Abstract
Purpose
Attention Deficit Hyperactivity Disorder (ADHD) is a common childhood neurobehavioral disorder characterized by inattention, poor impulse control, and motor restlessness. Risk factors include familial stressors, anxiety disorders, learning disabilities, heritability, abnormal brain development, and dopamine polymorphisms. Children with an orofacial cleft (OFC) history are more likely to experience familial stressors, anxiety disorders, learning disabilities, and abnormal brain development. Given the overlap between OFC and ADHD, a conceptual model is presented proposing that OFC children may be more likely to exhibit ADHD symptoms. Using pilot data we explored possibilities of these relationships.
Design
This cross-sectional pilot study included 29 children with OFC or a first-degree relative with OFC recruited through a cleft research registry.
Methods
A parental self-report Disruptive Behavior Disorder Scale (DBD) collected data on children’s ADHD symptoms. Saliva or whole blood samples were collected from children and parents for DNA analyses. Three ADHD-associated dopamine polymorphisms within DRD4, DRD2, and DAT 1 genes were genotyped. Tests for associations between presence of OFC and dopamine polymorphisms were conducted. Mixed effects models tested whether OFC children with dopamine polymorphisms had more ADHD symptoms.
Results
The DRD4 4-repeat allele was associated with increased inattentive ADHD symptoms (p=.03). Having the DRD2 Taq1A1 allele and OFC indicated fewer (p=.02) inattentive ADHD symptoms. In addition, OFC children were significantly less likely to have the DAT1 10-repeat allele (p=.04).
Conclusions
This pilot study indicates further investigation of dopamine genes and ADHD among OFC children in a larger sample is warranted, particularly for relationships with inattentive ADHD.
Keywords: dopamine polymorphism, nonsyndromic orofacial cleft, attention deficit hyperactivity disorder
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) (Kliegman, 2011) and Nonsyndromic Orofacial Clefting (OFC), primarily cleft lip and cleft palate without the presence of any other abnormalities (Wyszynski, 2002), are among the most common disorders of childhood (Kliegman, 2011; Wyszynski, 2002). Although very different disorders, given that ADHD is neurobehavioral and OFC is a congenital malformation, they have many similar psycho-social implications. Both disorders have also been associated with various genetic polymorphisms (Faraone & Mick, 2010; Nussbaum, McInnes, & Willard, 2007) and often, a child with OFC is diagnosed with ADHD (Richman, Ryan, Wilgenbusch, & Millard, 2004). However, the literature is lacking studies that examine a genetic relationship between ADHD and OFC.
Background
Attention deficit hyperactivity disorder (ADHD) is a neurobehavioral disorder that is characterized by inattention, poor impulse control, and motor restlessness (Kliegman, 2011). It is the most common childhood neurobehavioral disorder, affecting three to five percent of school-aged children (Kliegman, 2011) and can be classified as inattentive type, predominantly hyperactive-impulsive type, and combined type (Association, 2013). ADHD often persists into adulthood creating the potential for educational underachievement, employment difficulties, relationship difficulties, and substance abuse. Given its prevalence and lasting impact, it has become the most widely studied mental disorder among children. Extensive research efforts, have suggested that ADHD is the result of multiple environmental, developmental, and hereditable factors (Kliegman, 2011).
Developmentally, ADHD children are often missing normal asymmetry in the brain and have a five to ten percent reduction in prefrontal cortex and basal ganglia brain structures. These brain structures are responsible for housing an abundance of dopamine receptors (Kliegman, 2011). As such, several genes involved with dopamine signals and reuptake have been associated with ADHD (Waldman & Gizer, 2006). These genes include but are not limited to DRD4, DRD2, and DAT1. Research suggests that ADHD is associated with the DRD4 4 repeat allele—in which there is a randomly repeated DNA sequence of the 4 allele (Bidwell, et al., 2011; Fossella, et al., 2002; Smith, et al., 2003). It also provides evidence of an ADHD association with a presence of the DRD2 Taq1 A1 allele (Comings, et al., 1991; Drtilkova, et al., 2008; Kopeckova, et al., 2008; Sery, et al., 2006; Smith, et al., 2003) as well as the randomly repeated DNA sequence of the DAT1 10 repeat allele (Bellgrove, Hawi, Kirley, Gill, & Robertson, 2005; Bidwell, et al., 2011; Cook, et al., 1995; Kopeckova, et al., 2008; Waldman, et al., 1998).
