Abstract
The Polycomb group protein Bmi-1 is an essential regulator of cellular senescence and is believed to function largely through the direct repression of the Ink4a/Arf locus. However, concurrent deletion of Ink4a/Arf does not fully rescue the defects detected in Bmi-1−/− mice, indicating that additional Bmi-1 targets remain to be identified. The expression of the chromatin associated Sin3B protein is stimulated by oncogenic stress, and is required for oncogene-induced senescence. Here we demonstrate that oncogenic stress leads to the dissociation of Bmi-1 from the Sin3B locus, resulting in increased Sin3B expression and subsequent entry into cellular senescence. Furthermore, Sin3B is required for the senescent phenotype and elevated levels of reactive oxygen species elicited upon Bmi-1 depletion. Altogether, these results identify Sin3B as a novel direct target of Bmi-1, and establish Bmi-1-driven repression of Sin3B as an essential regulator of cellular senescence.
Keywords: Senescence, Transcription, Polycomb: Bmi1, Chromatin, Sin3
Introduction
Cellular senescence, an irreversible cell cycle arrest triggered by stress, is believed to prevent damaged or mutated cells from proliferating uncontrollably (1, 2). Several studies have shown that the expression of senescence markers is associated with low proliferation in a wide variety of cancer preneoplastic lesions including lung adenoma, melanocytic naevi and PanIN (3-5). For these reasons, cellular senescence has been long considered a tumor suppressor mechanism (1, 6, 7). This hypothesis was further supported by the demonstration that genetic inactivation of proteins involved in the establishment of senescence promotes tumorigenesis in vivo (8, 9). While the cellular factors that contribute to cellular senescence in response to oncogene activation remain for the most part unknown, numerous chromatin modifiers and chromatin-associated proteins have been shown to directly participate in the establishment and maintenance of the senescent phenotype (10).
Polycomb group (PcG) proteins are evolutionary conserved proteins that regulate cell fate decisions, and mammalian PcG proteins are components of complexes termed Polycomb repressive complexes (PRC1 and PRC2) (11, 12). PRC1 and PRC2 are targeted to specific loci and repress transcription through specific histone covalent modifications and chromatin compaction (13). Bmi-1, a PRC1 component, was initially identified as a c-myc cooperating oncogene in the induction of B-cell lymphoma (14, 15). Mice genetically inactivated for Bmi-1 exhibit numerous defects including growth retardation, hematological abnormalities, neurologic deficiencies, and premature death (16-18). At the cellular level, mouse embryonic fibroblasts (MEFs) deficient for Bmi-1 exhibit impaired S-phase progression and premature cellular senescence correlating with the accumulation of the products of the Ink4a/Arf locus, p16Ink4a and p19Arf (19). The cellular senescence phenotype elicited upon Bmi-1 depletion can be rescued by simultaneous inactivation of Ink4a/Arf in the mouse (19). Later studies established that in proliferating cells, Bmi-1 binds throughout the Ink4a/Arf locus, directly repressing the expression of p16Ink4a and p19Arf (20).
Bmi-1−/− mice exhibit significant defects in various hematopoietic compartments including differentiation blocks in both T- and B-cell lineages (18, 19, 21, 22). Concurrent deletion of Bmi-1 and Ink4a/Arf partially restores some of the hematopoietic abnormalities elicited in Bmi-1−/−mice. However, Bmi-1−/− Ink4a/Arf−/− mice still display numerous defects, including reduced numbers of nucleated thymocytes and splenocytes and a hypocellular bone marrow (23, 24). These observations suggested that Bmi-1 has Ink4a/Arf-independent functions, and therefore additional targets to be identified. Indeed, recent work has demonstrated that Bmi-1 regulates lymphoid lineage commitment through the Ink4a/Arf-independent repression of specific transcription factors (22). Additionally, loss of Bmi-1 leads to the detrimental accumulation of reactive oxygen species (ROS) levels in hematopoietic cells, regardless of the Ink4a/Arf status (25). However, the molecular mechanism underlying the accumulation of ROS observed in Bmi-1−/− hematopoietic cells remains to be identified.
