Summary
A major component of sex-allocation theory, the Trivers-Willard Model (TWM), posits that sons and daughters are differentially affected by variation in the rearing environment. In many species, the amount of parental care received is expected to have differing effects on the fitness of males and females. When this occurs, the TWM predicts that selection should favour adjustment of the offspring sex ratio in relation to the expected fitness return from offspring. However, evidence for sex-by-environment effects is mixed and little is known about the adaptive significance of producing either sex.
Here, we test whether offspring sex ratios vary according to predictions of the TWM in the house wren (Troglodytes aedon, Vieillot). We also test the assumption of a sex-by-environment effect on offspring using two experiments, one in which we manipulated age-differences among nestlings within broods, and another in which we held nestling age constant but manipulated brood size.
As predicted, females with high investment ability over-produced sons relative to those with lower ability. Males were also over-produced early within breeding seasons. In our experiments, the body mass of sons was more strongly affected by the sibling-competitive environment and resource availability than that of daughters: males grew heavier than females when reared in good conditions but were lighter than females when in poor conditions.
Parents rearing broods with 1:1 sex ratios were more productive than parents rearing broods biased more strongly towards sons or daughters, suggesting that selection favours the production of mixed-sex broods. However, differences in the condition of offspring as neonates persisted to adulthood, and their reproductive success as adults varied with the body mass of sons, but not daughters, prior to independence from parental care. Thus, selection should favour slight but predictable variations in the sex ratio in relation to the quality of offspring that parents are able to produce.
Offspring sex interacts with the neonatal environment to influence offspring fitness, thus favouring sex-ratio adjustment by parents. However, increased sensitivity of males to environmental conditions, such as sibling rivalry and resource availability, reduces the fitness returns from highly male-biased broods.
Keywords: family life, life history, sex allocation, sex ratio, sibling rivalry
Introduction
Variation in the ontogenetic environment often has long-term effects on phenotypic development, and can have profound consequences for individual life-history trajectories (Lindström 1999; Monaghan 2008). Recent work has revealed that variation in the neonatal environment can also have sex-specific consequences for offspring fitness (Wilkin & Sheldon 2009; Jones, Nakagawa & Sheldon 2009), and such differences in environmental sensitivity have critical implications for sex-allocation theory (Trivers & Willard 1973; Hewison & Gaillard 1999; Uller 2006; Koskela et al. 2009). For example, if one sex is more sensitive than the other to harsh conditions early in life, mothers should over-produce the less-sensitive sex when resources are in short supply, as this will maximize the number of offspring that survive to reproduce (Myers 1978). However, when effects of variation in the rearing environment persist to affect adult reproduction, sex ratios should also be adjusted according to the anticipated reproductive success of offspring if the quality of the rearing environment differentially affects the reproductive value of sons and daughters (Trivers & Willard 1973). Originally conceived by Trivers and Willard (1973), this concept has subsequently been expanded to a generalized Trivers-Willard Model (TWM) that can be used to predict how selection will favour the adjustment of offspring sex ratios in relation to environmental conditions (Cockburn, Legge & Double 2002; West 2009; Komdeur 2012).
The TWM rests upon several important assumptions that must be true for selection to favour sex-ratio adjustment: (i) variation in maternal condition is transferred to offspring during the period of parental care, (ii) differences in the condition of offspring at independence from parental care persist to adulthood, and (iii) these differences in condition have sex-specific effects on the reproductive success of offspring as adults. Although Trivers and Willard (1973) discussed the transfer of maternal condition to offspring, physical condition in the strictest sense has been shown to provide an incomplete picture of a mother’s ability to produce fit offspring (see, e.g., Sheldon & West 2004; West 2009; Pryke & Rollins 2012). Thus, the generalized TWM now encompasses a broader range of factors than simply a mother’s physical condition, and, for species with parental care, is related more generally to the parental ability to invest in offspring or the quality of the environment that parents are able to provide (West 2009). The interaction between offspring sex and environmental conditions on fitness is, thus, a critical element that should drive the evolution of adaptive sex allocation, with changes in the amount of parental care received having differing effects on sons and daughters (see also Sheldon 1998; Velando 2002; Love et al. 2005; Uller 2006; Saino et al. 2008; Pryke, Rollins & Griffith 2011). Traditionally, mothers that are able to rear high-quality offspring are predicted to over-produce sons with high reproductive potential at independence from parental care; when unable to produce high-quality offspring, mothers should produce daughters because their future reproductive success should be affected less by poor rearing conditions than that of sons (see also Sheldon 1998; Krist 2006; Robert, Schwanz & Mills 2010; Merkling et al. 2012; Pryke & Rollins 2012; but see Leimar 1996; Hewison & Gaillard 1999).
Williams (1979) suggested that a Trivers-Willard effect should produce sex-ratio distributions that are flatter and wider than the null expectation if sons and daughters are produced more frequently within families than predicted by chance (see also Harmsen & Cooke 1983). Such a result has seldom been demonstrated, implying constraints to sex-ratio adjustment (Harmsen & Cooke 1983; Westerdahl et al. 1997; Kölliker et al. 1999; Grindstaff et al. 2001; Postma et al. 2011; but see Svensson & Nilsson 1996; Bowers et al. 2013). It should be noted, however, that tests used to assess Williams’ (1979) prediction often lack power when clutch sizes are small (Ewen, Cassey & King 2003). Despite being an important precursor for adaptive sex allocation to occur, sex-specific environmental sensitivity may also help resolve this paradox and, in itself, act as a constraint to sex-ratio adjustment. For example, if sons are more sensitive than daughters to variation in environmental conditions, the overproduction of sons may actually be maladaptive as males face greater effects of sibling competition, resulting in selection for mixed-sex broods (see also Uller 2006).
In a population of house wrens, female and male nestlings are affected differently by age-related sibling competition created by the order in which they hatch from their eggs (Bowers, Sakaluk & Thompson 2011). Sons and daughters are similar, on average, in body mass, size, and plumage shortly after hatching and prior to leaving the nest, but first-hatching males grow faster and eventually obtain greater body mass than first-hatching females of the same age. The reverse is true for last-hatching males with an early size-disadvantage, as they grow more slowly and reach a lower body mass than females of similar age (Bowers, Sakaluk & Thompson 2011). In the current study, we first tested whether offspring sex ratios vary according to a mother’s ability to invest in offspring, as predicted by the TWM. We used the number of broods females produced within a given breeding season as a functional proxy for maternal investment ability, and used this to determine whether maternal abilities were associated with the sex ratio they produced (see Methods for further details). We predicted that females with above-average investment ability (i.e., those producing multiple broods of young within a single season) should overproduce males relative to females with a reduced ability to invest in offspring.
We then tested experimentally for a sex-by-environment interaction on offspring using two separate cross-fostering experiments. In one experiment, the “Competitive-hierarchy Experiment,” we manipulated sibling-competitive hierarchies by manipulating age-differences among nestlings within broods. In a second experiment, the “Brood-size Experiment,” we held the age of nestlings within broods constant, but manipulated brood size shortly after hatching to create relaxed and intense sibling-competitive environments for offspring. In both experiments, we predicted that the body mass of sons should be more strongly affected than that of daughters by the range of rearing conditions we created. We recently found that an experimentally induced increase in parental investment resulted in sons of above-average mass and size and daughters with enhanced immune responsiveness (Bowers et al. 2012). Thus, we predicted that sons reared under favourable conditions (i.e., older nestlings within broods and nestlings in small broods) would allocate their share of parental resources to increasing body mass and daughters to enhancing immune responsiveness, whereas sons should be more negatively affected than daughters by poorer rearing conditions (i.e., younger nestlings within broods and nestlings in enlarged broods). Although our hypothesis of a sex-by-environment interaction predicts that phenotypic differences among treatments should be sex-specific at the level of the individual, it also predicts that increases in the proportion of males within a brood should adversely affect their mass and survival as they face increased competition from other males. Indeed, if sons are more sensitive than daughters to environmental conditions early in life, mothers are predicted to overproduce daughters as the ability to produce high-quality offspring is reduced (Trivers & Willard 1973; Frank 1990). Thus, under normal conditions, female-biased broods may exhibit reduced nestling mass and recruitment, on average, because they are reared by mothers with a reduced ability to produce high-quality offspring (Trivers & Willard 1973; Frank 1990). However, if sons are more sensitive than daughters to environmental conditions, then male-biased broods should have reduced mass and recruitment because they contain relatively more of the more-sensitive sex (see also Myers 1978; Uller 2006). Therefore, we also tested for effects of the brood sex ratio on offspring size and survival, predicting that mixed-sex broods should exhibit enhanced nestling mass and survival relative to female- and male-biased broods. The TWM also postulates that differences in condition among offspring at independence from parental care persist to adulthood and affect reproduction sex-specifically (Trivers & Willard 1973; Hewison & Gaillard 1999). Thus, we tested whether this was true by monitoring the recruitment of offspring to the breeding population and testing whether the body mass of offspring prior to fledging correlated with that as an adult, and whether body mass prior to independence had sex-specific effects on adult reproductive success.