In comparison to ADHD, nonsyndromic OFC is among the most common congenital malformations and affects approximately 1 infant per 500 to 1,000 births worldwide (Wyszynski, 2002). Embryological development of OFC is derived from neural crest cells controlled by signaling from the brain. As such, a malformation of the brain creates disrupted signals resulting in the OFC defect (Sperber, Sperber, & Guttmann, 2010). Children with OFC are at risk for a variety of medical, cognitive (Wyszynski, 2002), and psychosocial complications (Collett & Speltz, 2006) and are 30% to 40% more likely to experience familial stressors, learning disabilities, and anxiety disorders (Wyszynski, 2002). Additionally, research evidence suggests that OFC children have abnormally small brains with a substantial decrease in the frontal lobe (Nopoulos, Langbehn, Canady, Magnotta, & Richman, 2007). Further investigation also indicates that OFC children are often over diagnosed with ADHD (Richman, et al., 2004).
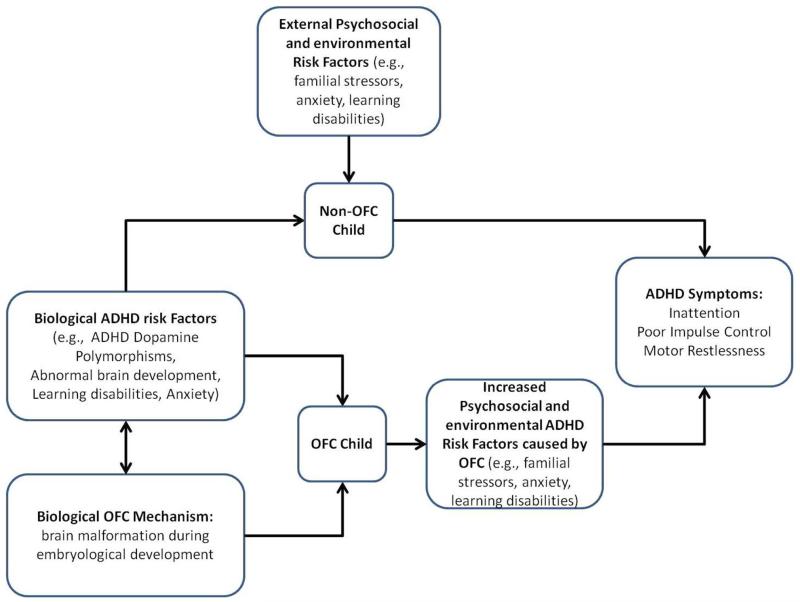
Given the overlap between OFC and ADHD, we propose a conceptual model (Figure 1) which hypothesizes that: 1) OFC children would be more likely to have an ADHD associated dopamine polymorphism, and 2) having both OFC and a dopamine polymorphism was associated with either a greater likelihood of ADHD diagnosis or increased ADHD symptoms. We then use pilot data to explore these proposed relationships and to determine whether there is enough evidence to warrant further research and subject recruitment in this area.
Figure 1.
OFC and ADHD conceptual model. This model demonstrates the overlap between OFC and ADHD suggesting a potential relationship.
Materials and Methods
Subjects
With Institutional Review Board approval, existing DNA samples for genotype data collection and existing phenotype data from the study “Extending the Phenotype of Nonsyndromic Orofacial Clefts” (RO1DE016148) were used. The premise of the parent study was to determine if non-clefted members of cleft families were more likely to have cleft-related phenotypes than controls. The parent study utilized a descriptive cross sectional design in which participants were seen only once for a variety of phenotypic assessments including ADHD as well as providing a whole blood or saliva sample for DNA extraction. Given the invasive nature and discomfort associated with a blood draw, saliva sampling was used whenever possible since DNA from saliva sampling is of the same quality as DNA obtained from blood. Participants were recruited through a research registry at a University affiliated Cleft Craniofacial Center and included the affected OFC child, their parents, and their siblings.