We have recently uncovered a central role for the histone deacetylase (HDAC)-associated Sin3B protein in cell cycle withdrawal. While Sin3B is dispensable for cell survival and proliferation in primary fibroblasts, it is required for entry into quiescence upon serum withdrawal, and for entry into cellular senescence triggered by serial passaging or oncogenic activation (26, 27). Moreover, in cells rendered senescent in culture and in preneoplastic senescent lesions, the expression of Sin3B is transcriptionally upregulated (27). The mechanism underlying this regulation of Sin3B is unknown. Here we identify Sin3B as a target of Bmi-1, and demonstrate that Sin3B is essential for the cellular phenotypes elicited upon Bmi-1 inactivation.
Results and Discussion
Oncogenic activation of the Ras/BRaf pathway leads to Sin3B upregulation and cellular senescence
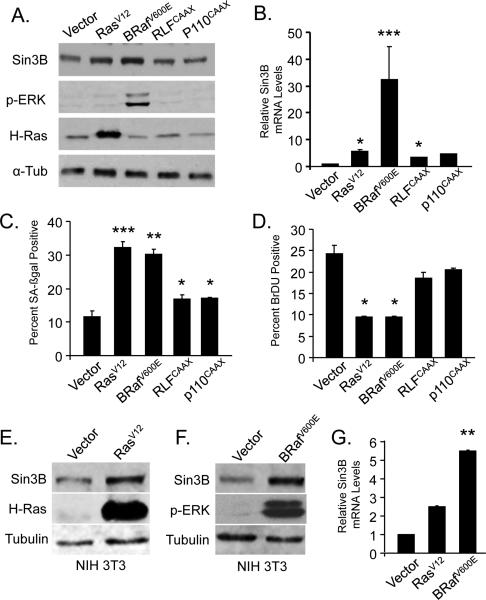
We previously demonstrated that Sin3B transcript and protein levels are upregulated in primary cells expressing an oncogenic form of H-Ras (RasV12) (27). To further delineate the downstream signaling pathway linking oncogenic Ras to Sin3B upregulation, activation of the three main Ras effector pathways was achieved in primary cells by retroviral infection (BrafV600E for the Raf pathway, RLFcaax for the Ral pathway, p110caax for the PI3K pathway). Ectopic expression of activated BRaf (BRafV600E) was sufficient to recapitulate the increased expression of Sin3B expression, both at the protein (Figure 1A) and the transcript level (Figure 1B). By contrast, expression of RLFcaax or p110caax only marginally increased Sin3B expression. Of note, expression of oncogenic BRaf resulted in a significantly higher activation of Sin3B expression compared to that observed following oncogenic Ras expression, consistent with the recent demonstration that activation of the PI3K pathway antagonizes Ras signaling as it relates to senescence (28). Importantly, activation of the MAPK pathway, but not activation of the RAL or the PI3K pathways, resulted in the initiation of the cellular senescence program, as demonstrated by increased SA-βGal positivity and decreased BrDU incorporation (Figure 1C and 1D), indicating that increased expression of Sin3B correlates with the activation of an oncogene-induced senescence program. However, ectopic expression of oncogenic H-Ras or BRaf in immortalized MEFs that are unable to undergo senescence, also led to Sin3B upregulation (Figure 1E, 1F and 1G), suggesting that Sin3B levels are modulated by oncogenic signals rather than cellular senescence per se.
Figure 1. Oncogenic activation of the Ras-Raf pathway promotes Sin3B upregualtion and cellular senescence.
(A) Western blot on whole cell extracts from primary wild-type MEFs infected with empty retrovirus (Vector) or retroviruses expressing H-RasV12, BRafV600E, RLFcaax or p110caax using the indicated antibodies. Antibodies used: Sin3B (Santa Cruz Biotechnology AK12), phosphorylated ERK (cell signaling 4377), H-Ras (Santa Cruz sc-520), alpha tubulin (Sigma T9026). Retroviral infections were preformed as previously described (27) (B) Relative Sin3B expression in wild-type MEFs infected with the indicated retroviruses as determined by qRT PCR (n=2) and reported to Beta-2-microglobulin (B2M). *p<0.5, ***p<0.001 as determined by a student's t test. Data represent mean ± SEM. (C) Quantification of the proportion of SA-ßgal positive cells in MEFs infected with the indicated retroviruses (n=2); at least 200 cells were counted per point. SA-ßGal staining was performed as previously described (27). *p<0.5, **p<0.01, ***p<0.001 as determined by a student's t test. Data represent mean ± SEM. (D) Quantification of the proportion of BrDU positive cells in MEFs infected with the indicated retroviruses (n=2); at least 200 cells were counted per point. BrdU staining was preformed as previously described (27). *p<0.05 as determined by a student's t test. Data represent mean ± SEM. (E) Western blot on whole cell extracts from NIH 3T3 cells infected with vector or RasV12 using the indicated antibodies. (F) Western blot on whole cell extracts from NIH 3T3 cells infected with vector or BRAFV600E using the indicated antibodies. (G) Relative Sin3B expression in NIH 3T3 cells infected with the indicated constructs as determined by qRT PCR (n=2) and reported to B2M. **p<0.01 as determined by a student's t test. Data represent mean ± SEM. (A to G) Cells were harvested 6 days following selection. MEFs were established as in ref. 27, cultured in 6% O2, and infected no later than passage 2.