Materials and methods
STUDY AREA AND SPECIES
We studied a population of house wrens breeding in secondary deciduous forest in McLean County, Illinois, USA (40.665°N, 88.89°W). Nestboxes (N = 820) were spaced 30 m apart along north-south transects separated by 60 m and mounted on 1.5-m poles atop 48.3-cm diameter aluminium predator baffles (Lambrechts et al. 2010 provide further details on nestboxes). House wrens are small (10–12 g), cavity-nesting songbirds with breeding grounds distributed across the mid-latitudes of North America, and birds in the study population winter in the southern United States and northern Mexico. Females and males do not show appreciable differences in return rate as recruits (Bowers et al. 2014a), and natal dispersal distances on the study site do not differ statistically between females (median distance = 674 m) and males (median distance = 608 m; Drilling and Thompson 1988). Upon arriving on the study area in spring, females select a mate that is defending a nest site and, after completing nest construction, lay a clutch of 4–8 eggs (Dobbs, Styrsky & Thompson 2006). Females produce one egg per day until their clutch is completed, laying a modal clutch size of 7 eggs for clutches in the first half of summer and 6 eggs in the second half of summer (Dobbs, Styrsky & Thompson 2006). Only females incubate eggs, but both parents provision offspring, and parents do not bias food delivery towards offspring of either sex (Albrecht & Johnson 2002). Females in the study population vary in the onset of incubation relative to clutch completion, which affects whether eggs hatch synchronously or asynchronously (initiating incubation prior to clutch completion results in asynchronous hatching, whereas delayed incubation until clutch completion results in synchronous hatching). In a given year, ca. 25–50% of clutches in the population hatch asynchronously, with eggs within a clutch hatching over two to three or, occasionally, four days; the remainder of clutches hatch synchronously, with all eggs of the clutch hatching within a day (Bowers, Sakaluk & Thompson 2011, 2013). The length of the nestling period is typically 15–17 days (Bowers, Sakaluk & Thompson 2013); the oldest, largest nestlings usually leave the nest first, with the rest of the brood following within a few hours. Some nestlings, typically small, underdeveloped runts, occasionally remain in the nest and die of starvation (Johnson 2014). House wrens are useful for studying sex-by-environment interactions on morphological and immunological traits because these generally do not differ, on average, for female and male nestlings; however, sex-differences can arise across a range of rearing conditions (Bowers, Sakaluk & Thompson 2011, Bowers et al. 2012; Thompson et al. 2014).
MATERNAL INVESTMENT ABILITY & BROOD SEX RATIOS, 2009–2011
We analysed brood sex ratios in relation to maternal investment ability using sex-ratio data of broods from the 2009–2011 breeding seasons (N = 1,377 offspring from 276 broods). Females in our study population are facultatively multi-brooded, with 50–70% of the females that complete a successful nesting attempt early in the breeding season attempting a second (Finke, Milinkovich & Thompson 1987; Dobbs, Styrsky & Thompson 2006; Bowers, Sakaluk & Thompson 2012). Whether or not a female produces multiple broods within years is strongly associated with her body condition, and, for females that produce multiple broods, those in good condition take less time to initiate their subsequent broods than those in poorer condition (Bowers, Sakaluk & Thompson 2012). Moreover, of the females in this analysis (N = 194 females from 2009–2011), those that were multi-brooded within a season were more likely to survive and return to breed the following year than females producing a single brood (generalized linear mixed model with maternal ID as a random effect and binary response: parameter estimate ± SE = 0.88 ± 0.38, F1, 215 = 5.24, P = 0.023). Thus, the number of broods a female produces within breeding seasons is a useful proxy for maternal investment ability, which we predicted would be positively associated with the proportion of male offspring that females produced.
We determined the sex of offspring within broods using blood samples (described in “General Field Procedures” below). We were unable to determine the sex of 15% of all the eggs that females produced because of offspring mortality prior to sexing and inviability of eggs, which raises the concern that brood sex ratios do not directly reflect the primary sex ratio. However, offspring mortality would have to be strongly sex-biased to create departures from the primary sex ratio (our data do not suggest that mortality in the nest is sex-biased, see below), and including incomplete broods in sex-ratio analyses should not affect the patterns observed (Fiala 1980; Krackow and Neuhaüser 2008; West 2009). Indeed, analysing modified forms of our dataset for this study assuming that all unsexed offspring were female or, alternatively, entirely male, had no effect on the results we report (the effects on sex ratios are still significant at P < 0.05).
GENERAL FIELD PROCEDURES
In all years, we visited nestboxes at least twice weekly to check for evidence of female settlement and clutch initiation. We then captured and identified females and males during incubation or early in the nestling-rearing stage by either capturing them inside nestboxes or using mist nets placed outside the box. Upon capture, we measured the body mass (± 0.1 g) and tarsus length (± 0.1 mm) of adults and ringed them with a unique U. S. Geological Survey aluminium leg ring; males received three additional coloured leg rings so they could be identified visually without needing to be recaptured.
We visited nests daily when hatching was expected and, for the cross-fostering experiments, we uniquely marked all nestlings within broods upon hatching by clipping their toenails. After cross-fostering (done blind with respect to nestling sex, see below), we monitored the subsequent development of nestlings and ringed them, weighed them, and measured their tarsus at 11 days of age. Nestling body mass and tarsus length generally reach asymptotic levels by this age (Lago, Johnson & Albrecht 2000), and body mass at this age positively predicts survival and recruitment into the breeding population (Bowers et al. 2014a). When nestlings were 11 days old, we drew a blood sample for molecular sexing and administered a phytohaemagglutinin (PHA) skin test to obtain a measure of cutaneous immune activity. Blood samples were stored in microcapillary tubes on ice while in the field and processed in the laboratory later the same day. After centrifuging blood samples and measuring haematocrit, a widely used measure of condition and health (Richner, Oppliger & Christe 1993; Ots, Murumägi & Hõrak 1998; Norte et al. 2008; Morrison, Ardia & Clotfelter 2009; Williams 2012; Ardia 2013), we stored red-blood cells for later use in molecular sex-identification of nestlings (for details of these procedures, see Forsman et al. 2010; Bowers, Sakaluk & Thompson 2011).
For the PHA test, we used a digital thickness gauge (Mitutoyo no. 547–500) to measure pre-injection web thickness as the mean of three measures, and we then injected the web intradermally with a 50-μL solution of PHA (Sigma, prod no. L8754) in phosphate-buffered saline (PBS; concentration = 5 mg PHA/mL PBS). Upon injection, PHA induces inflammation and swelling that includes immune cells derived from innate and adaptive axes of the immune system, and large swellings indicate a heightened level of immune activity (Martin et al. 2006, Vinkler et al. 2014). We measured the swelling 24 h post-injection, and used the difference between the mean of three pre- and post-injection measures as the PHA response. This immune response is thought to be energetically costly to produce and subject to a trade-off with other life-history functions (e.g., Martin, Scheuerlein & Wikelski 2003, Martin et al. 2006; Tschirren, Fitze & Richner 2003; Ardia 2005a,b), and the magnitude of the swelling is positively associated with long-term survival and lifetime reproductive success in our study population (Bowers et al. 2014a). Because we previously found no appreciable differences between the sexes using other immunological measures, such as the bactericidal activity of plasma or antibody production (Bowers et al. 2012), we focused in the current study on nestling responses to PHA injection and its potential trade-off with nestling growth (e.g., Fargallo et al. 2002; Tschirren, Fitze & Richner 2003; Dubiec, Cichoń & Deptuch 2006).
Not all offspring survived to leave the nest in the two experiments. Out of both experiments, a total of 85 of 758 offspring (11.2%) died prior to leaving the nest in non-depredated, non-abandoned broods, typically before four days of age. Most of the nestlings that died were removed from the nest by parents before we could collect them for molecular sexing (cases of complete nest failure prior to banding and bleeding nestlings were caused by nest depredation). The sex of nestlings that we did find dead in the nest did not differ from a 1:1 ratio (12 males, 15 females; binomial test P = 0.701). Although this suggests that sex-specific mortality did not occur, we cannot completely rule out the possibility that males and females died in the nest at unequal rates. As sex-specific effects of early rearing conditions may manifest themselves though differences in post-fledging survival or reproductive success later in life, we monitored the survival and recruitment of these offspring to the breeding population by attempting to catch all breeding adults in subsequent years.
COMPETITIVE-HIERARCHY EXPERIMENT
Throughout the 2011 breeding season, we exchanged randomly selected nestlings between brood dyads, placing a 4-day-old nestling into a 1-day-old brood and transferring a 1-day-old nestling from that brood into the 4-day-old’s brood, mimicking naturally occurring age differences among siblings within nests created by asynchronous hatching (Bowers, Sakaluk & Thompson 2011, 2013). Therefore, we created broods with a foster nestling that was either three days older (N = 53 broods, 47 of which successfully produced fledglings) or three days younger (N = 59 broods, 52 of which successfully produced fledglings) than their brood-mates (we obtained some younger nestlings from source nests not involved in the study). Thus, we held brood size constant before and immediately after our manipulation, creating experimentally “advantaged” and “disadvantaged” nestlings analogous to natural situations in which parents differentially invest in individuals within their brood (Jeon 2008). For example, early hatching nestlings within asynchronously hatched broods are typically much larger and heavier than their younger siblings within the nest, and these older nestlings are also heavier and larger than nestlings of the same age in synchronously hatched broods, in which all offspring are similar in body mass and size throughout nestling development (Bowers, Sakaluk & Thompson 2011).
In this experiment, we visited nests regularly to weigh nestlings when each was one, two, four, six, eight, 11, and 12 days of age. Approximately half of the younger foster nestlings did not survive to leave the nest (27 younger foster nestlings survived to fledging in the 52 successful nests that received one; none of the older foster nestlings died prior to fledging). Thus, we omitted the unmanipulated nestlings in broods where foster nestlings did not survive to be banded, measured, and sexed at 11 days of age to avoid inflating denominator degrees of freedom (see Data Analysis below). Including these unmanipulated nestlings in our analyses yields the same qualitative results (not shown). Because most offspring in a given brood were not cross-fostered, we calculated mean values for phenotypic traits of the males and females that were not cross-fostered, and used the mean values for these nestlings in our analysis of treatment effects to avoid inflating denominator degrees of freedom; analysing data for all individual nestlings yields the same qualitative results (not shown). For nestlings that were not cross-fostered, being reared with either an older or a younger foster nestling had no effect on their average body mass (F1, 64 = 0.40, P = 0.531), PHA response (F1, 62 = 2.08, P = 0.154), or haematocrit (F1, 29 = 0.03, P = 0.856); thus, we pooled these offspring into a single group for analysis.