The subject population was 100% Caucasian and consisted of 29 children from 16 families. Ages of the children ranged from 2 to 12 years, with a mean age of 6 years. Of the 29 children there were 14 males and 15 females. There were 8 OFC males and 12 OFC females. A total of 9 children had no cleft.
Disruptive Behavior Disorder Scale
The DBD is a parental-report questionnaire used to assess behaviors associated with ADHD and diagnose ADHD as inattentive, predominately hyperactive-impulse, and combined type (Barkley & Murphy, 2005). Parents rate ADHD symptoms as never true (0), sometimes true (1), often true (2), and always true (3).
Inattentive symptoms included inability to pay attention to details or makes careless mistakes in work or other activities; difficulty with sustained attention in tasks or play activities; does not seem to listen when spoken to directly; does not follow through on instructions and fails to finish work or chores; difficulty organizing tasks and activities; avoids or dislikes tasks that require sustained mental effort; often loses things necessary for tasks or activities; easily distracted by unimportant things; and forgetful in daily activities. Predominately hyperactive-impulsive type symptoms included: often fidgets with hands or feet or squirms in seat; difficulty remaining seated in classroom or other situations in which it is expected; runs or climbs excessively in situations in which it is inappropriate; difficulty playing or engaging in leisure activities quietly; always “on the go” or acts as if “driven by a motor”; talks excessively; blurts out answers before questions have been completed; difficulty waiting for and taking turns; and often interrupts or intrudes on others such as butting into conversations or games.
Since some evidence suggests that ADHD may be diagnosed more appropriately on a continuum of behavior (Bidwell, et al., 2011), the DBD was considered as both a categorical and dimensional measure of ADHD subtypes. For the categorical subtype measures, presence of at least 6 inattention symptoms were required for the inattention diagnosis, presence of at least 6 predominately hyperactive-impulsive symptoms were required for the hyperactive-impulsive diagnosis, and presence of at least 6 of both symptom subtypes were required for the combined type. In order, for a symptom to be considered “present” it had to be rated as “often true” or “always true”. For the continuous measures of each subtype, the numerical symptom values in each subscale were added together.
Genetic Analysis
Polymerase chain reaction (PCR) and size discriminating 2% agarose gel electrophoresis were utilized for the DRD4 VNTR and DAT1 VNTR genotyping. PCR primers and annealing conditions for DRD4 were 5′-CTT CCT ACC CTG CCC GCT CAT GCT GCT GCT CTA CTG G-3′ for the forward and 5′-ACC ACC ACC GGC AGG ACC CTC ATG GCC TTG CGC TC-3′ for the reverse primers with an annealing temperature of 70°C. PCR primers and annealing conditions for DAT1 were 5′-TGT GGT GTA GGG AAC GGC CTG AG-3′ for the forward and 5′-CTT CCT GGA GGT CAC GGC TCA AGG-3′ for the reverse primers with an annealing temperature of 59°C (Cheuk, Li, & Wong, 2006; Georgieva, et al., 2002). DRD2 was genotyped for the Taq A1 RFLP using PCR-RFLP and size discriminating 1% agarose gel electrophoresis. PCR primers and annealing conditions for DRD2 were 5′-CCG TCG ACC CTT CCT GAG TGT CAT CA-3′ for the forward and 5′-CCG TCG ACG GCT GGC CAA GTT GTC TA-3′ for the reverse primers with an annealing temperature of 65°C and digestion of the PCR product with TaqA1 prior to electrophoresis (Grandy, Zhang, & Civelli, 1993). All genotypes were double called by two individuals blind to the phenotype of the subjects. The raw data was evaluated for disparate genotyping calls and those that were unable to be resolved were re-genotyped.
