Abstract
Edmonston vaccine strains of measles virus (MV) have significant antitumor activity in mouse xenograft models of ovarian cancer. MV engineered to express the sodium iodide symporter gene (MV-NIS) facilitates localization of viral gene expression and offers a tool for tumor radiovirotherapy. Here, we report results from a clinical evaluation of MV-NIS in patients with taxol- and platinum-resistant ovarian cancer. MV-NIS was given intraperitoneally every 4 weeks for up to 6 cycles. Treatment was well tolerated and associated with promising median overall survival in these patients with heavily pretreated ovarian cancer; no dose-limiting toxicity was observed in 16 patients treated at high-dose levels (108–109 TCID50), and their median overall survival of 26.5 months compared favorably with other contemporary series. MV receptor CD46 and nectin-4 expression was confirmed by immunohistochemistry in patient tumors. Sodium iodide symporter expression in patient tumors after treatment was confirmed in three patients by 123I uptake on SPECT/CTs and was associated with long progression-free survival. Immune monitoring posttreatment showed an increase in effector T cells recognizing the tumor antigens IGFBP2 and FRα, indicating that MV-NIS treatment triggered cellular immunity against the patients' tumor and suggesting that an immune mechanism mediating the observed antitumor effect. Our findings support further clinical evaluation of MV-NIS as an effective immunovirotherapy.
Introduction
Ovarian cancer is the second most common malignancy of the female genital tract in the United States, causing an estimated 14,000 deaths in 2013 (1). Despite aggressive initial therapy, including debulking surgery followed by taxane/platinum-based regimens, the majority of the patients relapse. Although ovarian cancer is often initially sensitive to platinum-based chemotherapy, patients ultimately develop resistance. For resistant disease, patients are generally treated with agents such as liposomal doxorubicin (2, 3), topotecan (4), weekly paclitaxel (5, 6) or gemcitabine (2). Bevacizumab has also demonstrated some activity (7, 8), but most clinical trials in patients with platinum-resistant ovarian cancer report median overall survival (OS) in the order of 12 months or less (2–8). There is a pressing need for more effective treatments to improve the outcome of these patients.
Virotherapy is a treatment approach with mechanisms of action that are not cross resistant with chemotherapy. Moreover, virotherapy approaches with conditionally replicating viruses have the potential to overcome an important limitation of gene transfer approaches using nonreplicating vectors, i.e., their limited infection/ transduction efficiency (9). Because recurrent ovarian cancer remains confined in the peritoneal cavity in more than 80% of the patients, it provides a therapeutic opportunity for locoregional administration of novel therapeutics, including virotherapy agents. Despite promising preclinical work with different virotherapy agents (10), however, only a handful of clinical virotherapy trials have been reported. Early work with the conditionally replicating E1B attenuated adenovirus Onyx-015 in ovarian cancer showed no evidence of antitumor efficacy (11); this possibly reflected low expression levels of the native adenoviral receptor CAR (coxsackie-adenovirus-receptor) in ovarian tumors (12), a problem that a recently completed phase I trial with replicating adenovirus AD5.SSTR/TK.RGD (allowing CAR-independent infection; refs. 13, 14) attempted to overcome.
Measles virus (MV) is a negative-strand enveloped RNA virus (4), with six genes encoding 8 proteins (4). The H-protein is the surface glycoprotein that mediates MV attachment to its three known receptors, the CD46 molecule (15), the signaling lymphocyte activating molecule (SLAM) receptor (predominantly present on activated B, T cells, and monocytes; ref. 16), and the recently identified epithelial receptor nectin-4 (17). The F-protein is responsible for cell fusion following viral attachment. Cells infected by MV express F and H proteins on their membranes and, therefore, become highly fusogenic, causing fusion with uninfected neighboring cells, with the characteristic cytopathic effect of syncytia formation. Of note, natural infection with MV has been associated with spontaneous tumor regression in patients with Hodgkin's disease and non-Hodgkin's lymphoma (18, 19). Although the wild-type MV can lead to a potentially serious infectious disease, attenuated strains (vaccine strains) of MV have an outstanding safety record (20).
Of importance and in contrast to variable expression of receptors for other viral vectors, two of the three receptors for the MV are consistently expressed at high levels on ovarian tumors. This includes the CD46 receptor or complement cofactor protein (21), the expression of which allows tumor cells to evade complement-mediated lysis (22), and nectin-4 (23).
The sodium iodide symporter (NIS) is a membrane ion channel expressed on thyroid follicular cells that allows iodide trapping. NIS expression in thyroid tissue has been exploited for more than 50 years in clinical practice for thyroid imaging (with 123I or Technetium 99m), or ablation (with 131I), and for systemic therapy of well-differentiated thyroid malignancies (24). MV-NIS, a recombinant MV strain of the Edmonston vaccine lineage expressing the NIS gene, has the same vector backbone as the MV-CEA virus we tested in a recently completed phase I trial in patients with recurrent ovarian cancer (25) except for the transgenes (Supplementary Fig. S1). For MV-CEA, the extracellular domain of the human carcinoembryonic antigen (CEA) gene was inserted at position 1 upstream of the measles nucleocapsid (N) gene. For MV-NIS, the NIS cDNA was inserted downstream of the measles hemagglutinin (H) gene. Of note, transgene location for MV-NIS results in viral proliferation advantage as compared with MV-CEA, due to a transcriptional gradient in MV genome transcription (4). The latter also facilitates viral manufacturing in higher titers.
In in vitro experiments, MV-NIS induced the characteristic cytopathic effect of syncytia formation in ovarian cancer cells, but not in nontransformed cells such as normal human dermal fibroblasts or mesothelial cells, and led to concentration of radioiodine isotopes (125I) in infected ovarian cancer cells (26). In addition, MV-NIS had significant antitumor activity, both in subcutaneous and orthotopic SKOV3ip.1 models leading to growth arrest and significant prolongation of survival in orthotopic models (26). Imaging, following Tc-99M or 123I administration, demonstrated strong uptake in peritoneal tumors, suggesting that the NIS transgene could be used to localize viral gene expression.
Given the favorable performance features with MV-NIS, we launched a phase I/II trial in women with treatment-resistant ovarian cancer. The goals were to (i) determine the safety and tolerability of intraperitoneal administration of MV-NIS in patients with treatment-resistant ovarian cancer; (ii) assess, in a preliminary fashion, the antitumor efficacy of this approach by following CA-125 levels, radiographic response, time to progression, and survival; (iii) characterize expression of the MV receptors CD46 and nectin-4 in the patient tumor tissue; (iv) characterize viral gene expression using NIS expression as the surrogate; (v) assess viremia, viral replication and MV shedding, and persistence; and (f) determine humoral immune response to the injected virus.