BROOD-SIZE EXPERIMENT
During the second half of the 2011 breeding season, we cross-fostered nestlings shortly after hatching to create either small broods of four nestlings (N = 15 broods, 14 of which successfully produced a total of 54 offspring that survived to be measured at 11 days of age) or large broods of eight nestlings (N = 19 broods, 16 of which produced a total of 127 offspring surviving to 11 days of age). Treatments were randomly assigned to nests, and there was no difference between treatments prior to cross-fostering in clutch size (large broods = 6.0 ± 0.5 eggs, small broods = 5.9 ± 0.7 eggs; t32 = 0.86, P = 0.399) or brood size (large broods = 5.6 ± 0.2 nestlings, small broods = 5.7 ± 0.2 nestlings; t32 = 0.47, P = 0.645). Modal brood size is usually six nestlings during the time of the breeding season at which this experiment was conducted, and our treatments are within the natural range of variation in brood size. Not all broods began with six nestlings, thus we also obtained nestlings from source nests not involved in the study to bring some broods to four or to eight nestlings; all 19 of the large broods received at least one foster nestling to bring the brood size to eight, whereas four of the 16 small broods also contained one or more foster nestlings. Previous experiments on our study population showed that cross-fostering has no effect on nestling mass or survival (Finke, Milinkovich & Thompson 1987; Harper, Juliano & Thompson 1994). This was also true in the current study, as there was no difference between foster and non-foster nestlings in body mass (mixed-model ANOVA: F1, 158 = 0.15, P = 0.702) or survival to fledging (generalized linear mixed model with binary responses: F1, 183 = 0.13, P = 0.721). There was also no difference in the number of males and females that were cross-fostered (binomial test: χ2 = 0.02, P = 0.884), and brood sex ratios were similar for large and small broods (generalized linear model with binomial distribution: F1, 29 = 0.27, P = 0.609). Nestlings were cross-fostered within two days of hatching, and were never outside a nest for more than 10 min. We wanted nest-mates to be of comparable age and size at the time our treatment was imposed, so we delayed cross-fostering until the majority of eggs had hatched, which allowed us to create broods in which all nestlings were within 24 h of age. In this experiment, we visited nests to weigh nestlings when each was two, four, and 11 days of age. We were also able to observe parental food provisioning to the nest in this experiment as part of another study, which verified that nestlings in small broods had significantly higher per-nestling food availability than those in larger broods (Bowers et al. 2014b).
DATA ANALYSIS
We used SAS (version 9.3) for all analyses and all tests are two-tailed. To obtain standardized parameter estimates from analyses, we centred and standardized input variables prior to analysis (Schielzeth 2010). We first analysed brood sex ratios in relation to maternal investment ability. We included brood sex ratios from the 2009–2011 breeding seasons (N = 1,377 offspring from 276 broods) with the number of male offspring in a nest as the dependent variable and the number of sexed offspring as the binomial denominator (i.e. event/trial syntax) using a generalized linear mixed model (GLMM; PROC GLIMMIX in SAS) with maternal identity as a random effect. As a proxy for maternal investment ability, we used female reproductive effort (i.e., whether females produced a single brood or multiple broods within a season) as a fixed effect along with clutch-initiation date and year as fixed effects. We included maternal identity as a random effect to account for non-independence of females that produced multiple broods, but analysing a smaller subsample of nests that include only a female’s first brood of the season produces the same qualitative results (data not shown).
We then used general linear mixed models to analyse phenotypic variation among female and male nestlings in relation to their treatment groups in the two experiments. We included the nest of rearing as a random effect to account for the non-independence of offspring reared together within a nest, and we also included the nest of origin as a random effect to account for the relatedness of nestlings and maternal effects prior to cross-fostering. We tested for a treatment-by-sex interaction on nestling traits in both the competitive-hierarchy and brood-size experiments. Our analyses of nestling body mass focus on asymptotic mass at 11 days of age, and we included tarsus length at this age as a covariate. Forsman et al. (2010) recently showed that PHA responsiveness can covary with mass, so we included nestling body mass as a covariate when analysing PHA responses. We did not use a model-simplification procedure (i.e., remove non-significant interactions between treatment and sex) because we had predicted a priori an interaction between these variables in their effect on offspring traits. In the Competitive-hierarchy Experiment, we calculated the mean values for phenotypic traits (body mass, tarsus length, PHA responsiveness, and haematocrit) of the males and females that were not cross-fostered in a given nest, and used these mean values in our analyses to avoid inflating denominator degrees of freedom.
We also predicted that increases in the proportion of males within a brood should adversely affect their development and survival as they faced increased competition from other males. We tested this by pooling all nestlings from the two cross-fostering experiments and testing for a negative quadratic relationship between the brood sex ratio (i.e., the proportion of males within the brood, with males coded as 1 and females as 0) and the mass and number of nestlings fledged using linear mixed models, and recruitment to the breeding population using logistic regression with the nest of rearing and the nest of origin as random effects. We predicted that the relationship between the sex ratio and nestling mass and survival would be hump-shaped because broods that are primarily female-biased are predicted to be produced by mothers with below-average ability to invest in young, whereas male-biased broods should exhibit reduced mass and survival because of greater sensitivity of males than of females to sibling competition. Although the number of young sexed within a brood can often covary with the sex ratio, such that small broods are more likely to exhibit a sex ratio biased towards sons or daughters, we controlled for variation in brood size when analysing effects of the sex ratio. Of the broods with 0–25% male or 75–100% male offspring (N = 32 broods), there were two broods for which we could sex only a single nestling, and analysing effects of the brood sex ratio without these nests produces the same qualitative results (not shown).
Among the 673 offspring that survived to leave the nest in the two experiments, 30 recruited to the breeding population (11 females and 19 males). Recruitment rate in this study was typical for our study population, and was similar to that reported for other populations of house wrens (e.g., Kendeigh 1941; Poirier, Whittingham & Dunn 2004). Long-term data from the study population suggest that variation in recruitment is largely driven by variation in survival; although some offspring surviving to adulthood likely breed away from the study site, our data suggest that this is unrelated to environmental or phenotypic variation (Bowers et al. 2014a). Thus, a lack of data on dispersers should not introduce a bias in our results for recruits (Bowers et al. 2014a). Pooling all offspring across the two experiments, we tested for an effect of early rearing conditions on recruitment using logistic regression with the quality of the rearing environment as a fixed effect and the nest of rearing and nest of origin as random effects. For this analysis, we considered older offspring in the competitive-hierarchy experiment and nestlings in small broods from the brood-size experiment as being reared in high-quality conditions, the non-foster nestlings in the competitive-hierarchy experiment as being in typical rearing conditions (the presence of either an older or a younger foster sibling had no effect on these nestlings; see above), and younger offspring and those in large broods as experiencing poor-quality rearing conditions. We did not have sufficient replication among recruits to test for sex-specific treatment effects on adult reproduction (only two neonates from poor rearing conditions recruited to the breeding population), but we were able to test for a sex-specific effect of body mass prior to independence from parental care on adult reproduction, a major assumption of the TWM. For this analysis, we pooled offspring from the three breeding seasons (N = 60 recruits that hatched as nestlings from 2009–2011), and tested whether nestling body mass at 11 days of age was correlated with their body mass as adults using a general linear model that included sex, breeding stage upon capture as an adult (i.e., incubation or nestling rearing), and the day of year and time of day on which the adults were captured as covariates. Using this dataset, we also tested for sex-specific effects of offspring body mass prior to fledging on adult reproduction. We first used a logistic regression to test whether nestling body mass affected whether offspring would produce multiple broods of offspring in their first breeding season, and then used a linear model to analyse the number of offspring that these recruits successfully fledged from their nests as adults.
Results
OFFSPRING SEX RATIOS
Brood sex ratios were positively associated with maternal investment ability (Table 1; Fig. 1). As predicted, females rearing multiple broods within a year produced a slight but significant male-bias (proportion male = 0.561 ± 0.016, mean ± SE) relative to females rearing a single brood (proportion male = 0.475 ± 0.022; Table 1; Fig. 1). Clutch-initiation date also had an effect on brood sex ratios, with broods produced earlier within breeding seasons having a male-bias relative to broods produced later (Table 1).
Table 1.
Effects on brood sex ratios (proportion of offspring that were male). Maternal investment ability represents females that produced a single brood within the breeding season or those that produced multiple broods (see text). Data were centred and standardized to z-scores for analysis. Significant effects appear in bold type.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
| Intercept | 0.25 ± 0.07 | |||
| Maternal investment ability | 9.54 | 1, 272 | 0.002 | |
| Single-broodeda | −0.35 ± 0.11 | |||
| Clutch-initiation date | −0.13 ± 0.06 | 4.69 | 1, 272 | 0.031 |
| Year | 0.05 ± 0.05 | 1.01 | 1, 272 | 0.316 |
relative to multi-brooded females.
Fig. 1.