Statistical Analysis
SAS version 9.3 was used for statistical analysis. Fisher’s exact testing was used to explore if OFC children were more likely to have an ADHD associated dopamine polymorphism than non-OFC children (Table 1). In order to analyze whether having both a dopamine polymorphism and an OFC was associated with an increased risk for ADHD, we first tested whether any participants had categorically defined inattentive and/or predominately hyperactive ADHD—none did so we did not use this measure in further analyses. Instead, we used linear mixed effects models that accounted for correlation of participants within each family to determine whether having both a gene polymorphism and an OFC was associated with either the inattentive or predominately hyperactive-impulsive ADHD continuous subtype measure. Although testing whether the interaction of OFC and the dopamine polymorphism was our primary aim, if this interaction was not significant we also tested whether the main effects of OFC and the dopamine polymorphisms were associated with the ADHD continuous subtype measures. If significant associations were identified, we further broke down the continuous measure and explored associations for each of the individual symptoms within the subtype measure. All models controlled for age and gender, and accounted for familial relationship (Table 2).
Table 1. Genotype Frequencies and Percents with Affected Status.
| Polymorphism Genotype |
Total Sample % (n) |
Affected Sample % (n) |
Unaffected Sample % (n) |
|---|---|---|---|
| DRD4 (N=25) | |||
| 4/4 (vs. X/X, 4/X or X/4) | 72.00 (18) | 77.78 (14) | 57.14 (4) |
| DRD2 (N=24) | |||
| A1/A1, A1/A2, or A2/A1 (vs. A2/A2) |
45.83 (11) | 50 (8) | 37.50 (3) |
| DAT 1 (N=25) a | |||
| 10/10 (vs. 9/9, 9/10 or 10/9) |
44.00 (11) | 29.41 (5) | 75.00 (6) |
Note. X = 2 or 3
Affected children are significantly less likely to have the 10/10 genetic polymorphism as compared to unaffected children (Fisher’s exact test; p=.04).
Table 2. Multivariable Mixed effects models of ADHD as outcome using family as cluster, controlling for age and gender.
| B | SE | p-value | |||
|---|---|---|---|---|---|
|
Inattentive ADHD Subscale
| |||||
| Model with DAT1 | |||||
| Affected | −2.0768 | 1.8960 | .315 | ||
| DAT1 | −1.4805 | 2.1324 | .514 | ||
| DAT1*Affected | 3.7959 | 2.2350 | .140 | ||
| Model with DRD2 | |||||
| Affected | 3.25 | .83 | .01 | ||
| DRD2 | 3.61 | .80 | .01 | ||
| Affected*DRD2 | −5.93 | 1.84 | .02 | ||
| Model with DRD4 | |||||
| Affected | 3.0281 | 1.1197 | .035 | ||
| DRD4 | 2.8044 | 0.9913 | .03 | ||
| Affected*DRD4 | −2.6714 | 1.6059 | .15 | ||
|
| |||||
|
Hyper Impulsive ADHD Subscale
| |||||
| Model with DAT1 | |||||
| Affected | −3.3063 | 3.4810 | .38 | ||
| DAT1 | −5.0858 | 3.4997 | .20 | ||
| Affected*DAT1 | 4.3100 | 4.1331 | .34 | ||
| Model with DRD2 | |||||
| Affected | 0.2511 | 1.9092 | .90 | ||
| DRD2 | 1.7111 | 2.1140 | .46 | ||
| Affected*DRD2 | −1.1206 | 3.1418 | .74 | ||
| Model with DRD4 | |||||
| Affected | 4.3971 | 1.7721 | .05 | ||
| DRD4 | 1.5409 | 1.5795 | .37 | ||
| Affected*DRD4 | −4.0594 | 2.5194 | .68 | ||
Results
DRD4 Results
The DRD4 4 repeat allele was present in 72% of the sample including 14 children with OFC and 4 without. No significant relationship was found between having an OFC and having the DRD4 4-repeat allele (Fisher’s exact test, p=.36). Mixed-effects model results indicated that having both OFC and the DRD4 4-repeat allele was not associated with either inattentive or predominately hyperactive-impulsive ADHD. However, having the DRD4 4-repeat allele was shown to be associated with having a higher inattentive ADHD score after controlling for age, gender, and OFC status (β=1.47, p=.03). In particular, the DRD4 4-repeat allele was associated with higher scores on the “avoids or dislikes tasks that require sustained mental effort” (β=.41, p=.03) and “difficulty organizing tasks and activities” (β=.32, p=.006) inattentive ADHD symptoms after controlling for OFC status, age, and gender.