Patients and Methods
Patient selection
Eligible patients had persistent, recurrent, or progressive epithelial ovarian cancer or primary peritoneal cancer after prior treatment with platinum compounds and taxanes. Histologic confirmation of the original or recurrent tumor was required. Patients had to be ≥18 years old with adequate hematologic, liver, and kidney function, as defined by absolute neutrophil count (ANC) ≥ 1,500/mL; platelets ≥ 100,000/mL; hemoglobin ≥ 9 gm/dL; total bilirubin ≥ upper limit of normal; and creatinine ≥1.5 × upper limit of normal. Patients had to be immune to MV as shown by antimeasles IgG levels ≥ 20 ELISA units/mL, determined by enzyme immunoassay (Diamedix). Exclusion criteria included platinum-sensitive disease; Eastern Cooperative Oncology Group performance status of 3 or 4; chemotherapy, immunotherapy, or biologic therapy ≤4 weeks before study entry. Patients were also excluded if they had an HIV-positive test or history of other immunodeficiency, organ transplantation, history of chronic hepatitis B or C, intraabdominal disease >8 cm at the time of registration, intrahepatic disease, or disease beyond the peritoneal cavity. This study was approved by the Mayo Clinic Institutional Review Board, and all participants provided written informed consent.
Treatment
Construction of the MV-NIS virus has been previously described (27): a schematic representation of the MV-NIS genome is included in Supplementary Fig. S1. Clinical lots of the virus were produced by the Mayo Clinic Gene and Virus Therapy Shared Resource (GVTSR). During the course of the study, a new FDA-approved vector production methodology was developed by GVTSR employing HeLa as the producer cell line, which allowed production of clinical grade vector in higher titers as compared with the original Vero cell–based methodology (28). All patients underwent laparotomy or laparoscopy, for placement of the intraperitoneal catheter (Bard Access Systems). Peritoneal adhesions were lysed if technically possible. If ascites was present, it was drained through the peritoneal catheter before the viral administration. Patients received infusion of the MV-NIS diluted in 500 mL of normal saline over 30 minutes. Treatment was repeated monthly for up to 6 cycles, provided that toxicity was acceptable and there was no evidence of disease progression.
Experimental design and statistical analysis
The standard cohorts-of-three design (29) was applied. There were two dose levels (108 and 109 TCID50). Dose levels were determined based on the MV-CEA trial results and impacted by manufacturing limitations at the time of trial initiation. Three patients were treated per dose level and observed for 4 weeks before accrual to the next higher dose level was initiated. Intrapatient dose escalation was not allowed. Toxicity was assessed using Common Terminology Criteria Version 3.0. Dose-limiting toxicity was defined as grade ≥3 hematologic toxicity except for grade 3 ANC lasting <72 hours, elevation of serum creatine ≥2× the baseline, any other nonhematologic toxicity grade ≥3, viremia lasting for ≥6 weeks from last viral administration, grade 2 symptomatic bronchospasm or urticaria, and any grade 3 or higher allergic reactions. Following completion of the dose escalation phase of the trial, 10 patients were treated at the MTD to better characterize treatment safety and efficacy. Time to progression was defined as date of study enrollment to date of progressive disease; overall survival (OS) was defined as date of study enrollment to date of death or last follow-up in living patients. Time to progression and OS were summarized using a Kaplan–Meier approach.
Laboratory evaluation
Before treatment, patients had a history and physical exam performed, as well as a complete blood count (CBC), prothrombin time (PT) and activated partial thromboplastin time (aPTT), chemistry group, urinalysis, chest X-ray, HIV testing, CA-125 measurements, and electrocardiogram. CBC, chemistry group, PT, and aPTT were repeated on day 8, day 15, and before retreatment (cycles 2–6). In addition, peritoneal aspirates (or peritoneal lavage samples if no ascites) were obtained at baseline, day 3, day 8, and before all subsequent cycles. The peritoneal aspirate was tested for the presence of the virus by Vero cell overlay and quantitative reverse transcription PCR (RT-PCR), and anti-MV IgG antibodies. Patients' blood, urine, and mouth gargle specimens were tested for the presence of the virus (viremia and shedding) at multiple time points (Supplementary Fig. S2). Patient's immune competence [CD4, CD8 counts, immunoglobulins, complement, delayed-typed hypersensitivity (DTH) reaction to Candida, purified protein derivative, tetanus, and trichophyton] and humoral immunity against the virus were also tested at multiple time points (Supplementary Fig. S2). NIS expression in infected tumor was assessed by 123I SPECT/CT imaging, as outlined in Supplementary Fig. S3.
Assessment of antitumor response
Response Evaluation Criteria in Solid Tumors criteria (30) were applied for response assessment. CT or MRI and CA-125 measurements were done at baseline and before retreatment on cycles 2 to 6.
Detection and quantitation of MV N-gene RNA by QRT-PCR in peripheral blood mononuclear cells, mouth gargle, and urine specimens
Total RNA was extracted using either Trizol reagent (Invitrogen; Cat # 15596-026) and ethanol precipitation (urine and mouth gargle specimens) or the PaxGene Blood RNA Kit (Qiagen; Cat # 762164). Blood for isolation of PBMC's was collected using the PAXgene Blood RNA tubes as recommended by the manufacturer. Briefly, the QRT-PCR assay has been optimized for primers and probe, with Invitrogen-ABI TaqMan One-Step RT-PCR Master Mix Reagent and run on the Roche480 machine. The 50-µL qRT-PCR reaction volume was used to amplify a 61 base pair MVN genomic RNA target, in the presence of 0.3 mmol/L each forward primer (5′-GGGTTGGCCGGTTGGA3′), reverse primer (5′-AGAAGCCAGGGAGAGCTACAGA3′) and a 0.2 mmol/L Blackhole Quencher labeled probe (5′-/56-FAM/TGGGCAGCTCTCGCATCACTTGC3BHQ_1/-3′), 4 mmol/L MgCl, and 1 µg or a maximum volume of 5 µL of total RNA isolate. One cycle of RT reaction (30 minutes at 48°C) is applied followed by an activation step (10 minutes at 95°C), and 45 cycles of amplification (15 seconds at 95°C and 1 minute at 60°C), with fluorescence measured during the extension. A standard curve of 10-fold dilutions of an RNA fragment obtained in vitro transcription, containing 10 to 107 MV-N gene copies/mL, was used in the assay. Calculation of copy number was determined using the standard curve and the Roche480 machine Absolute quantification software.