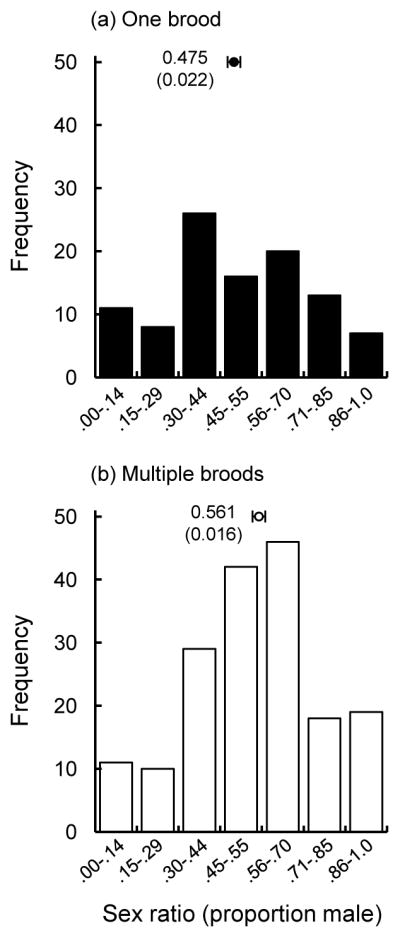
Offspring sex ratio (proportion of male offspring) in relation to maternal quality. Females producing a single brood of young within years (a) produced fewer males within their broods, on average, relative to females producing multiple broods of young (b). Points above histograms represent mean sex ratios ± SE, which are given.
COMPETITIVE-HIERARCHY EXPERIMENT
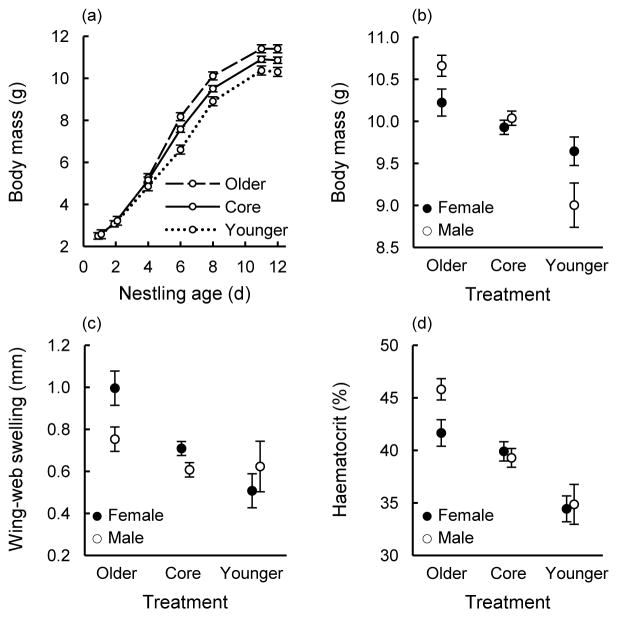
Nestlings quickly diverged in their growth trajectories after the implementation of the treatment, with older, competitively advantaged nestlings growing the fastest of the treatment groups and the younger, disadvantaged nestlings growing the slowest (Fig. 2a). Nestlings placed at a competitive advantage early on were heavier than their brood-mates for any age, and there was an interaction between treatment and sex in their effect on nestling mass on day 11 (Table 2; Fig. 2b). Older male nestlings became heavier than females in the same treatment (follow-up test: F1, 47 = 12.25, P = 0.001), but younger male nestlings tended to suffer disproportionately from their competitive disadvantage and were lighter than females of the same age (follow-up test: F1, 24 = 3.14, P = 0.080). There was also an interaction between treatment and nestling sex in their effect on PHA responsiveness (Table 2; Fig. 2c), as older females mounted disproportionately stronger immune responses to PHA injection than males in the same treatment (follow-up test: F1, 45 = 11.54, P = 0.001). Nestling haematocrit also differed sex-specifically across treatments (Table 2; Fig. 2d), with older males having higher haematocrit values than females in the same treatment (follow-up test: F1, 35 = 6.90, P = 0.013).
Fig. 2.
Results of the Competitive-hierarchy Experiment. Nestling (a) growth, (b) asymptotic body mass at 11 days of age, (c) cutaneous immune response to PHA injection, and (d) haematocrit in relation to treatment (least-squares means ± SE). “Core” nestlings are those that were not swapped among nests; we calculated a mean value for female and male core nestlings within each brood (N = 70 female and 70 male mean values, see text for details). For older nestlings, N = 19 females and 30 males; for younger nestlings, N = 20 females and 8 males.
Table 2.
Effects on nestlings in the Competitive-hierarchy and Brood-size Experiments. Data were centred and standardized to z-scores for analysis. Significant effects appear in bold type. See text for follow-up tests.
| Competitive-hierarchy Experiment | Brood-size Experiment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate ± SE | F | df | P | Estimate ± SE | F | df | P | ||
|
|
|
||||||||
| Body mass | Body mass | ||||||||
| Intercept | −1.71 ± 0.86 | Intercept | −0.70 ± 0.13 | ||||||
| Treatment | Treatment | ||||||||
| Oldera | 0.95 ± 1.20 | Large broodsb | −0.97 ± 0.17 | ||||||
| Corea | 1.15 ± 0.68 | Nestling sex | |||||||
| Nestling sex | Femalec | −0.41 ± 0.17 | |||||||
| Femalec | 0.81 ± 0.46 | Treat. × Sex | 0.61 ± 0.20 | 9.04 | 1, 146 | 0.003 | |||
| Treat. × Sex | −0.94 ± 0.47 | 7.98 | 2, 79 | < 0.001 | Tarsus length | 0.50 ± 0.05 | 99.36 | 1, 160 | < 0.001 |
| Tarsus length | 0.32 ± 0.11 | 9.03 | 1, 79 | 0.004 | Foster status | 0.71 | 1, 37.2 | 0.405 | |
| Fosterd | −0.09 ± 0.11 | ||||||||
| PHA responsiveness | PHA responsiveness | ||||||||
| Intercept | −2.76 ± 1.04 | Intercept | −0.37 ± 0.21 | ||||||
| Treatment | Treatment | 2.57 | 1, 67.3 | 0.114 | |||||
| Oldera | 3.61 ± 1.47 | Large broodsb | 0.53 ± 0.26 | ||||||
| Corea | 2.54 ± 0.84 | Nestling sex | 0.05 | 1, 155 | 0.83 | ||||
| Nestling sex | Femalec | 0.16 ± 0.24 | |||||||
| Femalec | 1.80 ± 0.63 | Treat. × Sex | −0.37 ± 0.29 | 1.69 | 1, 156 | 0.195 | |||
| Treat. × Sex | −1.47 ± 0.64 | 6.57 | 2, 70 | 0.002 | Body mass | 0.19 ± 0.08 | 5.79 | 1, 168 | 0.017 |
| Body mass | −0.07 ± 0.08 | 0.78 | 1, 70 | 0.381 | Foster status | 0.71 | 1, 37.9 | 0.405 | |
| Fosterd | 0.15 ± 0.15 | ||||||||
| Haematocrit | Haematocrit | ||||||||
| Intercept | −0.82 ± 0.30 | Intercept | 0.16 ± 0.21 | ||||||
| Treatment | 22.6 | 2, 35 | < 0.001 | Treatment | 4.19 | 1, 47.5 | 0.046 | ||
| Oldera | 1.75 ± 0.34 | Large broodsb | −0.33 ± 0.26 | ||||||
| Corea | 0.71 ± 0.32 | Nestling sex | 0.92 | 1, 166 | 0.338 | ||||
| Nestling sex | 1.81 | 1, 35 | 0.187 | Femalec | 0.26 ± 0.23 | ||||
| Femalec | −0.07 ± 0.36 | Treat. × Sex | −0.27 ± 0.27 | 0.98 | 1, 165 | 0.325 | |||
| Treat. × Sex | −0.60 ± 0.43 | 3.25 | 2, 35 | 0.051 | Foster status | 1.64 | 1, 44.8 | 0.206 | |
| Fosterd | 0.15 ± 0.12 | ||||||||
Core nestlings were not swapped among nests.
relative to younger nestlings.
relative to nestlings in small broods.
relative to male nestlings.
relative to nestlings that were not cross-fostered.
BROOD-SIZE EXPERIMENT
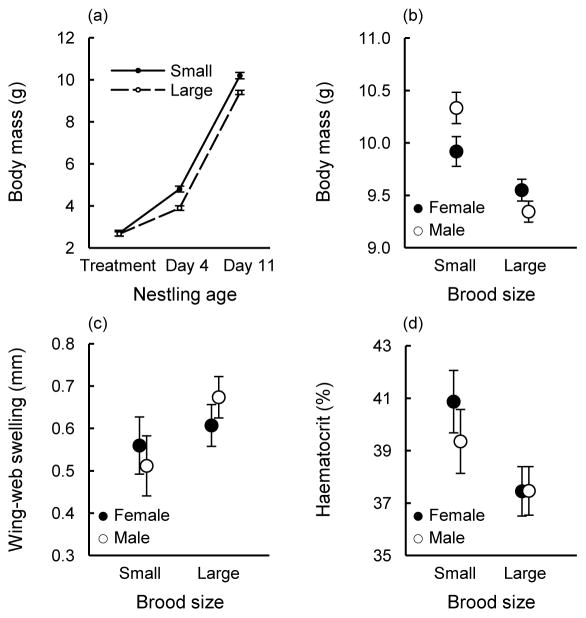
Nestlings quickly diverged in their growth trajectories after the implementation of the treatment, with nestlings in small broods experiencing reduced sibling competition and growing faster than nestlings in larger broods with increased resource competition (Fig. 3a). Nestlings in small broods were generally heavier than those in large broods, and there was an interaction between the brood-size treatment and nestling sex in their effect on individual body mass at 11 days of age (Table 2; Fig. 3b). Similar to the Competitive-hierarchy Experiment, males in this experiment were more strongly affected than females by the different levels of sibling competition; males were heavier than females in small broods (follow-up test: F1, 135 = 5.18, P = 0.024), but tended to be lighter than females in large broods (follow-up test: F1, 132 = 3.16, P = 0.078). There was no evidence of an interaction between brood size and nestling sex in their effect on immune responsiveness to PHA injection or on haematocrit (Table 2; Fig. 3c,d).