The PRC1 component Bmi-1 represses Sin3B expression in proliferating cells
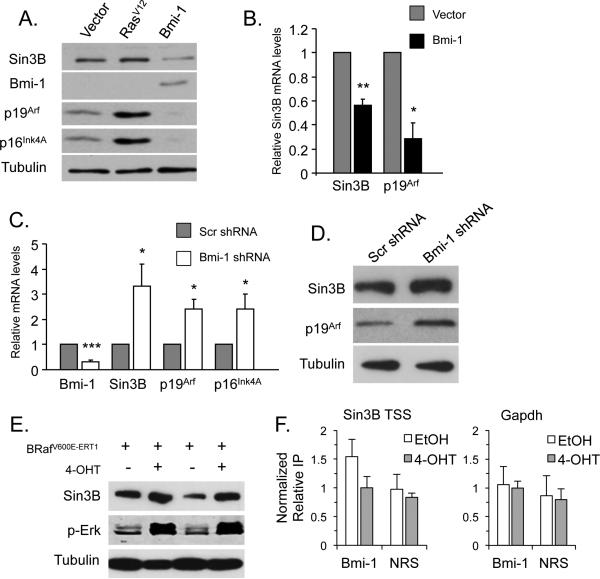
The results presented above indicate that the Sin3B locus is maintained in a relative transcriptionally repressed state in proliferating cells but becomes activated upon oncogene activation. These observations are reminiscent of what has been reported for the Ink4a/Arf locus, which is transcriptionally repressed in proliferating cells but becomes actively transcribed following oncogene activation. This repression is mediated in primary fibroblasts by the PcG protein Bmi-1 along with other PRC1 components such as Cbx7 and Cbx8 (20, 29-31), leading us to investigate whether Bmi-1 similarly regulates Sin3B expression. Indeed, ectopic expression of Bmi-1 strongly decreased both Sin3B transcript and protein levels in primary MEFs (Figure 2A and 2B). Importantly, Bmi-1 driven repression of Sin3B is specific, as the protein levels of the closely related Sin3A were not affected by Bmi-1 ectopic expression (Supplementary Figure 1A). Conversely, shRNA-mediated knockdown of Bmi-1 resulted in a significant increase of Sin3B mRNA and protein levels (Figure 2C and 2D). As Bmi-1 has been reported to prevent the occurrence of senescence, it remained possible that the Bmi-1-dependent modulation of Sin3B was merely due to senescence. We believe this to be unlikely, as Bmi-1 overexpression resulted in the downregulation of Sin3B levels in immortalized MEFs (NIH3T3), while Bmi-1 knockdown led to an increase in Sin3B transcript abundance in these cells (Supplementary Figure 2A and 2B). These results indicate that Sin3B is a bona fide downstream target of the Polycomb protein Bmi-1.
Figure 2. Bmi-1 directly represses Sin3B transcription.
(A) Western blot on whole cell extracts from wild-type MEFs infected with empty vector, RasV12, or Bmi-1 expressing retroviruses using the indicated antibodies. Bmi-1 (Millipore 05-637). (B) Relative expression of the indicated transcripts in wild-type MEFs infected with vector or Bmi-1 expressing retroviruses as determined by qRT PCR and reported to B2M (n=4). *p<0.05, **p<0.01 as determined by a student's t test. Data represent mean ± SEM. (C) Relative expression of indicated transcripts in wild-type MEFs infected with retroviruses encoding a scramble shRNA (Scr) or a Bmi-1 targeting shRNA as determined by qRT PCR and reported to B2M (n=4). *p<0.05, ***p<0.001 as determined by a student's t test. Data represent mean ± SEM. (D) Western blot on whole cell extracts from wild-type MEFs infected with retroviruses encoding a scramble shRNA or a Bmi-1 targeting shRNA using the indicated antibodies. p19Arf (Abcam ab80-25). (A to D) Cells were harvested 3 days following selection. (E) Western blot on whole cell extracts from MEFs infected with a retrovirus encoding BRafV600E-ERT1 2 days after 1uM 4-OHT addition using the indicated antibodies. (F) Chromatin immunoprecitation (ChIP) on the indicated loci (Sin3B TSS and Gapdh) in wild-type MEFs infected with a retrovirus encoding BRafV600E-ERT1 2 days after EtOH (white bars) or 4-OHT (gray bars) addition, with an antibody raised against Bmi-1 (Millipore 17-664) or non-reactive serum (NRS). Enrichments are displayed relative to input and normalized to 4-OHT treatment with Bmi-1 pull-down (n=6). ChIP experiments were performed as previously described (46). Data represent mean ± SEM.