Assessment of CD46 and nectin-4 expression in ovarian tumors
Immunohistochemistry for CD46
The primary antibody CD46 (EPR4014; AbCam; Cat# ab108307) was diluted 1:500, and slides were incubated overnight, at 4°C in a humidified chamber, then incubated with a Donkey Anti rabbit IgG-B biotinylated secondary antibody (Santa Cruz Biotech. Inc.; Cat # sc-2040) for 60 minutes at room temperature, followed by a detection step with Vectastain ABC and Peroxidase substrate DABkits (Vector Labs. Inc.; PK-6100 and Cat # SK-4100), counterstained using Accustain solution (Sigma; Cat # GSH-116), then dehydrated and mounted using Vecta- Mount H-5000 permanent mounting solution (Vector labs; Cat# H-5000).
Immunochemistry for nectin-4
The primary antibody Nectin4 MAB2659 (R&D Systems; Cat# AF2659) was diluted 1:500, and the slides were incubated overnight, at 4°C in a humidified chamber. For the next step, a secondary antibody reagent part of a Tissue Staining Goat HRP-DAB system kit was used (Abcam; Cat# CTS008) according to the manufacturer's instructions. After the detection step, the slides were counterstained using Accustain solution, dehydrated, and mounted using VectaMount H-5000 solution.
Assessment of humoral immune response against MV
Anti-MV IgG antibody levels were measured using the Diamedix Immunoassay, as per the manufacturer's instructions.
Assessment of cellular immune response against ovarian cancer antigens
IFNgamma and IL4 ELIspots were performed as previously described except that a 48-hour rather 10-day format was used (31). To detect tumor-specific T-cell immunity, a degenerate panel of peptides derived from either the folate receptor alpha (FRα; FR30, FR56, FR113, and FR238) or insulin-like growth factor binding protein 2 (IGFBP2; IGFBP2.17, IGFBP2.22, IGFBP2.249, and IGFBP2.293), as previously described, was used (32, 33). The plates were read on an AID ELISpot reader (Cell Technology, Inc.; reader software v.3.1.1.). A positive response was defined as a frequency that was both detectable (i.e., >1:100,000) and significantly (P < 0.05, two-tailed t test) greater than the mean of control no-antigen wells. Results are presented as the sum of the antigen-specific effector T cells for each peptide and each antigen.
Results
Patient characteristics
Sixteen patients with recurrent ovarian cancer were treated in this phase I trial. Table 1 summarizes the patients' characteristics. All participating patients had platinum-resistant disease and had been heavily pretreated having received a median of 4 chemotherapy regimens for recurrent disease.
Table 1.
Patient characteristics (N = 16)
| Age, y | |
| Mean (SD) | 59.4 (13.0) |
| Median | 57.5 |
| Performance score | |
| 0 | 11 (68.8%) |
| 1 | 5 (31.3%) |
| Ascites present, n (%) | |
| Absent | 3 (18.8%) |
| Slighta | 13 (81.3%) |
| Prior treatments | |
| Surgery | 16 (100.0%) |
| Radiation therapy | 2 (12.5%) |
| Chemotherapy | 16 (100%) |
| Prior chem. regimens (n) | |
| 1 | 2 (12.5%) |
| 2 | 3 (18.8%) |
| 3 | 2 (12.5%) |
| 4 | 2 (12.5%) |
| 5 | 3 (18.8%) |
| 6 | 1 (6.3%) |
| 7 | 1 (6.3%) |
| 8 | 2 (12.5%) |
Small amount present by imaging at study enrollment.
Toxicity
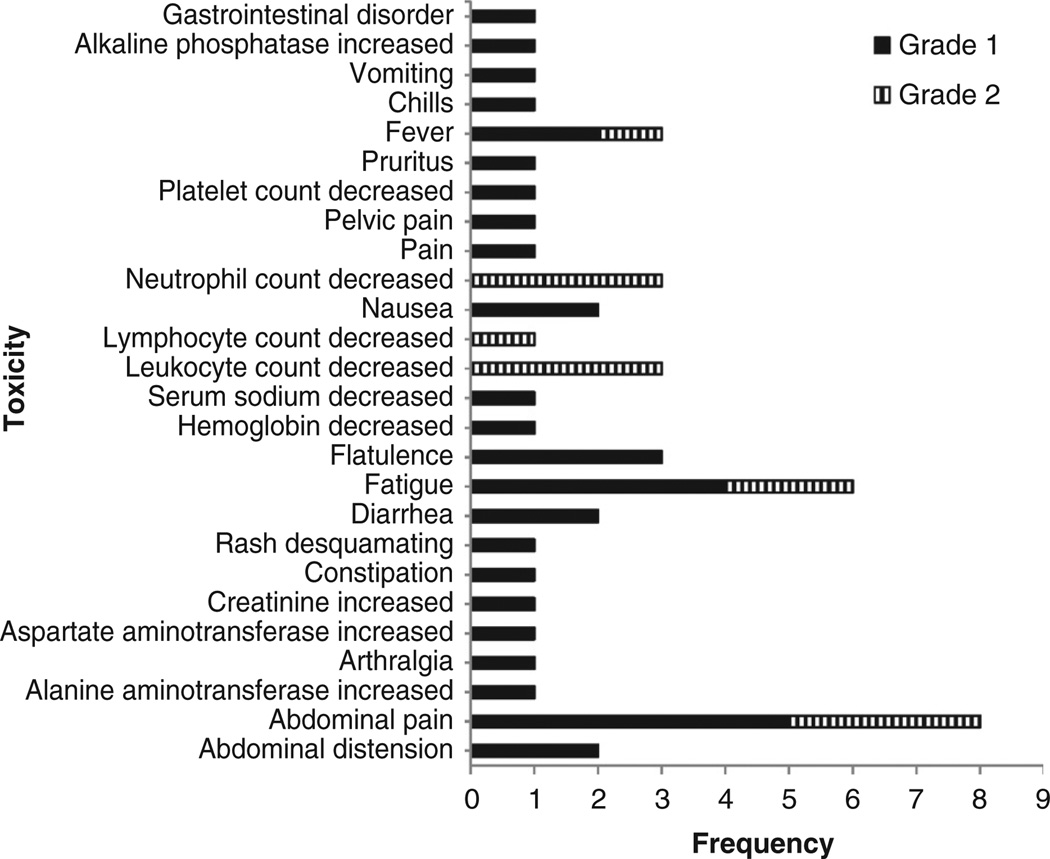
