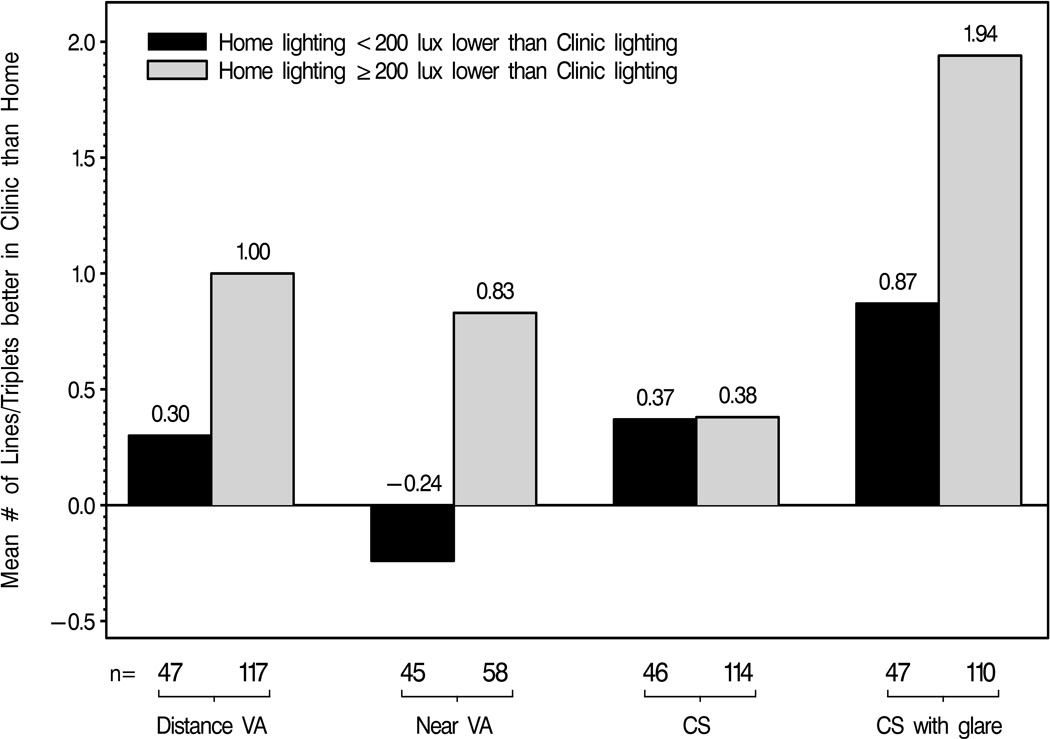
Higher lighting in the clinic compared to home was the strongest statistically significant factor associated with better vision in the clinic than home in univariate and multivariate analyses (p<0.05 for DVA, NVA, and CS with glare). Other factors associated with differences in vision scores between clinic and home for DVA, NVA, and CS with glare testing on univariate analyses (p<0.05) included: age, gender, race, occupation, and the GDS. In separate multiple regression models that included the aforementioned factors, better performance in the clinic than home (p<0.05) for DVA and NVA testing was male gender and for CS with glare was higher occupational level. Neither lighting, nor any other factor in the model, was significantly associated with differences in CS testing alone between clinic and home. Figure 3 illustrates the relationship between differences in vision between clinic and home and differences in lighting between clinic and home.
Figure 3.
Mean number of lines or triplets on vision testing measured better in clinic than home by differences in lighting between clinic and home for glaucoma and non-glaucoma participants. VA = Visual Acuity; CS = Contrast Sensitivity.
In a logistic regression analysis, clinically significant better vision in the clinic than home (≥ 2 lines/triplets difference) was associated with lower lighting in the home than clinic for DVA, NVA, and CS with glare testing (p<0.05). The odds that a patient with home lighting ≥ 200 lux lower than clinic lighting will read ≥ 2 lines/triplets better in clinic than home (p<0.05) was 2.9 (95% CI=1.1 to 7.5) for DVA, 4.6 (95% CI = 1.4 to 14.8) for NVA, and 4.2 (95% CI = 2.0 to 9.0) for CS with glare testing.