To explore the possibility that the Sin3B locus is a direct target of Bmi-1, we took advantage of the tamoxifen-inducible BRafV600E-ERT1 chimeric protein (32). In early passage MEFs expressing BRafV600E-ERT1, tamoxifen administration resulted in activation of the BRaf pathway, as evidenced by the accumulation of phosphorylated ERK1/2 (Figure 2E). More specifically, induction of the BRaf pathway led to the transcriptional activation of Sin3B, p16Ink4a, and p19Arf (Figure 2E and Supplementary Figure 3). Tamoxifen addition to wild-type MEFs did not alter Sin3B transcript or protein levels, demonstrating that the changes in Sin3B levels were due to BRaf activation (Supplementary Figure 4A and 4B). Interestingly, a time course study indicated that Sin3B upregulation is an early event following BRafV600E activation, and precedes the induction of established markers of senescence, including GLB1 and IL-6 (33-35) (Supplementary Figure 3). This observation is consistent with Sin3B upregulation being driven by oncogenic activation rather than by senescence itself. To examine the molecular mechanisms underlying Bmi-1 modulation of Sin3B expression, we performed Chromatin ImmunoPrecipitation (ChIP) experiments on primary MEFs, before or after BRafV600E activation. Prior to BRafV600E activation, Bmi-1 was found weakly but reproducibly enriched at the Sin3B transcription start site (TSS), compared to a control locus (gapdh) or a non relevant serum (NRS) (Figure 2F). Following BRafV600E activation, Bmi-1 was released from the Sin3B locus, coinciding with an increase in Sin3B transcription. Together, these experiments suggest that the repressor Bmi-1 is tethered to the Sin3B locus in proliferating cells, and released upon oncogenic stress, coinciding with its transcription.
Sin3B null cells are refractory to Bmi-1 knockdown induced cellular senescence
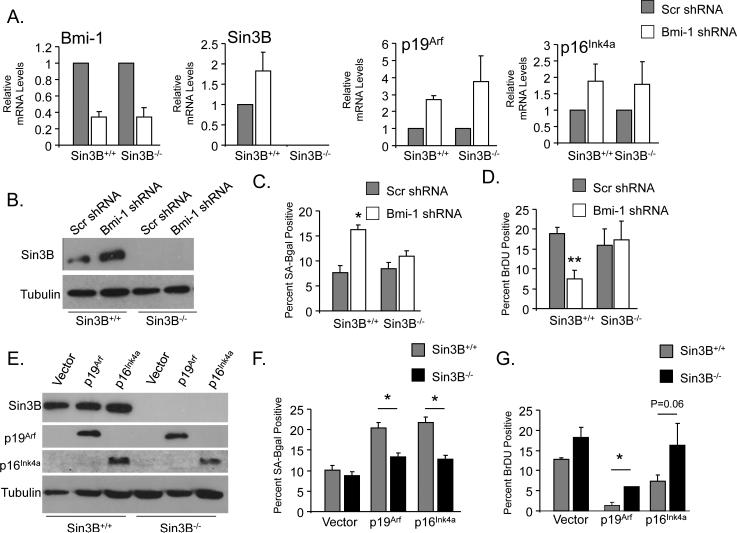
Bmi-1 depletion in primary fibroblasts leads to cellular senescence (19). Based on the demonstration that Sin3B is a direct target of Bmi-1 repression, and that Sin3B promotes replicative and oncogene-induced senescence (27), we investigated whether Sin3B upregulation contributed to the cellular senescence elicited upon a decrease in Bmi-1 levels. shRNA-mediated knockdown of Bmi-1 in early passage Sin3B+/+ and Sin3B−/− MEFs resulted in a 70% reduction in Bmi-1 transcript levels. As expected, Bmi-1 depletion correlated with an increase in Sin3B mRNA and protein levels only in wild-type MEFs, while an increase in p16Ink4a and p19Arf mRNA abundance was detected upon Bmi-1 knockdown in both Sin3B+/+ and Sin3B−/−MEFs (Figure 3A and 3B). In agreement with previous reports, decreasing Bmi-1 levels in wild-type MEFs resulted in a significant reduction in BrDU incorporation and an increase in SA-βGal positivity, indicative of premature entry in senescence (Figure 3C and 3D). Strikingly, genetic inactivation of Sin3B fully rescued the cell cycle withdrawal and premature entry into senescence elicited by Bmi-1 knockdown (Figure 3C and 3D). Sin3B inactivation prevented the entry into senescence elicited by Bmi-1 depletion despite the upregulation of p16Ink4a and p19Arf, indicating that the products of the Ink4a/Arf locus may be unable to modulate cell cycle exit in the absence of Sin3B. Indeed, similar to what was observed upon Bmi-1 knockdown, Sin3B was required for cell cycle withdrawal and entry into cellular senescence induced by p16Ink4a and p19Arf overexpression (Figure 3E, 3F and 3G).