Fig. 3.
Results of the Brood-size Experiment. Nestling (a) growth, (b) asymptotic body mass at 11 days of age, (c) cutaneous immune response to PHA injection, and (d) haematocrit in relation to the brood-size treatment (least-squares means ± SE). For small broods, N = 28 females and 26 males; for large broods, N = 61 females and 66 males.
PERSISTENT EFFECTS ON OFFSPRING
We predicted that broods with 1:1 sex ratios would have enhanced offspring performance, on average, relative to female- or male-biased broods. This was indeed the case, as the mass of 11-day-old nestlings, the number of nestlings fledged, and the recruitment of nestlings into the breeding population were highest for broods containing equal numbers of females and males after controlling for variation in brood size (Table 3; Fig. 4).
Table 3.
Relationship between brood sex ratio and offspring body mass, number of young fledged, and recruitment to the breeding population. Data were centred and standardized to z-scores for analysis. Significant effects appear in bold type.
| Nestling body mass | Estimate ± SE | F | df | P |
| Intercept | 0.11 ± 0.09 | |||
| Brood sex ratio | −0.00 ± 0.06 | 0.00 | 1, 168 | 0.944 |
| Brood sex ratio2 | −0.10 ± 0.04 | 7.25 | 1, 181 | 0.008 |
| Brood size | −0.14 ± 0.06 | 5.22 | 1, 157 | 0.024 |
| Number of young fledged | Estimate ± SE | F | df | P |
| Intercept | 0.35 ± 0.07 | |||
| Brood sex ratio | 0.03 ± 0.06 | 0.20 | 1, 127 | 0.659 |
| Brood sex ratio2 | −0.14 ± 0.04 | 12.01 | 1, 127 | < 0.001 |
| Brood size | 0.76 ± 0.06 | 150.41 | 1, 127 | < 0.001 |
| Recruitment | Estimate ± SE | χ2 | df | P |
| Intercept | −2.87 ± 0.22 | |||
| Brood sex ratio | −0.10 ± 0.19 | 0.27 | 1 | 0.606 |
| Brood sex ratio2 | −0.25 ± 0.12 | 5.43 | 1 | 0.020 |
| Brood size | −0.23 ± 0.20 | 1.20 | 1 | 0.274 |
Fig. 4.
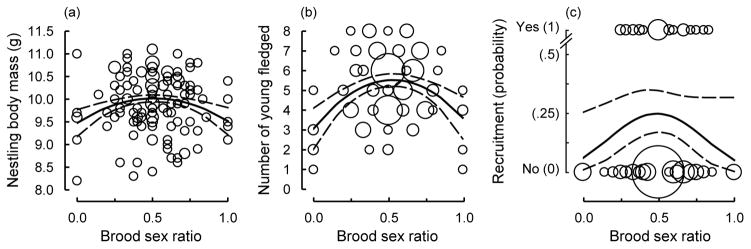
Nestling body mass (a, brood means), the number of young fledged (b), and recruitment to the breeding population (c) in relation to the brood sex ratio (proportion males). Recruitment is depicted at the level of the brood (i.e., broods with equal sex ratios have a 25% chance of producing a recruit). Bubble sizes are proportional to the number of overlapping data points, and curves represent the quadratic regression line ± 95% confidence limits.
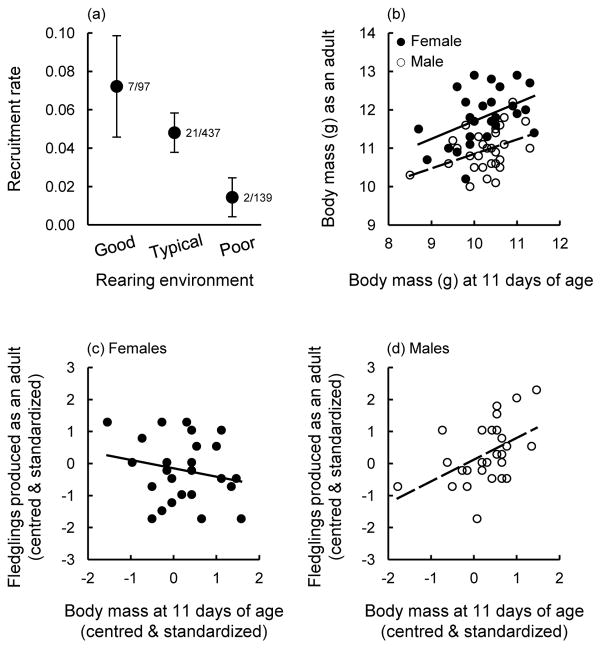
The quality of the rearing environment in the 2011 experiments also influenced the recruitment of offspring into the breeding population (logistic regression: , P = 0.039; Fig. 5a), with offspring in high-quality and normal rearing conditions each recruiting at a higher rate than those in poorer rearing conditions (post-hoc tests: P < 0.05; Fig. 5a). Among the offspring produced from 2009–2011 that recruited to breed in the population, we tested whether their body mass prior to independence correlated with their mass and reproductive success as adults. Body mass at 11 days of age positively predicted adult body mass for both females and males (Table 4, Fig. 5b). Whether or not individuals produced two or more broods of offspring in their first breeding season was influenced by body mass prior to independence, but in an interactive effect with offspring sex (Table 4); follow-up tests revealed a substantial effect of neonatal body mass for males (follow-up test: estimate ± SE = 2.07 ± 0.98, , P = 0.001) but no effect for females (follow-up test: estimate ± SE = –0.13 ± 0.60, , P = 0.823). There was also an interaction between body mass at this age and sex in their effect on the number of offspring successfully fledged as adults (Table 4; Fig. 5c,d). Males that were heavier than average prior to independence produced more fledglings than lighter males (follow-up test: estimate ± SE = 0.34 ± 0.11, F1, 24 = 9.41, P = 0.005; Fig. 5d); in contrast, the reproductive success of females was not influenced by their body mass at 11 days of age (follow-up test: estimate ± SE = –0.04 ± 0.11, F1, 23 = 0.15, P = 0.702; Fig. 5c).
Fig. 5.
(a) Recruitment of offspring from the 2011 cross-fostering experiments in relation to the quality of the rearing environment (means ± SE; see text for details). (b) Adult body mass in relation to body mass at 11 days of age for nestlings produced from 2009–2011 (raw data are plotted). (c,d) The number of offspring fledged as adults by (c) female and (d) male offspring produced from 2009–2011 in relation to their body mass prior to independence at 11 days of age (data are centred and standardized). In (b-d) solid and dashed lines represent the regression lines for females and males, respectively.
Table 4.
Lasting effects on offspring performance. Data were centred and standardized to z-scores for analysis.Significant effects appear in bold type. See text for follow-up tests.
| Correlation between body mass as a nestling and as an adult | Estimate ± SE | F | df | P |
| Intercept | −1.00 ± 0.19 | |||
| Body mass on day 11 | 0.47 ± 0.14 | 11.93 | 1, 54 | 0.001 |
| Sex | 23.45 | 1, 54 | < 0.001 | |
| Femalea | 0.93 ± 0.19 | |||
| Breeding stage | 4.52 | 1, 54 | 0.038 | |
| Incubationb | 0.49 ± 0.23 | |||
| Day of year | −0.07 ± 0.10 | 0.60 | 1, 54 | 0.443 |
| Time of day | −0.13 ± 0.07 | 3.47 | 1, 54 | 0.068 |
| Probability of producing multiple broods | Estimate ± SE | χ2 | df | P |
| Intercept | −0.34 ± 0.54 | |||
| Body mass on day 11 | 2.07 ± 0.98 | |||
| Sex | ||||
| Femalea | −0.40 ± 0.73 | |||
| Body mass × Sex | −2.23 ± 1.13 | 5.06 | 1 | 0.025 |
| Breeding date | −0.77 ± 0.39 | 4.82 | 1 | 0.028 |
| Number of fledglings produced | Estimate ± SE | F | df | P |
| Intercept | 2.00 ± 0.08 | |||
| Body mass on day 11 | 0.33 ± 0.11 | |||
| Sex | ||||
| Femalea | −0.25 ± 0.12 | |||
| Body mass × Sex | −0.41 ± 0.15 | 7.27 | 1, 48 | 0.010 |
| Breeding date | −0.22 ± 0.06 | 12.70 | 1, 48 | < 0.001 |
relative to male offspring.
relative to the nestling-provisioning stage.
Discussion
In both experiments, the body mass of males was more strongly affected by our treatments than that of females, but the immune responses to PHA injection were affected to a greater extent for females than for males in the Competitive-hierarchy Experiment. Haematocrit was also more strongly affected for males than for females in the Competitive-hierarchy Experiment. Although the meaning and significance of variation in haematocrit as a measure of condition or health state has been debated (Dawson & Bortolotti 1997; Fair, Whitaker & Pearson 2006; Norte et al. 2008), it appears to be a valid measure of condition, at least for some species. For example, variation in haematocrit among young in some species is associated with parental food-provisioning (Santangeli et al. 2012). Among nestling house wrens, haematocrit is positively correlated with nestling body condition, but not with immune activity (Forsman et al. 2010), and nestlings with haematocrit values comparable to the older nestlings within broods (Fig. 2d) have the highest average recruitment to the breeding population (Bowers et al. 2014a). The experimental results on nestling traits in the current study are consistent with an earlier descriptive study in which we detected a sex-specific effect of hatching order on nestling asymptotic body mass; although similar in mass shortly after hatching, first-hatching males were heavier and last-hatching males were lighter than similarly aged females (Bowers, Sakaluk & Thompson 2011). Indeed, daughters (i.e., the less-sensitive, more-robust sex) are more likely to be produced than sons among later-laid eggs of the clutch in house wrens (Albrecht 2000; Bowers, Sakaluk & Thompson 2011, 2014) and in other species ( Nager et al. 1999; Badyaev et al. 2002; Carranza 2004; Ležalová et al. 2005; but see Cichoń, Dubiec & Stoczkom 2003; Johnson et al. 2005; Bowers et al. 2013), which is consistent with the prediction that sex-specific environmental sensitivity should select for mothers that over-produce the more-robust sex in sub-optimal settings (Myers 1978).