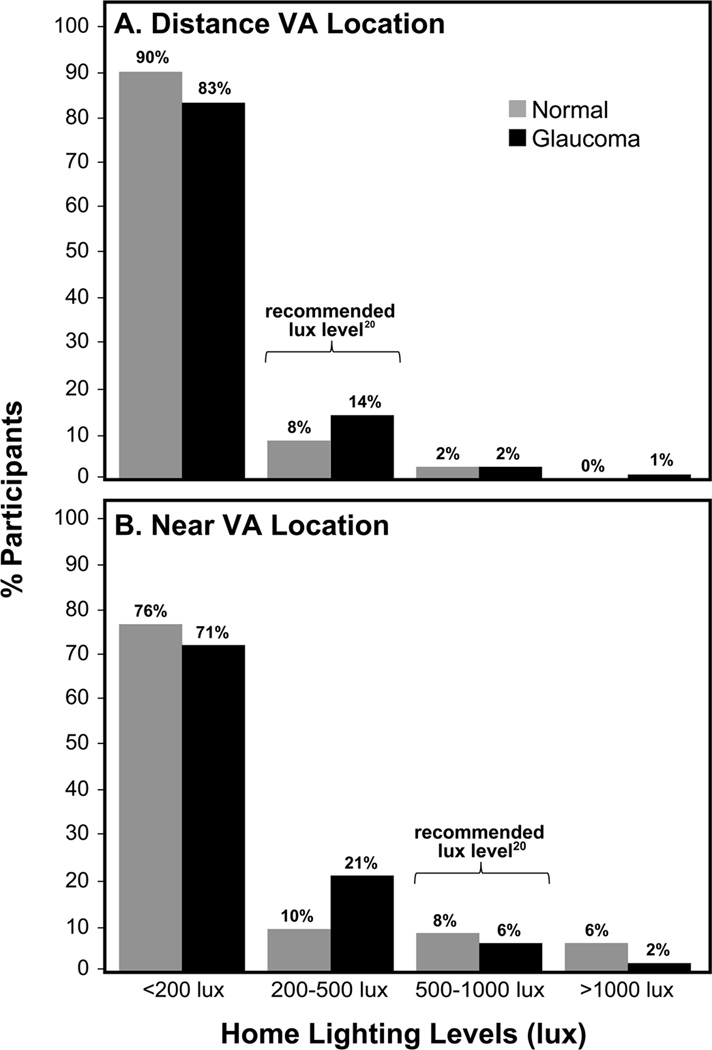
Lighting was significantly higher in the clinic than home in locations tested for DVA and NVA for both glaucoma and non-glaucoma groups (p<0.001, Table 3). Median lighting levels for all participants was 4.3 times higher in the clinic than home for DVA testing locations (4.0 for glaucoma and 4.6 for non-glaucoma groups) and 2.8 times higher in the clinic than home for NVA testing locations (2.8 for glaucoma and 3.2 for non-glaucoma groups). For NVA testing, home lighting was higher than clinic lighting in 21% of participants. Figure 4 displays the proportion of glaucoma and non-glaucoma participants with home lighting levels below, within, and above the recommended lighting levels20 in locations tested for DVA and NVA. Home lighting levels were below those recommended for 85% and 90% of the total sample in locations tested for DVA and NVA, respectively. There were no statistically significant differences in home lighting by glaucoma severity (p>0.10).
Table 3.
Median Lighting (lux) for Distance VA and Near VA Testing Locations in the Clinic and Home for Glaucoma and Non-glaucoma Participants.
| Location | Glaucoma | Non-glaucoma | All | |||
|---|---|---|---|---|---|---|
| Clinic (n) | Home (n) | Clinic (n) | Home (n) | Clinic (n) | Home (n) | |
| Lux at Distance VA location: median, range | 335.5* 223.5 – 548.0 (126) |
84.8 5.0 – 1435.0 (126) |
333.5* 252.5 – 417.5 (49) |
72.0 9.5 – 864.0 (49) |
335.0* 223.5 – 548.0 (175) |
78.0 5.0 – 1435.0 (175) |
| Lux at Near VA location: median, range | 357.8* 207.5 – 532.0 (112) |
127.8 6.5 – 1538.0 (112) |
363.5* 214.5 – 543.5 (43) |
114.0 8.0 – 1268.0 (43) |
358.5* 207.5 – 543.5 (155) |
126.0 6.5 – 1538.0 (155) |
VA = Visual Acuity
Significant difference in mean lighting between clinic and home, p<0.001, matched pair t-test.