Given the excellent safety of the closely related MV-CEA virus in our other recently completed phase I trial (25), only the highest viral doses tested in the MV-CEA trial were explored in the MV-NIS study (108 and 109 TCID50). Three patients received treatment at the 108 TCID50 dose level and three at the 109 TCID50 dose level. An additional 10 patients were then treated at the 109 TCID50 MTD expansion dose to better characterize the safety of the proposed phase II dose, and in a preliminary manner, assess antitumor efficacy. No significant or dose-limiting toxicity was observed. Figure 1 summarizes cycle 1 toxicity for all study patients; all observed toxicities were grade 1 and 2. Most common toxicities in all cycles were abdominal discomfort (grade 1: 5 patients, 31%; grade 2: 3 patients, 19%), fatigue (grade 1: 4 patients, 25%; grade 2: 2 patients, 12.5%), fever (grade 1: 2 patients, 12.5%; grade 2: 1 patient, 6%), and neutropenia, (grade 1: 3 patients, 19%; grade 2: 3 patients, 19%). There was one incident of grade 3 neutropenia and bilirubin elevation in a patient who received 109 TCID50 following the second treatment cycle. This patient experienced no significant toxicity in the first cycle; she, however, developed a probable allergic reaction during the second treatment cycle consisting of grade 2 hypotension, followed by grade 2 fever spikes with rigors, grade 3 elevation of direct bilirubin and, grade 3 neutropenia. Blood and urine cultures and QRT-PCR of blood, urine, and mouth gargle specimens ruled out an infectious etiology, including MV infection. Of note, this patient was treated with a viral lot prepared with the original (Vero cell based) production methodology. No allergic reactions were observed in any of the subsequent 13 patients who received virus prepared with the newer HeLa cell–based methodology (28). Immunosuppression has been observed following wild-type MV infection and can be associated with DTH suppression, bacterial infections, and reactivation of tuberculosis (34). Immunosuppression is, however, very infrequent following measles vaccination (35). Similar to the MV-CEA trial, in this study no evidence of treatment-induced immunosuppression was observed. Specifically, there were no treatment-related infections and no significant change in CD4, CD8, immunoglobulin, or complement levels (data not shown). In addition, no patient developed suppression of an initially positive DTH reaction.
Figure 1.
Adverse events possibly, probably, or definitely related to treatment in cycle 1.
Efficacy
Best objective response was stable disease in 13 of 16 patients (81%); this included 2 of 3 patients treated at dose level 1 and 11 of 13 patients at dose level 2. Responses of the tumor marker CA-125 were observed in 2 patients, both treated with 109 TCID50. Median duration of stable disease was 67 days, (range, 54–277 days). Median OS for study patients was 26.6 months, 95% confidence interval: 16.3–37.3 months. This correlates with the observed median OS of 38.4 months in patients who received the higher doses of 108 and 109 TCID50 in the MV-CEA trial (25) and compares favorably with the observed OS in contemporary trials targeting the same platinum-resistant patient population, including trials employing bevacizumab-based regimens, which ranges from 6 to 12 months (3, 5–8). Of note, survival outcomes in the completed MV-CEA trial (25) were dose dependent with patients receiving 108 TCID50 or higher doses of the virus surviving significantly longer (median OS, 38.4 months) as compared with patients who received lower doses (median OS, 10.6 months). Table 2 summarizes outcomes in both trials according to viral dose received.
Table 2.
Outcomes in MV-CEA and MV-NIS trials are impacted by dose level
| Patients (N) | Median TTP (range, months) |
Median OS (range, months) |
|
|---|---|---|---|
| MV-CEA (all dose levels) | 21 | 1.8 (0.7–9.1) | 12.3 (1.3–83.5+) |
| MV-CEA (103–107 TCID50) | 15 | 1.8 (0.7–9.1) | 10.6 (1.3–79.9) |
| MV-CEA (108–109 TCID50) | 6 | 3.7 (1.8–7.5) | 38.4 (7.2+–83.5+) |
| MV-NIS (108–109 TCID50) | 16 | 2.1 (0.9–25.7) | 26.5 (7.0–44.4+) |
| MV-CEA or NIS (108–109TCID50) | 22 | 2.7 (0.9–25.7) | 29.3 (7.0–83.5+) |
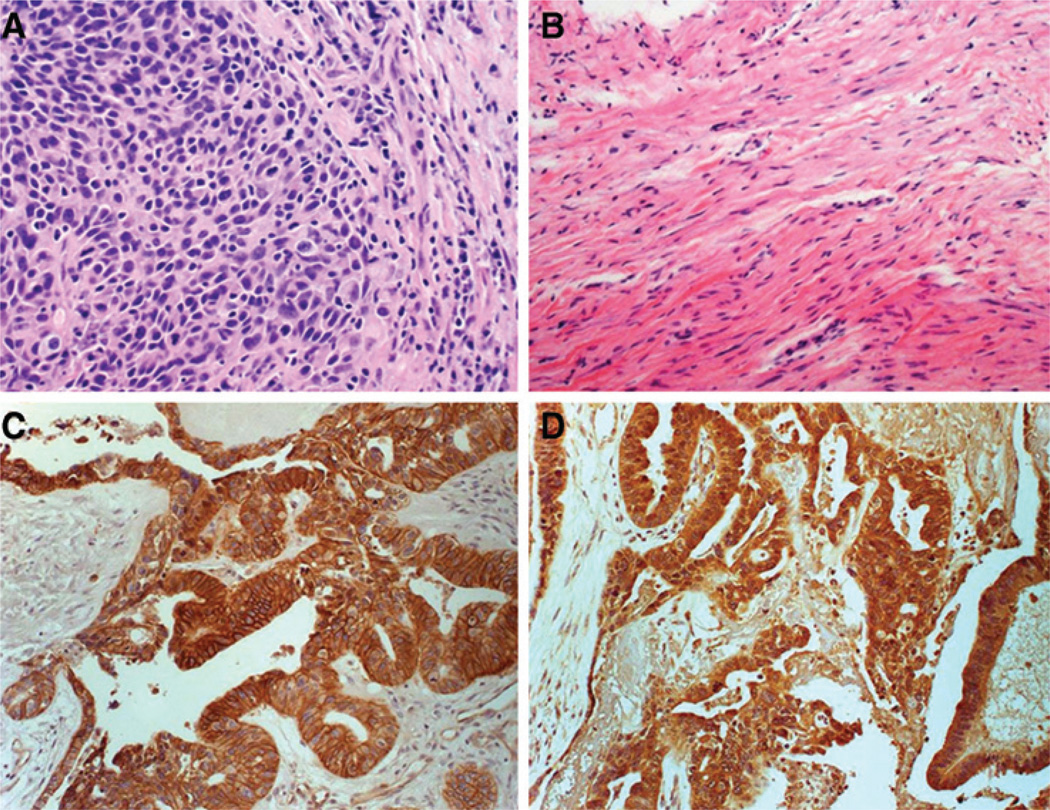