Sons and daughters appear to differ in how they allocate parental resources between growth and other competing demands, including components of the immune system (Fig. 2b,c; see also Fargallo et al. 2002; Tschirren, Fitze & Richner 2003; Dubiec, Cichoń & Deptuch 2006; Bowers et al. 2012). Such a sex-specific trade-off between growth and immunodevelopment could potentially be facilitated by developing gonads and increased testosterone in male nestlings that promotes the redistribution of resources away from immune function and towards growth (e.g., Folstad & Karter 1992; Casto, Nolan & Ketterson 2001; but see Hasselquist et al. 1999; Love et al. 2008). Regardless of the potential mechanisms generating sex-specific effects, sex-differences in growth and immune function have important implications for sex-allocation theory and sex-ratio variation. Although differences in offspring condition prior to independence may persist and have sex-specific effects on adult reproductive success (Fig. 5), nestlings with highest immune responsiveness prior to independence also have above-average longevity and lifetime reproductive success (Bowers et al. 2014a). If reproductive success is more strongly dependent upon body condition for males than for females, then large sons should have higher reproductive value than daughters of similar size. However, if sons mate indiscriminately, then producing high-quality daughters may actually maximize maternal fitness if sons are likely to mate with low-quality females that produce low-quality grandoffspring (Leimar 1996). Thus, whether sons of above-average body mass and size or daughters with above-average immune responsiveness maximize maternal fitness needs further study.
Sex-ratio bias has been well-documented in a variety of taxa, but little is known about the adaptive significance of producing either sex (Uller 2006; West 2009). Most studies to date have been conducted on sexually size-dimorphic species with differing energetic demands for the growth of sons and daughters, for which it is logical to assume that differences in the costs of producing either sex should provide an impetus for mothers to adjust the sex ratio (e.g., Carranza 2004; Weatherhead & Teather 1991; Sheldon & West 2004; Martin & Festa-Bianchet 2011; Carranza & Polo 2012). Alternatively, sex-ratio bias may also be favoured when environmental variation and parental investment affect the reproductive value of sons and daughters differently (Trivers & Willard 1973). This should be the case regardless of overall differences in the energetic costs of producing either sex (Hewison & Gaillard 1999), particularly in systems with intense intrasexual competition among males, as is the case in our study species. House wren males are highly territorial as adults, and larger and more attractive males typically outcompete other males for breeding territories and mates (Kendeigh 1941; Johnson & Kermott 1990; DeMory, Thompson & Sakaluk 2010); there is also evidence that males occasionally kill other adult males in conflicts over limiting breeding sites (Belles-Isles & Picman 1987). Thus, the persistent effects of neonatal condition on adult condition and reproductive success that we detected should select for mothers that produce sons and daughters according to their ability to rear high-quality, fit offspring (Trivers & Willard 1973).
Experimental demonstration of sex-by-environment effects has often proven elusive, particularly in natural populations (Hewison & Gaillard 1999; Uller 2006; Jones, Nakagawa & Sheldon 2009), and remains a top priority in the field (West 2009). For example, Sheldon et al. (1998) did not detect sex-specific effects of brood size on measures of nestling mass and size per se in the collared flycatcher (Ficedula albicollis), whereas Rosivall et al. (2010) found that growth of body mass and feathers was more adversely affected for males than for females using a similar experiment on the same species. In the current study, the condition of offspring as neonates and the brood environment had long-term effects on offspring recruitment and reproductive success. Thus, the morphological development and fitness of offspring may vary sex-specifically across a range of rearing conditions, and some effects may be manifested later in life (see also McDonald, Olsen & Cockburn 2005; Berthouly et al. 2008; Bogdanova & Nager 2008; Boncoraglio, Martinelli & Saino 2008; Bonisoli-Alquati et al. 2008; Sockman et al. 2008; Nicolaus et al. 2009; Wilkin & Sheldon 2009; Tilgar et al. 2010; Hogle & Burness 2014), with implications for adaptive sex-ratio variation and an optimal brood sex ratio.
Chromosomal sex determination is not regarded as a major constraint to sex-ratio adjustment (West & Sheldon 2002), particularly in birds, as females are heterogametic and, thus, may have some level of physiological control over the sex of their offspring (Pike & Petrie 2003; Alonso-Alvarez 2006; Rutkowska & Badyaev 2008; Pryke et al. 2014). However, assuming that female birds can adjust the sex of their offspring according to ecological conditions, why do they not show more extreme sex-ratio biases than is frequently observed? If sex-allocation theory is correct in that sons tend to be over-produced in certain contexts and daughters in others, then among-nest variation in sex ratios should be greater than the null binomial expectation (Williams 1979; Harmsen & Cooke 1983). Williams (1979) was among the first to suggest that a Trivers-Willard effect should produce distributions of clutch sex-ratios that are flatter and wider than the binomial expectation (see also Harmsen & Cooke 1983). Paradoxically, such a result has seldom been demonstrated, implying constraints to sex-ratio adjustment (but see Svensson & Nilsson 1996; Ewen, Cassey & King 2003; Bowers et al. 2013). However, lack of deviation from the null distribution does not necessarily mean that sex-ratio adjustment does not occur (Gowaty 1991). Indeed, it is possible that multiple selective forces act on the sex ratio, or that in many cases the underlying assumptions of the TWM are not met, resulting in weak net selection away from the null expectation (Cockburn, Legge & Double 2002; West 2009; Komdeur 2012). In the current study, multi-brooded mothers produced male-biased broods relative to those produced by single-brooded mothers, yet sex-ratio distributions were not skewed as strongly towards either sex as might be expected in the absence of physiological constraints to sex-ratio adjustment (Fig. 1). Although mothers should overproduce daughters as their ability to produce high-quality offspring is reduced and overproduce sons when able to produce high-quality offspring, our findings suggest that, when sons are more sensitive than daughters to variation in environmental conditions, the overproduction of sons may actually be maladaptive relative to the production of 1:1 sex ratios. There is a conflict between the benefits that sons accrue through increased investment from high-quality mothers and the costs that those sons face from increased competition with their brothers, with the net result being that broods with 1:1 sex ratios are more productive than female- or male-biased broods (Fig. 4). Thus, detection of extra-binomial sex-ratio variation may have remained elusive, not because of physiological constraints to sex-ratio adjustment per se, but because parental fitness is maximized when sons and daughters are produced in nearly equal frequency, selecting ultimately for mixed-sex broods and a reduction in sex-ratio variation.
Acknowledgments
We thank the 2009-2013 Wren Crews for field assistance; the ParkLands Foundation (Merwin Preserve), the Illinois Great Rivers Conference of the United Methodist Church, and the Sears and Butler families for the use of their properties; and Eduardo S. A. Santos, Ben Sheldon, and two anonymous reviewers for helpful comments on the manuscript. All research activities complied with current laws of the United States of America, and were in accordance with the Illinois State University Institutional Animal Care and Use Committee (Protocol Nos. 10-2009, 05-2010, 04-2013), United States Geological Survey banding permit 09211, and United States Fish and Wildlife Service collecting permit MB692148-0. Financial support was provided by NSF grants IBN-0316580 and IOS-0718140; NIH grant R15HD076308-01; the School of Biological Sciences, Illinois State University; and student-research grants from the American Ornithologists’ Union, the American Museum of Natural History’s Frank M. Chapman Memorial Fund, the Sigma Xi Society, the Champaign County Audubon Society, and the Beta Lambda Chapter of the Phi Sigma Biological Honor Society.
Footnotes
Data Accessibility
Data and supporting information are archived at the Dryad Digital Repository, doi: 10.5061/dryad.mg6t7 (Bowers et al. 2014c).