Figure 4.
Home lighting in testing locations for Distance VA (A) and Near VA (B) for glaucoma and non-glaucoma participants. VA = Visual Acuity.
In multiple regression analysis, the only factor associated with lower home lighting for DVA was higher number of co-morbidities (partial r=−0.21, p<0.05). Factors associated with lower home lighting for NVA were younger age (partial r=0.19, p<0.05) and self-reported African American race (partial r=−0.16, p<0.05). Home lighting for NVA was below recommended levels in 98% of African Americans and 87% of non-African Americans.
Comment
A patient’s visual function in their home is clearly important, yet only a handful of studies have assessed vision or visual function in the home.4, 21–23 In addition, only a few studies4,21 have evaluated whether vision measured in the clinic has strong ecological validity (i.e. approximates vision measured at home). In our comprehensive study, vision (DVA, NVA, CS, and CS with glare) measured in the clinic was statistically significantly better than vision measured at home for glaucoma patients, regardless of disease severity, and non-glaucoma patients.
In our study sample, more than half of all glaucoma and non-glaucoma participants measured DVA better in clinic than home and almost one-third of glaucoma participants read ≥2 lines better in the clinic. This disparity between clinic and home was even larger for the advanced glaucoma group with over one-third of this group reading ≥3 lines better in clinic than home. Our findings are comparable to a prior study of low vision patients24, and also suggest that older adults with early or no glaucoma measure better DVA in the clinic than home. Furthermore, our results are clinically significant given the association between a 2-line difference on ETDRS and a clinically meaningful 5-point difference on the NEI-VFQ-25 (National Eye Institute Visual Function Questionnaire 25).10 Thus, patients measuring ≥2 lines better DVA in the clinic than home (i.e. 29% glaucoma patients and 44% advanced glaucoma patients in our sample) are likely not functioning at their maximum visual potential in their home.
A high proportion of participants measured better NVA in the clinic than home, with approximately one-fifth of all participants reading ≥2 lines better in clinic than home. In our study, NVA was measured under standardized diffuse lighting in the clinic and customary lighting for near work, including direct task lighting, in the home. Twenty-one percent of participants had higher lighting in the home compared to clinic, likely due to direct task lighting in the home. The use of direct task lighting, as opposed to diffuse lighting, in the clinic may have resulted in even better NVA scores in the clinic than our report. Since many clinicians use direct task lighting for NVA testing, the difference in NVA between clinic and home (clinic better than home) may be even greater than suggested by our study.
This study was the first to compare clinic and home measurements of CS and CS with glare. Our results suggest that older adults with and without glaucoma may be experiencing greater difficulty with contrast sensitivity and glare in their homes than measured in the clinic. The largest differences occurred for CS with glare testing, with approximately three-quarters of all participants performing better in clinic than home and a half of all participants reading ≥2 triplets better in the clinic than home. These findings may be most relevant for patients who may not meet standard requirements for cataract surgery by glare testing in the clinic despite significant visual difficulties from glare in their home. In our study, glare testing was measured with the CS chart, thus our findings may not be directly applicable to glare testing with DVA charts, as routinely performed in the clinic.
The results from our study challenge the assumption that vision measured in the clinic is equivalent to vision at home. Clinician awareness of this potential discrepancy in vision between clinic and home may help explain disjunctions between clinical testing and a patient’s report of visual difficulty in their home. A report from the Salisbury Eye Evaluation Study suggests a good correlation (r=0.52 to 0.86) between performance-based tasks in the clinic and home in 19 visually impaired patients and 78 ocular normals.23 Interestingly in this report, performance-based tasks in the home were overall better than those measured in the clinic (reading with the only statistically significant difference) and lighting was not a predictor of home performance. Functional tasks routinely performed under home lighting may be easier for patients to complete in the home than in the clinic due to reasons including familiarity and lower stress in the home. We report on differences in clinical measures of vision, which likely requires equal expertise and exposure between clinic and home. While our results seem contrary to this prior report, both suggest that vision or visual function in the clinic may be different than in the home. Vision measurements in the home, as opposed to in the clinic, are recommended to more accurately evaluate a patient’s visual function in the home.