Despite our initial hypothesis that a heavier tumor burden would facilitate oncolysis and antitumor effect, we have observed very intriguing evidence of clinical efficacy in patients with low disease burden as highlighted in the following study patient example. The patient was diagnosed with grade 3 serous papillary carcinoma, underwent hysterectomy, salpingo-oophorectomy, and tumor debulking followed by standard adjuvant chemotherapy consisting of intravenous paclitaxel and carboplatin. After four chemotherapy treatment cycles, the patient was found to have progressive disease in the pelvic area and was referred to our institution. At that time she had optimal secondary debulking performed with microscopic residual disease remaining at the end of the procedure. Following intraperitoneal port placement, patient proceeded to receive six cycles of intraperitoneal MV-NIS. As per the trial eligibility criteria, the patient was measles immune at study entry, with a high measles IgG titer of 94.8 EU/mL (positive ≥ 20 EU/mL). The patient's tumor marker CA-125 decreased from 70 U/mL pretreatment to 20 U/mL posttreatment. SPECT/CT imaging showed NIS expression with 123I uptake in sites of pelvic implants, indicating viral gene expression in areas of residual disease. Following six treatment cycles with MV-NIS, a second look laparoscopy was performed as prespecified per protocol and multiple biopsies were obtained. There was no viable tumor remaining; only fibrosis and residual sclerotic reaction was observed. Figure 2 depicts the pathologic findings at baseline (2A) as well as the complete absence of viable tumor (pathologic CR) at the time of the second look laparotomy (2B). The patient remained disease free for 25 months, at which time she presented with extra abdominal relapse consisting of metastatic disease in a pericardial lymph node.
Figure 2.
A, pre MV-NIS treatment biopsy from perirectal region showing high-grade serous ovarian carcinoma. B, abdominal wall nodule biopsy following six cycles of MV-NIS in the same patient. There is dense fibrosis, but no evidence of viable tumor, indicating a pathologic complete response to treatment. C and D, representative images showing overexpression of MV receptors CD46 (C) and nectin-4 (D) in tumors of study patients (brown staining).
Expression of the MV-NIS receptor CD46 and nectin-4 in tumor specimens
Immunohistochemical analysis of baseline tumor samples from study patients showed moderate or high expression of CD46 and nectin-4 MV receptors in all patients (Fig. 2C and D, respectively). Specifically, 3 of 14 (21%) patients had moderate CD46 expression and 10 of 14 (71%) patients had high CD46 expression, whereas 1 of 14 patients (7%) had moderate nectin-4 expression and 13 of 14 (93%) had high nectin-4 expression. Of note, the 1 patient who was negative for CD46 had high nectin-4 expression.
Assessment of viral biodistribution and shedding
There was no evidence of shedding as tested by quantitative RT-PCR in mouth gargle and urine specimens for any of the study patients at the prespecified time points (Supplementary Fig. S2), and no detection of viral genomes in peripheral blood.
Assessment of immune response to MV
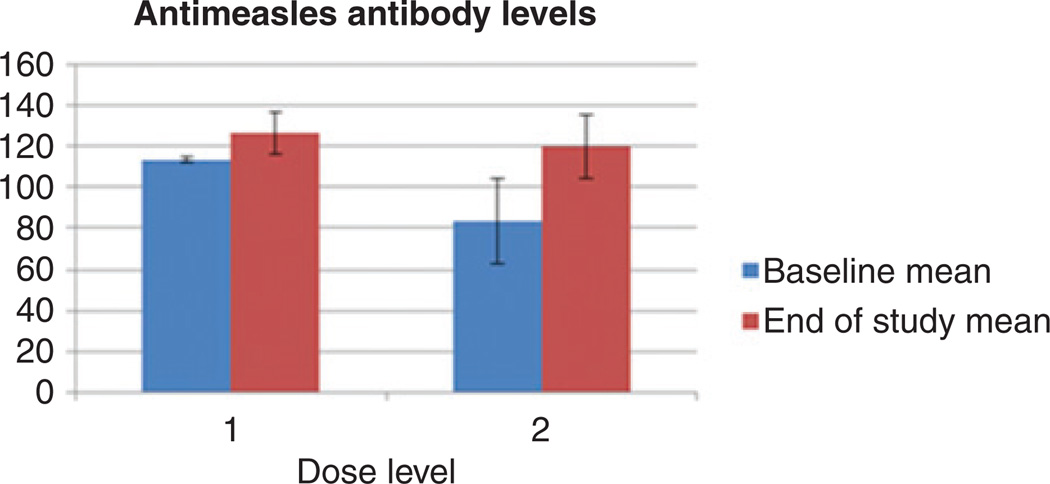
Figure 3 depicts mean serum antimeasles antibody levels in the serum at baseline and on study completion according to dose levels. As per study eligibility, all patients were measles immune at baseline. There was no significant change in the measles antibody titers in blood and peritoneal fluid (data not shown) during the course of the trial, as compared with baseline.
Figure 3.
No significant change in anti-MV antibody titers was observed following MV-NIS treatment. Results are presented per dose level.