Figure 3. Sin3B is required for Bmi-1 knockdown induced senescence.
(A) Relative expression of the indicated transcripts in Sin3B+/+ or Sin3B−/− MEFs infected with a retroviruses encoding a scramble shRNA (Scr) or a Bmi-1 targeting shRNA as determined by qRT PCR and reported to B2M (n=3). Data are relative to either Sin3B+/+ or Sin3B−/− Scr shRNA. Data represent mean ± SEM. (B) Western blot on whole cell extracts from Sin3B+/+ or Sin3B−/−MEFs infected with retroviruses encoding a scramble shRNA (Scr) or a Bmi-1 targeting shRNA using the indicated antibodies. (C) Proportion of BrDU positive cells in Sin3B+/+ or Sin3B−/− MEFs infected with retroviruses encoding a scramble shRNA (Scr) or a Bmi-1 targeting shRNA. At least 200 cells were counted in duplicate (n=4). *p<0.05 as determined by a student's t test. Data represent mean ± SEM. (D) Proportion of Sa-ßGal positive cells in Sin3B+/+ or Sin3B−/−MEFs infected with retroviruses encoding a scramble shRNA (Scr) or a Bmi-1 targeting shRNA. At least 200 cells were counted in duplicate (n=4). **p<0.01 as determined by a student's t test. Data represent mean ± SEM. (E) Western blot on whole cell extracts from Sin3B+/+ or Sin3B−/−MEFs infected with retroviruses encoding vector, p19Arf, or p16Ink4a using the indicated antibodies. p16Ink4a (Santa Cruz Biotechnology). (F) Proportion of BrDU positive cells in Sin3B+/+ or Sin3B−/− MEFs infected with retroviruses encoding vector, p19Arf, or p16Ink4a. At least 200 cells were counted in duplicate (n=2). *p<0.05 as determined by a student's t test. Data represent mean ± SEM. (G) Proportion of Sa-ßGal positive cells in Sin3B+/+ or Sin3B−/− MEFs infected with retroviruses encoding vector, p19Arf, or p16Ink4a. At least 200 cells were counted in duplicate (n=2). *p<0.05 as determined by a student's t test. Data represent mean ± SEM. (A to G) Cells were harvested 6 days following selection.
Sin3B is required for the elevated ROS levels in Bmi-1 depleted cells
Recent work has demonstrated that Bmi-1 prevents the detrimental accumulation of ROS in both neurons and hematopoietic cells (25, 36). Increases in ROS are a primary cause of cellular senescence in MEFs as culturing MEFs in low oxygen conditions prevents entry into cellular senescence (37). Furthermore, ROS accumulation contributes to the senescence phenotype upon oncogenic stimuli (38, 39). We have previously demonstrated that Sin3B directly and specifically represses the transcription of several nuclear-encoded mitochondrial genes in differentiated myotubes (40). Since Sin3B−/− MEFs are resistant to premature senescence elicited by oncogene signaling and Bmi-1 depletion, we hypothesized that Sin3B levels may modulate ROS accumulation downstream of Bmi-1.