We found that higher lighting in the clinic compared to a patient’s home was the most significant factor associated with better vision in the clinic than home for DVA, NVA, and CS with glare testing. Male gender (for NVA and DVA) and higher occupational level (for CS with glare) were other factors significantly associated with better vision in the clinic than home. These relationships, however, are not nearly as strong nor readily comprehensible as the one between lighting and vision testing. Furthermore, it is unclear why lighting or any other factor in our analyses was not found to be associated with differences in CS testing between clinic and home.
While our findings of low lighting in the homes of older adults may not be surprising, the degree of difference in lighting between the clinic and home and the proportion of patients with home lighting below the standard recommendations are striking. Median lighting levels in the home were over 4 times lower than in the clinic in locations tested for DVA and nearly 3 times lower than in the clinic in locations tested for NVA, despite the use of diffuse lighting in the clinic for NVA testing. In addition, home lighting levels were below those recommended20 for the majority of all participants, regardless of glaucoma severity, in locations tested for DVA (85% of participants) and NVA (90% of participants). These findings are significant given that older adults spend approximately 80% of their day in their homes.24 Compared to a study from 1979,25 we report home lighting levels to be only slightly higher in areas used for distance vision (20–30 lux vs. 78 lux) and lower in areas used for near vision (177 lux vs. 126 lux).
Home lighting is a modifiable factor affecting vision and visual function in the home. Increased home lighting has been associated with better vision in older adults with4 and without22,26 vision impairment as well as improved reading acuity and rate27, improved activities of daily living and quality of life28, and a potential reduction in falls29. Older adults may rate their home lighting as adequate despite being below the recommended levels.30 Public health awareness and patient education of the importance of home lighting may improve visual function of many older adults in their homes. General guidelines regarding lighting are available at http://www.lrc.rpi.edu/programs/lightHealth/AARP/index.asp; accessed August 22, 2012.31
Although our results suggest a relationship between higher lighting and better vision, increased home lighting is not recommended for all patients. Certain patients may prefer lower levels of lighting due to reduced glare or difficulties with light/dark adaptation. While recommendations of increased home lighting may improve visual function in many patients, a client-centered, individualized, in-home evaluation by an occupational therapist or referral to a low vision specialist may be most beneficial to this subset of patients.
Other factors, besides lighting, may play a role in differences in vision testing between clinic and home. Patients may associate testing in the clinic with physician visits, where they are motivated to perform optimally to please their physician, receive a “good report”, or avoid further treatment. Such a conditioned response, likely associated with increased anxiety in a physician’s office, also occurs with blood pressure measurements, where the term “white-coat hypertension” refers to blood pressure measurements higher in the clinic than ambulatory settings.1,2 Alternatively, increased relaxation in the home environment may contribute to better cognitive testing in the home than clinic.3 Perhaps patients perform better on vision testing in the clinic due to a “white-coat motivation syndrome” and/or perform worse in the home due to increased relaxation and thus less motivation to perform as well as in the clinic.
This report is from a larger study that comprehensively evaluates visual function and quality of life in patients with and without glaucoma. The strengths of our study include the randomization between clinic and home visits, standardized testing, high inter-grader reliability, and examiner-masking to participant diagnosis. In addition, our study sample was relatively large and encompassed all stages of glaucoma and ocular normals. Participants in our study were recruited from a tertiary care center and consist of fairly well-educated, cognitively intact, English-speaking older adults of mainly Caucasian and African American descent. Participants had no other major ocular co-morbidities and were able to complete reliable and repeatable visual fields. Our results, therefore, may not be generalizable to populations differing in these characteristics. While it is difficult to ascertain, vision and lighting in the homes of patients refusing participation (47% of eligible patients), particularly those with advanced glaucoma, may differ from those participating in the study. Although our intent was to evaluate a patient’s vision in their “natural” environment, the presence of an examiner in the home may have resulted in better patient performance due to increased efforts to please the examiner or worse patient performance due to anxiety of a stranger in their home. In addition, participants may have had different examiners or the same examiner conducting the clinic and home visits, potentially inducing measurement variability or measurement bias, respectively. Efforts to decrease measurement variability and bias were made through a standardization exam and a didactic course on test administration for all examiners prior to data collection. Lastly, this is a cross-sectional study, thus the directional influences of lighting on vision should be studied prospectively.