Detection of the NIS transgene
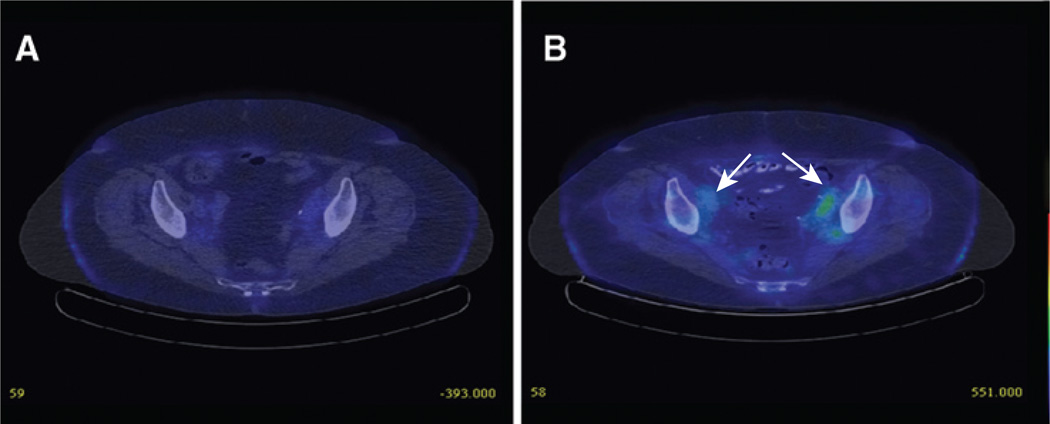
123I SPECT/CT imaging was performed at baseline of cycles 1 and 2 and on days 3, 8, and 15 of cycle 1. In case of a positive scan, additional imaging was obtained on day 25. Imaging during cycle 2 was performed only if positive results were obtained in cycle 1 at corresponding time points. NIS expression as imaged by 123I uptake was observed in 3 of the 13 patients treated at the 109 TCID50 dose level. Figure 4 shows representative imaging in one of the study patients. The patient's 123I scans were positive on days 8 and 15 of cycle 1 and days 8, 15, and 21 of cycle 2, indicating viral gene expression in pelvic tumor deposits following repeat administration of the virus, despite preexisting humoral immunity to MV. Imaging was negative at baseline both at cycle 1 and before retreatment at cycle 2. A second patient had 123I positivity, indicating NIS expression, on day 8 of cycle 1, and the third patient on day 15 of cycle 1.
Figure 4.
NIS expression as imaged by I123 uptake in one of the study patients; I123 scan was negative at baseline (A) but became positive on day 8 of cycle 1 (B).
Treatment with MV-NIS augments endogenous immunity against tumor antigens
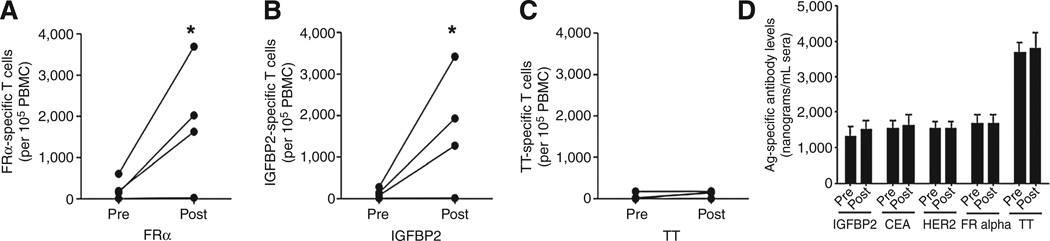
Although our initial phase I studies were not designed to collect and preserve T cells for functional analysis, the observed clinical benefit in some of the study patients in the context of minimal residual disease raised the possibility that in addition to oncolysis, other mechanisms such as the development of an antitumor immune response might be contributing to the favorable clinical outcomes. We therefore collected pre- and posttreatment specimens in a subgroup of study patients to investigate the possible immunotherapeutic potential of this approach and address the vaccine effects of MV and the role of antitumor immunity in the clinical efficacy of MV treatment. Figure 5 shows the results of an IFNγ ELIspot analysis of the specimens obtained from 4 MV-NIS–treated patients, demonstrating that MV treatment activates tumor antigen–specific T cells. In that experiment, peripheral blood T cells were stimulated with a pool of HLA-DR epitopes derived from the FRα or IGFBP2. Both of these antigens are highly expressed in a high percentage of patients with ovarian cancer and some patients with ovarian cancer demonstrate natural immunity to these antigens (32, 33). As shown, patients treated with MV-NIS augmented immune responses to both antigens but not to tetanus, which was added as a control for specificity of the immune response. Similar results were obtained when an IL4 ELISPOT analysis for the same antigens was performed. In addition, pre- and posttreatment sera samples, available from 31 patients that received either MV-CEA or MV-NIS, were tested to detect antibodies targeting FRα, HER-2, CEA, and IGFBP2; no responses to any of the antigens were generated. Collectively, the generation of IFNγ T-cell response and no antibody response would suggest that MV treatment predominantly elicits the generation of Th1-mediated immunity, despite the presence of IL4-secreting T cells.
Figure 5.
Generation of Th1 immune responses in patients with ovarian cancer undergoing treatment with MV. A–C, mean pretreatment and posttreatment IFNγ ELIspot responses for four treated patients against tumor antigens and tetanus toxoid (TT). Each symbol is calculated from 12 replicates. D, mean pre- and posttreatment antibody responses to various purified tumor antigens as well as tetanus toxoid. Each bar represents the mean (SEM) levels antibodies calculated from duplicate samples from 31 patients with ovarian cancer treated with MV.
Discussion
This trial confirmed the safety of an engineered MV strain expressing the NIS transgene, given intraperitoneally, for the treatment of recurrent ovarian cancer and demonstrated early evidence of antitumor activity. No dose-limiting toxicity was observed in doses as high as 109 TCID50. The most common toxicities were mild (grade 1–2, abdominal pain, fatigue, fever, and neutropenia). This is consistent with the excellent safety record of the oncolytic MV platform in other malignancies (independent of the route of administration), including intratumoral and resection cavity (recurrent head and neck, recurrent glioblastoma; ref. 36), intravenously with or without cyclophosphamide (multiple myeloma; refs. 37, 38), and intrapleurally (mesothelioma; ref. 39).
The observed median OS of 26.5 months in this group of heavily pretreated patients (median of four chemotherapy regimens for recurrent disease) is compelling. Of note, this survival outcome is very similar to the OS in the MV-CEA trial at comparable dose levels (38.4 months at doses 108 TCID50 or higher vs. only 10.6 months for patients who received lower MV-CEA doses). The survival of patients treated with MV compares favorably with contemporary series of novel therapeutics in patients with platinum-resistant or refractory ovarian cancer where the observed OS ranges between 6 to 12 months (7, 40–43). It is of note that this promising outcome was observed in the context of preexisting antiviral immunity (Fig. 3).