DRD2 Results
DRD2 genotyping indicated the presence of the Taq1A1 allele in 46% of the sample including 8 children with OFC and 3 without. No significant relationship was found between having an OFC and having the DRD2 Taq1A1 allele (Fisher’s exact test, p=.68). Mixed-effects model findings for DRD2 suggested that having an OFC with the presence ofTaq1A1 allele is significantly associated with having fewer inattentive ADHD symptoms (ß= −5.93, SE=1.84 p = .02). Further analyses indicated no relationship with having an OFC and a Taq1A1 allele and any individual inattentive ADHD symptoms. No increased risk was found for predominately hyperactive-impulsive ADHD among OFC children with the presence of a Taq1A1allele.
DAT 1 Results
DAT 1 genotyping showed the presence of the 10-repeat allele in 44% of the sample including 5 children with OFC and 6 without. Results also indicated that OFC children were significantly less likely to have the 10-repeat allele (Fisher’s exact test; p=.04). Furthermore, mixed-effects modeling indicated that having both OFC and the DAT1 10-repeate allele was not associated with inattentive or predominately hyperactive ADHD. Neither the OFC main effect nor the DAT1 10-repeat allele main effect was significantly associated with the DBD scale.
Discussion
Previous research has suggested an association with the DRD4 4- repeat allele and ADHD (Bidwell, et al., 2011; Fossella, et al., 2002; Smith, et al., 2003). This association was also observed in our sample though it was based on a continuum of ADHD symptomatology rather than traditional categorical DBD diagnosis confirmation. Although participants in this investigation did not have a categorical diagnosis of ADHD, results indicated a significant association between the 4-repeat allele and the inattentive ADHD subtype particularly with the symptoms of “avoids or dislikes tasks that require sustained mental effort” and “difficulty organizing tasks and activities”. These findings are similar to Bidwell et al (2011), who also found an association between the 4-repeat allele and increased ADHD inattentive symptoms. We did not observe any association between having the DRD4 4-repeat allele and OFC. As such, children with both an OFC and the DRD4 4-repeat allele were not more likely to have an increased or a decreased risk of ADHD.
The findings which indicate that having an OFC and the presence of a DRD2 Taq1A1 could be protective of the Inattentive ADHD based on a continuum of symptomsare novel. However, they are contradictory to previous investigations that found a relationship between Taq1A1 and ADHD (Comings, et al., 1991; Drtilkova, et al., 2008; Kopeckova, et al., 2008; Sery, et al., 2006; Smith, et al., 2003). Since previous investigations did not focus on the OFC populationthese results may indicate a potential gene-gene interaction between genes associated with clefting and the having Taq1A1 allele creating a decreased risk.
Finally, the results indicating that OFC children are significantly less likely to have a DAT1 10-repeat allele are novel. Although the DAT1 findings suggest there may be a link between OFC and the 10-repeat allele, they do not support results from previous investigations suggesting an association between the 10-repeat allele and ADHD in general (Bellgrove, Hawi, Kirley, Gill, & Robertson, 2005; Bidwell, et al., 2011; Cook, et al., 1995; Kopeckova, et al., 2008; Waldman, et al., 1998). The decreased incidence of an OFC child having the DAT1 10-repeat allele suggests that OFC polymorphisms occurring with this dopamine polymorphism is unlikely. Again this may suggest a gene-gene interaction in that the DAT1 10-repeat allele is protective of OFC development. Or rather, that these two polymorphisms do not commonly occur together among those families with clefting. Failure to have an association between the 10-repeat allele and ADHD may be attributed to sample size as well as the sample population since this polymorphism was found significantly less among the OFC children. As such, these findings may change with a larger sample size.