In summary, distance and near visual acuity, contrast sensitivity, and contrast sensitivity with glare may be better in the clinic than home for older adults with and without glaucoma. This discrepancy may be due, in part, to poor home lighting. Clinician awareness of these results may ease confusion regarding inconsistencies between a patient’s stated visual difficulties and their clinical exam and facilitate discussions promoting increased home lighting and in-home evaluations. Finally, the results from our study challenge the ecological validity of vision testing in the clinic and emphasize the importance of measuring vision in the home to accurately evaluate visual function of older adults in their home.
Acknowledgements
The authors thank Drs. Edward Barnett, Michael Kass, and Carla Siegfried for assistance in patient recruitment, Wanda Panick and graduate students from the Program in Occupational Therapy at Washington University School of Medicine (years 2006 to 2009) for data collection and entry, and Carolyn Baum for her consultation. Drs. Anjali Bhorade and Mae Gordon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by awards from the National Eye Institute (1K23EY017616-01), Pfizer, American Glaucoma Society, Harvey A. Friedman Center for Aging and Dr. Morris grant 5K07AG2116405, unrestricted grants from Research to Prevent Blindness and NIH Vision Core Grant P30 EY02687 and the Washington University Institute of Clinical and Translational Sciences Multidisciplinary Clinical Research Career Development Program (KL2 TR000450).
The funding organizations listed above had no role in the design or conduct of this research.
Contributor Information
Anjali M. Bhorade, Email: bhorade@vision.wustl.edu.
Monica S. Perlmutter, Email: perlmutterm@wusm.wustl.edu.
Brad Wilson, Email: wilson@vrcc.wustl.edu.
Jamie Kambarian, Email: kambarianj@vision.wustl.edu.
Sidney Chang, Email: sid_chang@yahoo.com.
Melike Pekmezci, Email: melike123@hotmail.com.
Mae Gordon, Email: mae@vrcc.wustl.edu.
References
- 1.Pickering T, Davidson K, Gerin W, Schwartz JE. Masked Hypertension. Hypertension. 2002;40:795–796. doi: 10.1161/01.hyp.0000038733.08436.98. [DOI] [PubMed] [Google Scholar]
- 2.Pickering T. Blood pressure measurement and detection of hypertension. The Lancet. 1994;344:31–35. doi: 10.1016/s0140-6736(94)91053-7. [DOI] [PubMed] [Google Scholar]
- 3.Ward HW, Ramsdell JW, Jackson JE, et al. Cognitive function testing in comprehensive geriatric assessment. A comparison of cognitive test performance in residential and clinic settings. Journal of the American Geriatrics Society. 1990;38:1088–1092. doi: 10.1111/j.1532-5415.1990.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 4.Silver JH, Gould ES, Irvine D, Cullinan TR. Visual acuity at home and in eye clinics. Transactions of the ophthalmological societies of the United Kingdom. 1978;98:262–266. [PubMed] [Google Scholar]
- 5.Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. American Journal of Ophthalmology. 2006;141:24–30. doi: 10.1016/j.ajo.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 6.Rubin GS, Munoz B, Bandeen-Roche K, West SK. Monocular versus binocular visual acuity as measures of vision impairment and predictors of visual disability. Investigative ophthalmology & visual science. 2000;41:3327–3334. [PubMed] [Google Scholar]
- 7.Ferris F, Kassoff A, Bresnick G, Bailey I. New visual acuity charts for clinical research. American Journal of Ophthalmology. 1982;94:91–96. [PubMed] [Google Scholar]
- 8.Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Investigative ophthalmology & visual science. 1991;32:422–432. [PubMed] [Google Scholar]
- 9.International Council of Ophthalmology (ICO) Visual Acuity Measurement Standard. [Accessed February 10, 2012];1984 (Available at: http://archive.icoph.org/pdf/ICOVisualAcuity1984.pdf.) [Google Scholar]
- 10.McKean-Cowdin R, Varma R, Hays RD, et al. Longitudinal changes in visual acuity and health-related quality of life: the Los Angeles Latino Eye study. Ophthalmology. 2010;117:1900–1907. 7 e1. doi: 10.1016/j.ophtha.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miskala PH, Hawkins BS, Mangione CM, et al. Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization--SST Report No. 1. Archives of ophthalmology. 2003;121:531–539. doi: 10.1001/archopht.121.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelli D, Robson J, Wilkins A. The design of a new letter for measuring contrast sensitivity. Clinical Vision Science. 1988;2:187–199. [Google Scholar]