It is also interesting that the long median OS in study patients was associated with a relatively short median time to progression. Although the latter could reflect the very aggressive imaging schedule followed in this trial with CTs obtained every month, the survival benefit could also be indicative of a different mechanism of action, beyond oncolysis, contributing to the antitumor effect, such as an immune-based mechanism. Although the correlative analysis in this trial was designed to examine the oncolytic mechanism of action, several findings suggest immune-mediated antitumor activity. Specifically, there were CA-125 responses in MV-CEA–treated patients even at doses as low as 103 TCID50 (25); moreover, the clinical benefit that patients derived (including pathologic complete response) in the context of microscopic residual disease (see Results section) raises the possibility of an immune-mediated antitumor effect. Although immunologic analysis could be performed for only a small subgroup of study patients, the immune response data support this hypothesis. The results shown in Fig. 5 indicate the development of a Th1 response against ovarian cancer antigens such as FRα and IGBP2.
The collective analysis of the MV-CEA and MV-NIS trial data indicates a dose effect in the observed antitumor activity and impact on survival, which suggests that improvement of oncolysis could further optimize results with MV-based therapy. This hypothesis is further strengthened by recent data deriving from an MV trial in myeloma, where responses of disseminated disease were accomplished following intravenous administration of high MV-NIS doses to patients lacking neutralizing antibodies against MV (38). Given the fact that the majority of patients with ovarian cancer have neutralizing antibodies against the virus (38), we are studying means to avoid immune capture of the virus and thus facilitate its delivery to the tumor, such as using mesenchymal stem cells for viral delivery (44).
On the basis of the results of the phase I/II study of MV-NIS reported here, we have designed a randomized phase II trial comparing intraperitoneal administration of MV-NIS to treating physician's chemotherapy of choice for patients with recurrent ovarian cancer and low disease burden, where the virus has a higher likelihood of also working as effective immunotherapy. Outcomes to be evaluated in the study, in addition to efficacy and toxicity assessment, include patient reported outcomes such as quality of life given the favorable toxicity profile of the virus as compared to the known significant side effects of standard chemotherapy approaches employed in ovarian cancer treatment. This trial will also include a prospective immunologic analysis, and thus it is expected to provide additional information on a possible immune-mediated mechanism of action of MV-NIS and guide future steps including combinatorial strategies with other immunomodulatory approaches such as immune checkpoint blockade (45, 46).
In summary, intraperitoneal administration of MV-NIS in patients with recurrent ovarian cancer was associated with compelling survival outcomes and merits further prospective testing. Moreover, this study has generated mechanistic hypotheses regarding a novel immune-based mechanism of MV action that we are planning to explore in additional trials.
Supplementary Material
Acknowledgments
The authors thank the trial coordinator Janet Lensing and patients and their families. They also thank Raquel Ostby for her help with article preparation.
Grant Support
This research was support by NCI/NIH grants P50CA 136393, R01CA 136547, P30CA 15083, NCRR U54RR 24150, and UL1 TR000135.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
M.J. Federspiel has ownership interest (including patents) in Magnis Therapeutics LLC. S.J. Russell is CEO and has ownership interest (including patents) in Magnis Therapeutics LLC. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: E. Galanis, P.J. Atherton, K.L. Knutson, W.A. Cliby, L.C. Hartmann
Development of methodology: E. Galanis, P.J. Atherton, K.L. Knutson, S.J. Russell, K.W. Peng
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): E. Galanis, K.L. Knutson, S.C. Dowdy, P. Haluska Jr, I. Aderca, M.S. Block, J. Bakkum-Gamez, M.J. Federspiel, K.R. Kalli, G. Keeney, L.C. Hartmann
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): P.J. Atherton, M.J. Maurer, K.L. Knutson, W.A. Cliby, A. Oberg, M.S. Block, J. Bakkum-Gamez, L.C. Hartmann
Writing, review, and/or revision of the manuscript: E. Galanis, P.J. Atherton, M.J. Maurer, K.L. Knutson, S.C. Dowdy, W.A. Cliby, P. Haluska Jr, A. Oberg, M.S. Block, J. Bakkum-Gamez, M.J. Federspiel, K.R. Kalli, G. Keeney, K.W. Peng, L.C. Hartmann
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): K.L. Knutson
Study supervision: E. Galanis
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol. 2008;26:890–896. doi: 10.1200/JCO.2007.13.6606. [DOI] [PubMed] [Google Scholar]
- 3.Vergote I, Finkler N, del Campo J, Lohr A, Hunter J, Matei D, et al. Phase 3 randomised study of canfosfamide (Telcyta, TLK286) versus pegylated liposomal doxorubicin or topotecan as third-line therapy in patients with platinum-refractory or -resistant ovarian cancer. Eur J Cancer. 2009;45:2324–2332. doi: 10.1016/j.ejca.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Lamb RA, Kolakofsky D, editors. Paramyxoviridae: the viruses and their replication. 4th ed. Philadelphia, PA: Lippincott-Raven Publishers; 2001. [Google Scholar]
- 5.Markman M, Blessing J, Rubin SC, Connor J, Hanjani P, Waggoner S. Phase II trial of weekly paclitaxel (80mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;101:436–440. doi: 10.1016/j.ygyno.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Thigpen JT, Blessing JA, Ball H, Hummel SJ, Barrett RJ. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. J Clin Oncol. 1994;12:1748–1753. doi: 10.1200/JCO.1994.12.9.1748. [DOI] [PubMed] [Google Scholar]
- 7.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 8.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 9.Hartkopf AD, Fehm T, Wallwiener D, Lauer U. Oncolytic virotherapy of gynecologic malignancies. Gynecol Oncol. 2011;120:302–310. doi: 10.1016/j.ygyno.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Raki M, Rein DT, Kanerva A, Hemminki A. Gene transfer approaches for gynecological diseases. Mol Ther. 2006;14:154–163. doi: 10.1016/j.ymthe.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Vasey PA, Shulman LN, Campos S, Davis J, Gore M, Johnston S, et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20:1562–1569. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- 12.Rocconi RP, Numnum TM, Stoff-Khalili M, Makhija S, Alvarez RD, Curiel DT. Targeted gene therapy for ovarian cancer. Curr Gene Ther. 2005;5:643–653. doi: 10.2174/156652305774964668. [DOI] [PubMed] [Google Scholar]