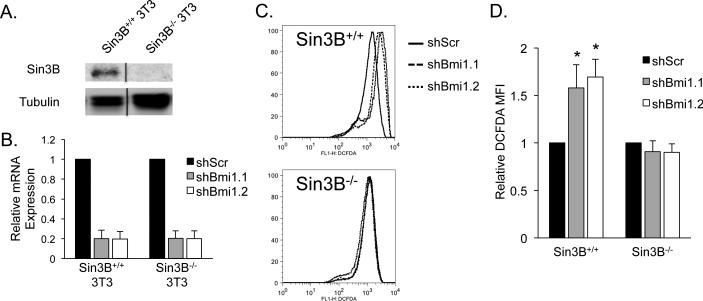
To separate the accumulation of ROS resulting from entry in senescence from that caused specifically by Bmi-1 depletion, we generated immortalized Sin3B+/+ and Sin3B−/− murine fibroblasts using a 3T3 protocol (Figure 4A). These cells are unable to undergo cellular senescence upon Bmi-1 depletion (data not shown). As previously reported in multiple cell types (25, 36), lowering Bmi-1 levels using two independent shRNAs increased the levels of intracellular ROS in Sin3B+/+ 3T3s. Strikingly, Sin3B−/− 3T3s fail to exhibit increased ROS accumulation upon Bmi-1 depletion (Figure 4B, 4C, and 4D).
Figure 4. Bmi-1 depletion causes a Sin3B dependent increase in ROS in fibroblasts.
(A) Western blot on whole cell lysates of Sin3B+/+ and Sin3B−/− 3T3 cells using Sin3B (Santa Cruz Biotechnology AK12) and alpha-tubulin (Sigma T9026) antibodies. Lysates were run noncongruously on the same gel and were cropped together, indicated by the black line. (B) Bmi-1 relative expression following infection with either scramble shRNA (shScr) or one of two shRNA targeting Bmi-1 (shBmi1.1 or shBmi1.2). Expression values are related to B2M. Cells were harvested 9 days following selection (n=3). Data represent mean ± SEM. (C) Sin3B+/+ and Sin3B−/− 3T3 cells were infected with shScr, shBmi1.1, or shBmi1.2. Nine days following selection, 300,000 cells were incubated in 5uM DCFDA (Life Technologies) at 37°C for 5 minutes and immediately analyzed on a FACSCalibur (BD Biosciences). Dead cells were excluded with 7AAD. Data was analyzed on FlowJo (TreeStar). Shown is a representative histogram plot. (D) Quantification of (C). n=3. Data represent mean ± SEM. *p<0.05 as determined by a student's t test.
In summary, these results demonstrate that Sin3B is an essential regulator of the cell cycle withdrawal and the cellular consequences elicited upon Bmi-1 depletion. It could be argued that Sin3B−/− MEFs require additional mutations to proliferate in culture, which would confound our conclusion that Sin3B is required for entry into cellular senescence upon Bmi-1 depletion. However, our results do not support this interpretation. First, the proportion of midgestation Sin3B−/− embryos obtained from Sin3B heterozygous crosses is 25% indicating that no additional stochastic mutation is required for Sin3B−/− embryonic cells to grow in vivo (26). Second, Sin3B−/− MEFs proliferate similarly to wild-type MEFs at early passages and do not undergo proliferative crisis for at least twenty-six passages (26, 27). Therefore, the clonal selection of a stochastic mutation is not occurring in Sin3B−/− MEFs, in contrast to what has been observed for other knockout MEF lines (for example see ref. 41, 42). Along with the fact that our experiments were performed in primary MEFs at early passage, our data support the conclusion that Sin3B inactivation is sufficient to suppress the cellular senescent phenotype elicited by Bmi-1 depletion.