References
- Albrecht DJ. Sex ratio manipulation within broods of house wrens, Troglodytes aedon. Animal Behaviour. 2000;59:1227–1234. doi: 10.1006/anbe.1999.1420. [DOI] [PubMed] [Google Scholar]
- Albrecht DJ, Johnson LS. Manipulation of offspring sex ratio by second- mated female house wrens. Proceedings of the Royal Society of London B. 2002;269:461–465. doi: 10.1098/rspb.2001.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Alvarez C. Manipulation of primary sex-ratio: an updated review. Avian and Poultry Biology Reviews. 2006;17:1–20. [Google Scholar]
- Ardia DR. Individual quality mediates trade-offs between reproductive effort and immune function in tree swallows. Journal of Animal Ecology. 2005a;74:517–524. [Google Scholar]
- Ardia DR. Tree swallows trade off immune function and reproductive effort differently across their range. Ecology. 2005b;86:2040–2046. [Google Scholar]
- Ardia DR. The effects of nestbox thermal environment on fledging success and haematocrit in tree swallows. Avian Biology Research. 2013;6:99–103. [Google Scholar]
- Badyaev AV, Hill GE, Beck ML, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002;295:316–318. doi: 10.1126/science.1066651. [DOI] [PubMed] [Google Scholar]
- Belles-Isles JC, Picman J. Suspected adult intraspecific killing by house wrens. Wilson Bulletin. 1987;99:497–498. [Google Scholar]
- Berthouly A, Helfenstein F, Tanner M, Richner H. Sex-related effects of maternal egg investment on offspring in relation to carotenoid availability in the great tit. Journal of Animal Ecology. 2008;77:74–82. doi: 10.1111/j.1365-2656.2007.01309.x. [DOI] [PubMed] [Google Scholar]
- Bogdanova MI, Nager RG. Sex-specific costs of hatching last, an experimental study on herring gulls (Larus argentatus) Behavioral Ecology and Sociobiology. 2008;62:1533–1541. [Google Scholar]
- Boncoraglio G, Martinelli R, Saino N. Sex-related asymmetry in competitive ability of sexually monomorphic barn swallow nestlings. Behavioral Ecology and Sociobiology. 2008;62:729–738. [Google Scholar]
- Bonisoli-Alquati A, Martinelli R, Rubolini D, Saino N. Sex-specific effects of albumen removal and nest environment manipulation on barn swallow nestlings. Ecology. 2008;89:2315–2324. doi: 10.1890/07-1066.1. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Adaptive sex allocation in relation to hatching synchrony and offspring quality in house wrens. American Naturalist. 2011;177:617–629. doi: 10.1086/659630. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Experimentally increased egg production constrains future reproduction of female house wrens. Animal Behaviour. 2012;83:495–500. [Google Scholar]
- Bowers EK, Smith RA, Hodges CJ, Zimmerman LM, Thompson CF, Sakaluk SK. Sex-biased terminal investment in offspring induced by maternal immune challenge in the house wren (Troglodytes aedon) Proceedings of the Royal Society B. 2012;279:2891–2898. doi: 10.1098/rspb.2012.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Munclinger P, Bureš S, Kučerová L, Nádvorník P, Krist M. Cross-fostering eggs reveals that female collared flycatchers adjust clutch sex ratios according to parental ability to invest in offspring. Molecular Ecology. 2013;22:215–228. doi: 10.1111/mec.12106. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Sibling cooperation influences the age of nest-leaving in an altricial bird. American Naturalist. 2013;181:775–786. doi: 10.1086/670244. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Offspring sex ratio varies with clutch size for female house wrens induced to lay supernumerary eggs. Behavioral Ecology. 2014;25:165–171. [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014a doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Nietz D, Thompson CF, Sakaluk SK. Parental provisioning in house wrens: effects of varying brood size and consequences for offspring. Behavioral Ecology. 2014b doi: 10.1093/beheco/aru153. [DOI] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Data from: Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. Dryad Digital Repository. 2014c doi: 10.5061/dryad.mg6t7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza J. Sex allocation within broods: the intrabrood sharing-out hypothesis. Behavioral Ecology. 2004;15:223–232. [Google Scholar]
- Carranza J, Polo V. Is there an expected relationship between parental expenditure and sex ratio of litters or broods? Animal Behaviour. 2012;84:67–76. [Google Scholar]
- Casto JM, Nolan V, Jr, Ketterson ED. Steroid hormones and immune function: experimental studies in wild and captive dark-eyed juncos (Junco hyemalis) American Naturalist. 2001;157:408–420. doi: 10.1086/319318. [DOI] [PubMed] [Google Scholar]
- Cichoń M, Dubiec A, Stoczkom M. Laying order and offspring sex in blue tits Parus caeruleus. Journal of Avian Biology. 2003;34:355–359. [Google Scholar]
- Cockburn A, Legge S, Double MC. Sex-ratios in birds and mammals: can the hypotheses be disentangled? In: Hardy ICW, editor. Sex Ratios: Concepts and Research Methods. Cambridge University Press; Cambridge: 2002. pp. 266–286. [Google Scholar]
- Dawson RD, Bortolotti GR. Variation in hematocrit and total plasma proteins of nestling American kestrels (Falco sparverius) in the wild. Comparative Biochemistry and Physiology. 1997;117A:383–390. [Google Scholar]
- DeMory ML, Thompson CF, Sakaluk SK. Male quality influences male provisioning in house wrens independent of attractiveness. Behavioral Ecology. 2010;21:1156–1164. [Google Scholar]
- Dobbs RC, Styrsky JD, Thompson CF. Clutch size and the costs of incubation in the house wren. Behavioral Ecology. 2006;17:849–856. [Google Scholar]
- Drilling NE, Thompson CF. Mate switching in multi-brooded house wrens. Auk. 1991;108:60–70. [Google Scholar]
- Dubiec A, Cichoń M, Deptuch K. Sex-specific development of cell-mediated immunity under experimentally altered rearing conditions in blue tit nestlings. Proceedings of the Royal Society B. 2006;273:1759–1764. doi: 10.1098/rspb.2006.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen JG, Cassey P, King RAR. Assessment of the randomization test for binomial sex-ratio distributions in birds. Auk. 2003;120:62–68. [Google Scholar]
- Fair J, Whitaker S, Pearson B. Sources of variation in haematocrit in birds. Ibis. 2007;149:535–552. [Google Scholar]
- Fargallo JA, Laaksonen T, Pöyri V, Korpimäki E. Inter-sexual differences in the immune response of Eurasian kestrel nestlings under food shortage. Ecology Letters. 2002;5:95–101. [Google Scholar]
- Fiala KL. On estimating the primary sex ratio from incomplete data. American Naturalist. 1980;115:442–444. [Google Scholar]
- Finke MA, Milinkovich DJ, Thompson CF. Evolution of clutch size: an experimental test in the house wren (Troglodytes aedon) Journal of Animal Ecology. 1987;56:99–114. [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. American Naturalist. 1992;139:603–622. [Google Scholar]
- Forsman AM, Sakaluk SK, Thompson CF, Vogel LA. Cutaneous immune activity, but not innate immune responsiveness, covaries with mass and environment in nestling house wrens (Troglodytes aedon) Physiological and Biochemical Zoology. 2010;83:512–518. doi: 10.1086/649894. [DOI] [PubMed] [Google Scholar]
- Frank SA. Sex allocation theory for birds and mammals. Annual Review of Ecology and Systematics. 1990;21:13–55. [Google Scholar]
- Gowaty PA. Facultative manipulation of sex ratios in birds: rare or rarely observed? Current Ornithology. 1991;8:141–171. [Google Scholar]
- Grindstaff JL, Buerkle CA, Casto JM, Nolan V, Jr, Ketterson ED. Offspring sex ratio is unrelated to male attractiveness in dark-eyed juncos (Junco hyemalis) Behavioral Ecology and Sociobiology. 2001;50:312–316. [Google Scholar]
- Harmsen R, Cooke F. Binomial sex-ratio distribution in the lesser snow goose: a theoretical enigma. American Naturalist. 1983;121:1–8. [Google Scholar]
- Harper RG, Juliano SA, Thompson CF. Intrapopulation variation in hatching synchrony in house wrens: test of the individual-optimization hypothesis. Auk. 1994;111:516–524. [Google Scholar]
- Hasselquist D, Marsh JA, Sherman PW, Wingfield JC. Is avian humoral immunocompetence suppressed by testosterone? Behavioral Ecology and Sociobiology. 1999;45:167–175. [Google Scholar]
- Hewison AJM, Gaillard JM. Successful sons or advantaged daughters? The Trivers-Willard model and sex-biased maternal investment in ungulates. Trends in Ecology and Evolution. 1999;14:229–234. doi: 10.1016/s0169-5347(99)01592-x. [DOI] [PubMed] [Google Scholar]
- Hogle NC, Burness G. Sex-specific environmental sensitivity is transient in nestling tree swallows (Tachycineta bicolor) Journal of Ornithology. 2014;155:91–100. [Google Scholar]
- Jeon J. Evolution of parental favoritism among different-aged offspring. Behavioral Ecology. 2008;19:344–352. [Google Scholar]
- Johnson LS. House wren (Troglodytes aedon) In: Poole A, editor. The Birds of North America. 2. Cornell Lab of Ornithology and American Ornithologists’ Union; 2014. [Google Scholar]
- Johnson LS, Kermott LH. Possible causes of territory takeovers in a north-temperate population of house wrens. Auk. 1990;107:781–784. [Google Scholar]
- Johnson LS, Wimmers LE, Johnson BG, Milkie RC, Molinaro RL, Gallagher BS, Masters BS. Sex manipulation within broods of house wrens? A second look. Animal Behaviour. 2005;70:1323–1329. [Google Scholar]
- Jones KS, Nakagawa S, Sheldon BC. Environmental sensitivity in relation to size and sex in birds: meta-regression analysis. American Naturalist. 2009;174:122–133. doi: 10.1086/599299. [DOI] [PubMed] [Google Scholar]
- Kendeigh SC. Territorial and mating behavior of the house wren. Illinois Biological Monographs. 1941;18:1–120. [Google Scholar]
- Kölliker M, Heeb P, Werner I, Mateman AC, Lessells CM, Richner H. Offspring sex ratio is related to male body size in the great tit (Parus major) Behavioral Ecology. 1999;10:68–72. [Google Scholar]
- Komdeur J. Sex allocation. In: Royle NJ, Smiseth PT, Kölliker M, editors. The Evolution of Parental Care. Oxford University Press; Oxford: 2012. pp. 171–188. [Google Scholar]