- 13.Kim KH, Dmitriev I, O'Malley JP, Wang M, Saddekni S, You Z, et al. A phase I clinical trial of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in patients with recurrent gynecologic cancer. Clin Cancer Res. 2012;18:3440–3451. doi: 10.1158/1078-0432.CCR-11-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KH, Dmitriev IP, Saddekni S, Kashentseva EA, Harris RD, Aurigemma R, et al. A phase I clinical trial of Ad5/3-Delta24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol Oncol. 2013;130:518–524. doi: 10.1016/j.ygyno.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 16.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 17.Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VH, et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bluming AZ, Ziegler JL. Regression of Burkitt's lymphoma in association with measles infection. Lancet. 1971;2:105–106. doi: 10.1016/s0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- 19.Taqi AM, Abdurrahman MB, Yakubu AM, Fleming AF. Regression of Hodgkin's disease after measles. Lancet. 1981;1:1112. doi: 10.1016/s0140-6736(81)92286-8. [DOI] [PubMed] [Google Scholar]
- 20.Cutts FT, Markowitz LE. Successes and failures in measles control. J Infect Dis. 1994;170(Suppl 1):S32–S41. doi: 10.1093/infdis/170.supplement_1.s32. [DOI] [PubMed] [Google Scholar]
- 21.Bjorge L, Hakulinen J, Wahlstrom T, Matre R, Meri S. Complement-regulatory proteins in ovarian malignancies. Int J Cancer. 1997;70:14–25. doi: 10.1002/(sici)1097-0215(19970106)70:1<14::aid-ijc3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Oglesby TJ, White D, Tedja I, Liszewski K, Wright L, Van den Bogarde J, et al. Protection of mammalian cells from complement-mediated lysis by transfection of human membrane cofactor protein and decay-accelerating factor. Trans Assoc Am Physicians. 1991;104:164–172. [PubMed] [Google Scholar]
- 23.Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, Lopez M, et al. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol. 2010;134:835–845. doi: 10.1309/AJCPGXK0FR4MHIHB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–1463. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 25.Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasegawa K, Pham L, O'Connor MK, Federspiel MJ, Russell SJ, Peng KW. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res. 2006;12:1868–1875. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- 27.Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 28.Langfield KK, Walker HJ, Gregory LC, Federspiel MJ. Manufacture of measles viruses. Methods Mol Biol. 2011;737:345–366. doi: 10.1007/978-1-61779-095-9_14. [DOI] [PubMed] [Google Scholar]
- 29.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- 30.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 31.Karyampudi L, Formicola C, Erskine CL, Maurer MJ, Ingle JN, Krco CJ, et al. A degenerate HLA-DR epitope pool of HER-2/neu reveals a novel in vivo immunodominant epitope, HER-2/neu88–102. Clin Cancer Res. 2010;16:825–834. doi: 10.1158/1078-0432.CCR-09-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalli KR, Krco CJ, Hartmann LC, Goodman K, Maurer MJ, Yu C, et al. An HLA-DR-degenerate epitope pool detects insulin-like growth factor binding protein 2-specific immunity in patients with cancer. Cancer Res. 2008;68:4893–4901. doi: 10.1158/0008-5472.CAN-07-6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knutson KL, Krco CJ, Erskine CL, Goodman K, Kelemen LE, Wettstein PJ, et al. T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. J Clin Oncol. 2006;24:4254–4261. doi: 10.1200/JCO.2006.05.9311. [DOI] [PubMed] [Google Scholar]
- 34.Griffin D, Bellini W, editors. Measles virus. 3rd ed. New York: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 35.Okada H, Sato TA, Katayama A, Higuchi K, Shichijo K, Tsuchiya T, et al. Comparative analysis of host responses related to immunosuppression between measles patients and vaccine recipients with live attenuated measles vaccines. Arch Virol. 2001;146:859–874. doi: 10.1007/s007050170121. [DOI] [PubMed] [Google Scholar]
- 36. ClinicalTrials.gov [homepage on the Internet]. NCT00390299: viral therapy in treating patients with recurrent glioblastoma multiforme. Available from: www.clinicaltrials.gov/ct2/show/NCT00390299.
- 37. ClinicalTrials.gov [homepage on the Internet]. NCT00450814: vaccine therapy with or without cyclophosphamide in treating patients with recurrent or refractory multiple myeloma. Available from: www.clinical-trials.gov/ct2/show/NCT00450814.
- 38.Russell SJ, Federspiel MJ, Peng KW, Tong C, Dingli D, Morice WG, et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc. 2014;89:926–933. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ClinicalTrials.gov [homepage on the Internet]. NCT0153177: intrapleural measles virus therapy in patients with malignant pleural mesothelioma. Available from: www.clinicaltrials.gov/ct2/show/NCT01503177.
- 40.Markman M, Webster K, Zanotti K, Peterson G, Kulp B, Belinson J. Survival following the documentation of platinum and taxane resistance in ovarian cancer: a single institution experience involving multiple phase 2 clinical trials. Gynecol Oncol. 2004;93:699–701. doi: 10.1016/j.ygyno.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Colombo N, Kutarska E, Dimopoulos M, Bae DS, Rzepka-Gorska I, Bidzinski M, et al. Randomized, open-label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum- refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. J Clin Oncol. 2012;30:3841–3847. doi: 10.1200/JCO.2011.38.8082. [DOI] [PubMed] [Google Scholar]
- 42.Gordon AN, Tonda M, Sun S, Rackoff W. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Hanker LC, Loibl S, Burchardi N, Pfisterer J, Meier W, Pujade-Lauraine E, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23:2605–2612. doi: 10.1093/annonc/mds203. [DOI] [PubMed] [Google Scholar]
- 44.Mader EK, Maeyama Y, Lin Y, Butler GW, Russell HM, Galanis E, et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engeland CE, Grossardt C, Bossow S, Lay-Mees C, Shevchenko I, Umansky V, et al. Measles virus mediated immune checkpoint blockade enhances cancer immunovirotherapy. Mol Ther. 2014;22:s11. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardcastle J, Feng J, Kurokawa C, Domingo-Musibay E, Allen C, Johnson E, et al. Modulation of innate immunity with CTLA4 blockade in measles virotherapy for glioblastoma. Mol Ther. 2014;22:s247. (abstr 641). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.