Implications
To date there are no known published studies investigating ADHD dopamine polymorphisms among the OFC population. This pilot study served as a key, ground breaking step (Leon, Davis, & Kraemer, 2011) in the exploration of potential ADHD and OFC genetic relationships. As is often the case with pilot studies, ours was limited due to the sample size (Leon, Davis, & Kraemer) and absence of categorical ADHD diagnoses, which prompted analyses of ADHD types based on a continuum that is not traditionally done with the DBD. Given that our study was underpowered to find the types of interactions we were hypothesizing, the lack of significant findings suggests only that these results are inconclusive thus far. Hence, further investigation with a larger OFC population and presence of a categorical ADHD diagnosis or use of a continual ADHD measure is needed to continue exploring the relationship of DRD4, DRD2, and DAT1 on the risk of ADHD---inattentive ADHD and its’ symptomatology in particular. The OFC population is already at risk for medical, cognitive, and psychosocial complications. Determining the genetic association of ADHD dopamine polymorphisms among the OFC population may assist in accurate ADHD diagnoses. Earlier recognition, diagnosis, and intervention for ADHD may improve developmental outcomes. Consequently, prevention of ADHD complications such as risk taking behaviors, educational underachievement or employment difficulties, and relationship difficulties may improve quality of life.
Supplementary Material
Acknowledgments
This research was funded by T32 NR009759 “Targeted Research and Academic Training Program for Nurses in Genomics”, RO1DE016148 “Extending the Phenotype of Nonsyndromic Orofacial Clefts”, and by K01 MH096944 “Statistical Methods for Developing RDoC-Based Multidimensional Profiles”.
Contributor Information
Emily E. Stevens, College of Continuing and Professional Studies Chatham University, 222 Coolidge Hall, Woodland Road, Pittsburgh, PA 15232.
Meredith L. Wallace, Department of Psychiatry, School of Medicine, University of Pittsburgh, mel20@pitt.edu.
Yvette P. Conley, Department of Health Promotion and Development, School of Nursing; Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, yconley@pitt.edu.
Mary L. Marazita, Department of Oral Biology, and Director, Center for Craniofacial and Dental Genetics ; School of Dental Medicine; Professor, Department of Human Genetics, Graduate School of Public Health; Professor, Psychiatry, and Clinical and Translational Science, School of Medicine, University of Pittsburgh, marazita@pitt.edu.
References
- Association AP, editor. Diagnostic and Statistical Manual of Mental Disorders. 54 ed. American Psychological Association; Washington, DC: 2013. [Google Scholar]
- Barkley RA, Murphy KR. Attention-deficit hyperactivity disorder: a clinical workbook. 3rd ed. Guildford Press; New York: 2005. [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43(13):1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Willcutt EG, McQueen MB, DeFries JC, Olson RK, Smith SD, et al. A family based association study of DRD4, DAT1, and 5HTT and continuous traits of attention-deficit hyperactivity disorder. Behavioral Genetics. 2011;41:165–174. doi: 10.1007/s10519-010-9437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk DKL, Li SYH, Wong V. Exon 3 polymorphisms of dopamine D4 receptor (DRD4) gene and attention deficit hyperactivity disorder in chinese children. American Journal of Medical Genetics Part B. 2006;141B:907–911. doi: 10.1002/ajmg.b.30397. [DOI] [PubMed] [Google Scholar]
- Collett BR, Speltz ML. Social-emotional development of infants and young children with orofacial clefts. Infants and Young Children. 2006;19(4):262–291. [Google Scholar]