Our observations also indicate that oncogenic stress leads to the dissociation of the Bmi-1 protein from the Sin3B locus. Interestingly, it has been recently demonstrated that oncogenic activation promotes Bmi-1 phosphorylation, resulting in its dissociation from the chromatin fiber, thus providing a potential molecular basis for Bmi-1's release from the Sin3B locus upon oncogene activation (43, 44). In addition, our results indicate that ROS accumulation upon Bmi-1 depletion depends on the presence of Sin3B. How Sin3B prevents the accumulation of ROS is not entirely clear at this point. As we have recently demonstrated that Sin3B directly and specifically represses the transcription of several nuclear-encoded mitochondrial genes in differentiated myotubes (40), it is tempting to speculate that Sin3B levels and activities may alter mitochondrial metabolism, ultimately regulating ROS production. These observations indicate that Sin3B serves as the elusive molecular link between Bmi-1 and mitochondrial function. Finally, given the well-established pro-oncogenic function on Bmi-1 in human cancers, it will be interesting to assess the contribution of Sin3B expression in preventing cellular transformation. Since Sin3B deletion, unlike Ink4a/Arf deletion, does not sensitize to oncogenic transformation (27), it may therefore represent an ideal therapeutic target for mitigating the deleterious effects of a wide variety of stress, including those triggered by loss of Bmi-1 expression. Consistent with this hypothesis, we have recently demonstrated that Sin3B inactivation delays oncogenic Ras-driven tumorigenesis in a mouse model of pancreatic cancer (45). Based on the results presented here, we speculate that this property may be linked to the ability of Sin3B to modulate ROS levels in response to stress, a hypothesis that remains to be investigated.
Supplementary Material
Acknowledgements
We are grateful to all members of the David laboratory for helpful discussions during the preparation of the manuscript. We thank Drs. Sally Temple, Gabriella De Vita, Goberdhan Dimri, David Levy and Marty McMahon for the generous gifts of plasmids used in this study and Dr. Michael Garabedian for helpful discussions. We wish to acknowledge the Skirball Institute of Biomolecular Medicine for hosting our laboratory following Hurricane Sandy. All animal experiments were done in accordance with the guidelines of the National Institutes of Health (NIH) and were approved by the NYU School of Medicine Institutional Animal Care and Use Committee. This work was funded by the American Cancer Society (115014-RSG-08-054-01-GMC to GD), the National Institute of Health (5R01CA148639 and 5R21CA155736 to GD), the Irma T. Hirschl Charitable Trust (GD), the Samuel Waxman Cancer Research Foundation (GD) and a Feinberg NYU individual grant (GD). TD, DJC and AJB were supported by predoctoral NIH training grants T32CA009161 (TD, DJC) and T32GM066704 (AJB).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes & development. 2010;24(22):2463–79. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11(11):S27–31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 3.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436(7051):642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell ME, DeNicola GM, Martins CP, Jacobetz MA, Maitra A, Hruban RH, et al. Cellular features of senescence during the evolution of human and murine ductal pancreatic cancer. Oncogene. 2012;31(12):1599–608. doi: 10.1038/onc.2011.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–4. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 6.Dimauro T, David G. Ras-induced senescence and its physiological relevance in cancer. Current cancer drug targets. 2010;10(8):869–76. doi: 10.2174/156800910793357998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campisi J. Aging, cellular senescence, and cancer. Annual review of physiology. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpless NE, DePinho RA. Cancer: crime and punishment. Nature. 2005;436(7051):636–7. doi: 10.1038/436636a. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436(7051):725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David G. Regulation of oncogene-induced cell cycle exit and senescence by chromatin modifiers. Cancer biology & therapy. 2012;13(11):992–1000. doi: 10.4161/cbt.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richly H, Aloia L, Di Croce L. Roles of the Polycomb group proteins in stem cells and cancer. Cell death & disease. 2011;2:e204. doi: 10.1038/cddis.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell stem cell. 2010;7(3):299–313. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon JA, Kingston RE. Occupying chromatin: Polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Molecular cell. 2013;49(5):808–24. doi: 10.1016/j.molcel.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haupt Y, Alexander WS, Barri G, Klinken SP, Adams JM. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell. 1991;65(5):753–63. doi: 10.1016/0092-8674(91)90383-a. [DOI] [PubMed] [Google Scholar]
- 15.van Lohuizen M, Verbeek S, Scheijen B, Wientjens E, van der Gulden H, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991;65(5):737–52. doi: 10.1016/0092-8674(91)90382-9. [DOI] [PubMed] [Google Scholar]