- Koskela E, Mappes T, Niskanen T, Rutkowska J. Maternal investment in relation to sex ratio and offspring number in a small mammal: a case for Trivers and Willard theory? Journal of Animal Ecology. 2009;78:1007–1014. doi: 10.1111/j.1365-2656.2009.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krackow S, Neuhäuser M. Insights from complete-incomplete brood sex-ratio disparity. Behavioral Ecology and Sociobiology. 2008;62:469–477. [Google Scholar]
- Krist M. Should mothers in poor condition invest more in daughter than in son? Ethology Ecology & Evolution. 2006;18:241–246. [Google Scholar]
- Lago K, Johnson LS, Albrecht DJ. Growth of late-hatched, competitively disadvantaged nestling house wrens relative to their older, larger nestmates. Journal of Field Ornithology. 2000;71:676–685. [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, Barba E, et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithologica. 2010;45:1–26. [Google Scholar]
- Leimar O. Life-history analysis of the Trivers and Willard sex-ratio problem. Behavioral Ecology. 1996;7:316–325. [Google Scholar]
- Ležalová R, Tkadlec E, Oborník M, Śimek J, Honza M. Should males come first? The relationship between offspring hatching order and sex in the black-headed gull Larus ridibundus. Journal of Avian Biology. 2005;36:478–483. [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends in Ecology and Evolution. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- Love OP, Chin EH, Wynne-Edwards KE, Williams TD. Stress hormones: a link between maternal condition and sex-biased reproductive investment. American Naturalist. 2005;166:751–766. doi: 10.1086/497440. [DOI] [PubMed] [Google Scholar]
- Love OP, Salvante KG, Dale J, Williams TD. Sex-specific variability in the immune system across life-history stages. American Naturalist. 2008;172:E99–E112. doi: 10.1086/589521. [DOI] [PubMed] [Google Scholar]
- Martin JGA, Festa-Bianchet M. Sex ratio bias and reproductive strategies: what sex to produce when? Ecology. 2011;92:441–449. doi: 10.1890/09-2413.1. [DOI] [PubMed] [Google Scholar]
- Martin LB, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proceedings of the Royal Society of London B. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Functional Ecology. 2006;20:290–299. [Google Scholar]
- McDonald PG, Olsen PD, Cockburn A. Sex allocation and nestling survival in a dimorphic raptor: does size matter? Behavioral Ecology. 2005;16:922–930. [Google Scholar]
- Merkling T, Leclaire S, Danchin E, Lhuillier E, Wagner RH, White J, Hatch SA, Blanchard P. Food availability and offspring sex in a monogamous seabird: insights from an experimental approach. Behavioral Ecology. 2012;23:751–758. [Google Scholar]
- Monaghan P. Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison ES, Ardia DR, Clotfelter ED. Cross-fostering reveals sources of variation in innate immunity and hematocrit in nestling tree swallows Tachycineta bicolor. Journal of Avian Biology. 2009;40:573–578. [Google Scholar]
- Myers JH. Sex ratio adjustment under food stress: maximization of quality or numbers of offspring? American Naturalist. 1978;112:381–388. [Google Scholar]
- Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R. Experimental demonstration that offspring sex ratio varies with maternal condition. Proceedings of the National Academy of Sciences of the USA. 1999;96:570–573. doi: 10.1073/pnas.96.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaus M, Michler SPM, Ubels R, van der Velde M, Komdeur J, Both C, Tinbergen JM. Sex-specific effects of altered competition on nestling growth and survival: an experimental manipulation of brood size and sex ratio. Journal of Animal Ecology. 2009;78:414–426. doi: 10.1111/j.1365-2656.2008.01505.x. [DOI] [PubMed] [Google Scholar]
- Norte AC, Ramos JA, Araújo PM, Sousa JP, Sheldon BC. Health-state variables and enzymatic biomarkers as survival predictors in nestling great tits (Parus major): effects of environmental conditions. Auk. 2008;125:943–952. [Google Scholar]
- Ots I, Murumägi A, Hõrak P. Haematological health state indices of reproducing great tits: methodology and sources of natural variation. Functional Ecology. 1998;12:700–707. [Google Scholar]
- Pike TW, Petrie M. Potential mechanisms of avian sex manipulation. Biological Reviews. 2003;78:553–574. doi: 10.1017/s1464793103006146. [DOI] [PubMed] [Google Scholar]
- Poirier NE, Whittingham LA, Dunn PO. Males achieve greater reproductive success through multiple broods than through extrapair mating in house wrens. Animal Behaviour. 2004;67:1109–1116. [Google Scholar]
- Postma E, Heinrich F, Koller U, Sardell RJ, Reid JM, Arcese P, Keller LF. Disentangling the effect of genes, the environment and chance on sex ratio variation in a wild bird population. Proceedings of the Royal Society B. 2011;278:2996–3002. doi: 10.1098/rspb.2010.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryke SR, Rollins LA. Mothers adjust offspring sex to match the quality of the rearing environment. Proceedings of the Royal Society B. 2012;279:4051–4057. doi: 10.1098/rspb.2012.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryke SR, Rollins LA, Griffith SC. Context-dependent sex allocation: constraints on the expression and evolution of maternal effects. Evolution. 2011;65:2792–2799. doi: 10.1111/j.1558-5646.2011.01391.x. [DOI] [PubMed] [Google Scholar]
- Pryke SR, Rollins LA, Griffith SC, Buttemer WA. Experimental evidence that maternal corticosterone controls adaptive offspring sex ratios. Functional Ecology. 2014 doi: 10.1111/1365-2435.12232. [DOI] [Google Scholar]
- Richner H, Oppliger A, Christe P. Effect of an ectoparasite on reproduction in great tits. Journal of Animal Ecology. 1993;62:703–710. [Google Scholar]
- Robert KA, Schwanz LE, Mills HR. Offspring sex varies with maternal investment ability: empirical demonstration based on cross-fostering. Biology Letters. 2010;6:242–245. doi: 10.1098/rsbl.2009.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosivall B, Szöllösi E, Hasselquist D, Török J. Males are sensitive – sex-dependent effect of rearing conditions on nestling growth. Behavioral Ecology and Sociobiology. 2010;64:1555–1562. [Google Scholar]
- Rutkowska J, Badyaev AV. Meiotic drive and sex determination: molecular and cytological mechanisms of sex ratio adjustment in birds. Philosophical Transactions of the Royal Society B. 2008;363:1675–1686. doi: 10.1098/rstb.2007.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino N, de Ayala RM, Martinelli R, Boncoraglio G. Male-biased brood sex ratio depresses average phenotypic quality of barn swallow nestlings under experimentally harsh conditions. Oecologia. 2008;156:441–453. doi: 10.1007/s00442-008-0971-8. [DOI] [PubMed] [Google Scholar]
- Santangeli A, Hakkarainen H, Laaksonen T, Korpimäki E. Home range size is determined by habitat composition but feeding rate by food availability in male Tengmalm’s owls. Animal Behaviour. 2012;83:1115–1123. [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution. 2010;1:103–113. [Google Scholar]
- Sheldon BC. Recent studies of avian sex ratios. Heredity. 1998;80:397–402. [Google Scholar]
- Sheldon BC, West SA. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. American Naturalist. 2004;163:40–54. doi: 10.1086/381003. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, Merilä J, Lindgren G, Ellegren H. Gender and environmental sensitivity in nestling collared flycatchers. Ecology. 1998;79:1939–1948. [Google Scholar]
- Sockman KW, Weiss J, Webster MS, Talbott V, Schwabl H. Sex-specific effects of yolk-androgens on growth of nestling American kestrels. Behavioral Ecology and Sociobiology. 2008;62:617–625. [Google Scholar]
- Svensson E, Nilsson J-Å. Mate quality affects offspring sex ratio in blue tits. Proceedings of the Royal Society of London B. 1996;263:357–361. [Google Scholar]
- Thompson CF, Sakaluk SK, Masters BS, Johnson BGP, Forsman AM, Johnson LS. Condition-dependent sex difference in nestling house wren (Troglodytes aedon) response to phytohaemagglutinin injection. Canadian Journal of Zoology. 2014;92:1–7. [Google Scholar]
- Tilgar V, Mänd R, Kilgas P, Mägi M. Long-term consequences of early ontogeny in free-living great tits Parus major. Journal of Ornithology. 2010;151:61–68. [Google Scholar]
- Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Tschirren B, Fitze PS, Richner H. Sexual dimorphism in susceptibility to parasites and cell-mediated immunity in great tit nestlings. Journal of Animal Ecology. 2003;72:839–845. [Google Scholar]
- Uller T. Sex-specific sibling interactions and offspring fitness in vertebrates: patterns and implications for maternal sex ratios. Biological Reviews. 2006;81:207–217. doi: 10.1017/S1464793105006962. [DOI] [PubMed] [Google Scholar]
- Velando A. Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the blue-footed booby. Behavioral Ecology. 2002;13:443–449. [Google Scholar]
- Vinkler M, Svobodová J, Gabrielová B, Bainová H, Bryjová A. Cytokine expression in phytohaemagglutinin-induced skin inflammation in a galliform bird. Journal of Avian Biology. 2014;45:43–50. [Google Scholar]
- Weatherhead PJ, Teather KL. Are skewed fledgling sex ratios in sexually dimorphic birds adaptive? American Naturalist. 1991;138:1159–1172. [Google Scholar]
- West SA. Sex Allocation. Princeton University Press; Princeton, New Jersey, USA: 2009. [Google Scholar]
- West SA, Sheldon BC. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- Westerdahl H, Bensch S, Hansson B, Hasselquist D, von Schantz T. Sex ratio variation among broods of great reed warblers Acrocephalus arundinaceus. Molecular Ecology. 1997;6:543–548. doi: 10.1046/j.1365-294x.2000.01028.x. [DOI] [PubMed] [Google Scholar]
- Wilkin TA, Sheldon BC. Sex differences in the persistence of natal environmental effects on life histories. Current Biology. 2009;19:1998–2002. doi: 10.1016/j.cub.2009.09.065. [DOI] [PubMed] [Google Scholar]
- Williams GC. The question of adaptive sex ratio in outcrossed vertebrates. Proceedings of the Royal Society of London B. 1979;205:567–580. doi: 10.1098/rspb.1979.0085. [DOI] [PubMed] [Google Scholar]
- Williams TD. Physiological Adaptations for Breeding in Birds. Princeton University Press; Princeton, New Jersey, USA: 2012. [Google Scholar]