- Comings DE, Comings BG, Muhleman D, Dietz G, Shahbahrami B, Tast D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266(13):1793–1800. [PubMed] [Google Scholar]
- Cook EH, Jr., Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56(4):993–998. [PMC free article] [PubMed] [Google Scholar]
- Drtilkova I, Sery O, Theiner P, Uhrova A, Zackova M, Balastikova B, et al. Clinical and molecular-genetic markers of ADHD in children. Neuro Endocrinol Lett. 2008;29(3):320–327. [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33(1):159–180. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Yanhong W, Swanson JM, Praff DW, et al. Assessing the molecular genetics of attention networks. BMC Neuroscience. 2002;4(14):1–11. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva L, Dimitrova A, Nikolov I, Koleva S, Tsvetkova R, Owen MF, et al. Dopamine transporter gene (DAT1) VNTR, polymorphism in major psychiatric disorders: family-based association study in the Bulgarian population. Acta Psychiatry Scandinavia. 2002;105:396–399. doi: 10.1034/j.1600-0447.2002.1o174.x. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Zhang Y, Civelli O. PCR detection of the taqA RFLP at the DRD2 locus. Human Molecular Genetics. 1993;2(12):2197. doi: 10.1093/hmg/2.12.2197-a. [DOI] [PubMed] [Google Scholar]
- Kliegman RM, editor. Nelson Textbook of Pediatrics. 19 ed. Saunders, An Imprint of Elsevier; Philadelphia, PA: 2011. [Google Scholar]
- Kopeckova M, Paclt I, Petrasek J, Pacltova D, Malikova M, Zagatova V. Some ADHD polymorphisms (in genes DAT1, DRD2, DRD3, DBh, 5-HTT) in case-control study of 100 subject 6-10age. Neuro Endocrinol Lett. 2008;29(2):246–251. [PubMed] [Google Scholar]
- Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–629. doi: 10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Langbehn DR, Canady J, Magnotta V, Richman L. Abnormal brain structure in children with isolated clefts of the lip of palate. Archives of Pediatric and Adolescent Medicine. 2007;161(8):753–758. doi: 10.1001/archpedi.161.8.753. [DOI] [PubMed] [Google Scholar]
- Nussbaum RL, McInnes RR, Willard HF, editors. Thompson & Thompson Genetics in Medicine. 7th ed. Saunders; Philadelphia: 2007. [Google Scholar]
- Richman LC, Ryan S, Wilgenbusch T, Millard T. Overdiagnosis and medication for attention-deficit hyperactivity disorder in children with cleft: diagnostic examination and follow-up. Cleft Palate Craniofac J. 2004;41(4):351–354. doi: 10.1597/03-047.1. [DOI] [PubMed] [Google Scholar]
- Sery O, Drtilkova I, Theiner P, Pitelova R, Staif R, Znojil V, et al. Polymorphism of DRD2 gene and ADHD. Neuro Endocrinol Lett. 2006;27(1-2):236–240. [PubMed] [Google Scholar]
- Smith KM, Daly M, Fischer M, Yiannoutsos CT, Bauer L, Barkley R, et al. Association of the dopamine beta hydroxylase gene with attention deficit hyperactivity disorder: genetic analysis of the Milwaukee longitudinal study. Am J Med Genet B Neuropsychiatr Genet. 2003;119B(1):77–85. doi: 10.1002/ajmg.b.20005. [DOI] [PubMed] [Google Scholar]
- Sperber G, Sperber SM, Guttmann GD, editors. Craniofacial embryogenetics and development. 2nd ed. PMPH USA, Ltd.; Shelton, CT: 2010. [Google Scholar]
- Waldman ID, Gizer IR. The genetics of attention deficit hyperactivity disorder. Clin Psychol Rev. 2006;26(4):396–432. doi: 10.1016/j.cpr.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, et al. Association and linkage of the dopamine transporter gene and attention deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet. 1998;63:1767–1776. doi: 10.1086/302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski DF, editor. Cleft Lip and Palate From Origin to Treatment. Oxford University Press, Inc.; New York, NY: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.