- 16.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–7. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423(6937):302–5. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 18.van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes & development. 1994;8(7):757–69. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397(6715):164–8. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 20.Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21(5):525–30. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arranz L, Herrera-Merchan A, Ligos JM, de Molina A, Dominguez O, Gonzalez S. Bmi1 is critical to prevent Ikaros-mediated lymphoid priming in hematopoietic stem cells. Cell cycle. 2012;11(1):65–78. doi: 10.4161/cc.11.1.18097. [DOI] [PubMed] [Google Scholar]
- 22.Oguro H, Yuan J, Ichikawa H, Ikawa T, Yamazaki S, Kawamoto H, et al. Poised lineage specification in multipotential hematopoietic stem and progenitor cells by the polycomb protein Bmi1. Cell stem cell. 2010;6(3):279–86. doi: 10.1016/j.stem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E, et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes & development. 2005;19(12):1438–43. doi: 10.1101/gad.1299305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oguro H, Iwama A, Morita Y, Kamijo T, van Lohuizen M, Nakauchi H. Differential impact of Ink4a and Arf on hematopoietic stem cells and their bone marrow microenvironment in Bmi1-deficient mice. The Journal of experimental medicine. 2006;203(10):2247–53. doi: 10.1084/jem.20052477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459(7245):387–92. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David G, Grandinetti KB, Finnerty PM, Simpson N, Chu GC, Depinho RA. Specific requirement of the chromatin modifier mSin3B in cell cycle exit and cellular differentiation. Proc Natl Acad Sci U S A. 2008;105(11):4168–72. doi: 10.1073/pnas.0710285105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandinetti KB, Jelinic P, DiMauro T, Pellegrino J, Fernandez Rodriguez R, Finnerty PM, et al. Sin3B expression is required for cellular senescence and is up-regulated upon oncogenic stress. Cancer Res. 2009;69(16):6430–7. doi: 10.1158/0008-5472.CAN-09-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, et al. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Molecular cell. 2011;42(1):36–49. doi: 10.1016/j.molcel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard D, Martinez-Leal JF, Rizzo S, Martinez D, Hudson D, Visakorpi T, et al. CBX7 controls the growth of normal and tumor-derived prostate cells by repressing the Ink4a/Arf locus. Oncogene. 2005;24(36):5543–51. doi: 10.1038/sj.onc.1208735. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich N, Bracken AP, Trinh E, Schjerling CK, Koseki H, Rappsilber J, et al. Bypass of senescence by the polycomb group protein CBX8 through direct binding to the INK4A-ARF locus. The EMBO journal. 2007;26(6):1637–48. doi: 10.1038/sj.emboj.7601632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yap KL, Li S, Munoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Molecular cell. 2010;38(5):662–74. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12(19):2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187–95. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 34.Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133(6):1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 36.Chatoo W, Abdouh M, David J, Champagne MP, Ferreira J, Rodier F, et al. The polycomb group gene Bmi1 regulates antioxidant defenses in neurons by repressing p53 pro-oxidant activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(2):529–42. doi: 10.1523/JNEUROSCI.5303-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5(8):741–7. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275(5306):1649–52. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 39.Lee AC, Fenster BE, Ito H, Takeda K, Bae NS, Hirai T, et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. The Journal of biological chemistry. 1999;274(12):7936–40. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 40.van Oevelen C, Bowman C, Pellegrino J, Asp P, Cheng J, Parisi F, et al. The mammalian Sin3 proteins are required for muscle development and sarcomere specification. Mol Cell Biol. 2010;30(24):5686–97. doi: 10.1128/MCB.00975-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Cichetti G, Onda H, Koon HB, Asrican K, Bajraszweski N, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. Journal of Clinical Investigation. 2003;112(8):1223–33. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Jacob NK, Ladner KJ, Beg A, Perko JD, Tanner SM, et al. RelA/p65 functions to maintain cellular senescence by regulating genomic stability and DNA repair. EMBO Rep. 2009;10(11):1272–8. doi: 10.1038/embor.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Liu F, Yu H, Zhao X, Sashida G, Deblasio A, et al. Akt phosphorylates the transcriptional repressor bmi1 to block its effects on the tumor-suppressing ink4a-arf locus. Science signaling. 2012;5(247):ra77. doi: 10.1126/scisignal.2003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nacerddine K, Beaudry JB, Ginjala V, Westerman B, Mattiroli F, Song JY, et al. Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer. The Journal of clinical investigation. 2012;122(5):1920–32. doi: 10.1172/JCI57477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rielland M, Cantor DJ, Graveline R, Hajdu C, Mara L, de Diego Diaz B, et al. Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression. The Journal of clinical investigation. 2014 doi: 10.1172/JCI72619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jelinic P, Pellegrino J, David G. A novel mammalian complex containing Sin3B mitigates histone acetylation and RNA polymerase II progression within transcribed loci. Molecular and Cellular Biology. 2011;21(1):54–62. doi: 10.1128/MCB.00840-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.