- 13.Fillenbaum GG. Multidimensional functional assessment of older adults: The Duke Older Americans Resources and Services procedures. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 14.Hollingshead AB, Redlich FC. Social class and mental illness: a community study. 1958. American journal of public health. 2007;97:1756–1757. doi: 10.2105/ajph.97.10.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. The British journal of psychiatry : the journal of mental science. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 16.Katzman R, Brown T, Fuld P, et al. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. The American journal of psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 18.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 19.Alden D, Austin C, Sturgeon R. A correlation between the Geriatric Depression Scale long and short forms. Journal of gerontology. 1989;44:124–125. doi: 10.1093/geronj/44.4.p124. [DOI] [PubMed] [Google Scholar]
- 20.Rea M. Iesna Lighting Handbook. 8ed. New York, NY: Illuminating Engineering Society of North America; 1993. [Google Scholar]
- 21.Hawkins BS. Reliability of visual acuity measurements and screening under field conditions. Ophthalmic epidemiology. 1995;2:99–106. doi: 10.3109/09286589509057089. [DOI] [PubMed] [Google Scholar]
- 22.Albert SM, Bear-Lehman J, Burkhardt A. Disparities between ambient, standard lighting and retinal acuities in community-dwelling older people: Implications for disability. Journal of American Geriatric Society. 2006;54:1713–1718. doi: 10.1111/j.1532-5415.2006.00922.x. [DOI] [PubMed] [Google Scholar]
- 23.West SK, Rubin GS, Munoz B, et al. Assessing functional status: correlation between performance on tasks conducted in a clinic setting and performance on the same task conducted at home. The Salisbury Eye Evaluation Project Team. The journals of gerontology Series A, Biological sciences and medical sciences. 1997;52:M209–M217. doi: 10.1093/gerona/52a.4.m209. [DOI] [PubMed] [Google Scholar]
- 24.Horgas AL, Wilms HU, Baltes MM. Daily life in very old age: everyday activities as expression of successful living. The Gerontologist. 1998;38:556–568. doi: 10.1093/geront/38.5.556. [DOI] [PubMed] [Google Scholar]
- 25.Cullinan TR, Silver JH, Gould ES, Irvine D. Visual disability and home lighting. Lancet. 1979;1:642–644. doi: 10.1016/s0140-6736(79)91082-1. [DOI] [PubMed] [Google Scholar]
- 26.Richards OW. Effects of luminance and contrast on visual acuity, ages 16 to 90 years. American journal of optometry and physiological optics. 1977;54:178–184. doi: 10.1097/00006324-197703000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Bowers AR, Meek C, Stewart N. Illumination and reading performance in age-related macular degeneration. Clinical & experimental optometry : journal of the Australian Optometrical Association. 2001;84:139–147. doi: 10.1111/j.1444-0938.2001.tb04957.x. [DOI] [PubMed] [Google Scholar]
- 28.Brunnstrom G, Sorensen S, Alsterstad K, Sjostrand J. Quality of light and quality of life--the effect of lighting adaptation among people with low vision. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) 2004;24:274–280. doi: 10.1111/j.1475-1313.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 29.Connell BR, Wolf SL. Environmental and behavioral circumstances associated with falls at home among healthy elderly individuals. Atlanta FICSIT Group. Arch Phys Med Rehabil. 1997;78(2):179–186. doi: 10.1016/s0003-9993(97)90261-6. [DOI] [PubMed] [Google Scholar]
- 30.Bakker RIY, Lachs MS. Lighting levels in the dwellings of homebound older adults. Journal of Housing for Elderly. 2004;18:17–27. [Google Scholar]
- 31.Lighting the Way: a Key to Independence. Troy, NY: Lighting Research Center at Rensselaer Polytechnic Institute; 2001. [Accessed August 22, 2012]. Available at: http://www.lrc.rpi.edu/programs/lightHealth/AARP/index.asp. [Google Scholar]