Abstract
Prospective epidemiological studies found that generalized anxiety disorder (GAD) can impair immune function and increase risk for cardiovascular disease or events. Mechanisms underlying the physiological reverberations of anxiety, however, are still elusive. Hence, we aimed to investigate molecular processes mediating effects of anxiety on physical health using blood gene expression profiles of 336 community participants (157 anxious and 179 control). We examined genome-wide differential gene expression in anxiety, as well as associations between nine major modules of co-regulated transcripts in blood gene expression and anxiety. No significant differential expression was observed in women, but 631 genes were differentially expressed between anxious and control men at the false discovery rate of 0.1 after controlling for age, body mass index, race, and batch effect. Gene set enrichment analysis (GSEA) revealed that genes with altered expression levels in anxious men were involved in response of various immune cells to vaccination and to acute viral and bacterial infection, and in a metabolic network affecting traits of metabolic syndrome. Further, we found one set of 260 co-regulated genes to be significantly associated with anxiety in men after controlling for the relevant covariates, and demonstrate its equivalence to a component of the stress-related conserved transcriptional response to adversity profile. Taken together, our results suggest potential molecular pathways that can explain negative effects of GAD observed in epidemiological studies. Remarkably, even mild anxiety, which most of our participants had, was associated with observable changes in immune-related gene expression levels. Our findings generate hypotheses and provide incremental insights into molecular mechanisms mediating negative physiological effects of GAD.
1. Introduction
Individuals with generalized anxiety disorder (GAD) experience daily excessive, uncontrollable, and often irrational worry.1 GAD is fairly common with a lifetime prevalence of 5.7%.2 Prospective epidemiological studies have found that GAD is a risk factor for cardiovascular diseases and cardiac events over many ensuing years.3-6 For instance, a prospective cohort study following 1015 patients for a mean of 5.6 years found that GAD was associated with a 62% higher rate of cardiovascular events (defined as stroke, transient ischemic attack, heart failure, myocardial infarction, or death) after comorbid conditions, including major depressive disorder, hypertension, history of myocardial infarction, diabetes, congestive heart failure, stroke, cardiac disease severity, medication use, and age have been accounted for.4 Another prospective study of 49,000 young Swedish men followed for 37 years found multi-adjusted hazard ratios for coronary heart disease and acute myocardial infarction to be 2.17 and 2.51 respectively for anxious men.5 Interestingly, a variety of potential mediators for the association of GAD and cardiovascular diseases and events have been examined, including C-reactive protein level, heart rate variability, smoking, medication non-adherence, and physical inactivity, but they did not explain the association.4 Why anxiety increases risks of cardiovascular disease and events is still poorly understood.
Along the same lines, several studies have shown that that psychological stress, such as feeling stressed, anxious, or depressed, has a negative impact on immune functions, including reducing immune response to influenza7,8 or pneumococcal pneumonia vaccines,9 reactivating latent herpes virus10, and increasing severity and duration of infectious diseases.11-13 For example, in a prospective study of 83 healthy young adults, those who felt more stressed had a slower rate of production of antibody titer and less maintenance of the produced antibody titer to the influenza vaccination over the 4-month follow-up period.7 Consistently, an epidemiological study found that having any anxiety disorder in the past year was associated with 1.7 times higher risk of having infectious conditions such as tuberculosis or bronchitis after comorbid depressive disorders, substance use disorders, and relevant sociodemographic factors have been adjusted for.14 Understanding biological mechanisms by which psychological stress influences immune function has important clinical implications in treatment and prevention.
Hence, we aimed to investigate molecular mechanisms underlying these observed physiological consequences of anxiety symptoms using peripheral blood gene expression profiles of anxious and control individuals. Specifically, we performed a genome-wide differential gene expression analysis in anxiety versus control groups, followed by gene set enrichment analysis to gain insights into biological mechanisms of the differentially expressed genes. Additionally, we examined associations between anxiety and the nine highly conserved major axes of covariation in blood gene expression, also followed by gene set enrichment analysis.
The notion of major axes of covariance in blood gene expression arose from observations that gene expression profiles in human tissues typically have complex and pervasive co-regulation patterns.15,16 To capture the covariance structure of peripheral blood gene expression, Chaussabel and colleagues queried multiple blood gene expression datasets for conserved modules of transcripts that differ by disease state.15 These modules were further refined by Preininger and colleagues into nine major axes of covariation which are consistently observed in whole blood from healthy adults.16 Each of the nine major axes of covariation consists of 99 to 1028 strongly co-regulated genes.16 The nine axes collectively capture 37% to 51% of the blood transcriptomic variance depending on the population. These nine axes were shown to be highly repeatable across six studies that were carried out in different geographical locations (Atlanta, Morocco, Finland, Australia, and England).16 These axes were also differentially expressed under a wide variety of disease conditions, and in response to environmental and genetic stimuli.16,17
2. Methods
2.1 Study participants
Participants were recruited by the Center for Health Discovery and Well-being (CHDWB), which was established by Emory University in 2008 as a research center to evaluate the effectiveness and utility of a health and prevention-focused rather than disease-focused care setting. Participants of the CHDWB were Emory University employees who were randomly selected and invited to participate. Inclusion criteria were 18 years of age or older and being able to give informed consent. Exclusion criteria consisted of a) current malignant neoplasm, b) history of malignancy during the previous five years, c) having an acute illness in the two weeks prior to baseline assessment, d) hospitalization in the preceding year due to acute or chronic disease or psychiatric disorder, e) significant change in a chronic medical condition (such as hypertension or diabetes) requiring new medication, and f) history of substance abuse or alcoholism.18 A total of 546 of the approximately 700 participants were included in this study on the basis of availability of gene expression data.
2.2 Anxiety phenotype
Anxiety symptoms were assessed with the GAD-7 scale,19 a well-validated and efficient tool for screening and assessing symptoms of generalized anxiety disorder. The GAD-7 has been validated both in the general population20 and primary care setting19 showing excellent internal consistency (Croncbach α=0.92), and very good test-retest reliability (intraclass correlation = 0.83). Its score ranges from 0-21, with higher scores reflecting more anxiety symptoms. Additionally, the GAD-7 is not only highly sensitive (up to 92%) and specific (up to 82%) in detecting generalized anxiety disorder, but also very good at detecting social anxiety disorder and post-traumatic stress disorder when compared with diagnoses made via interview by mental health professionals.19 Further, the GAD-7 scores were shown to be strongly correlated with WHO-DAS-II disability score (r=0.704; p<0.001), particularly for Participation in Society (r=0.741), Understanding Communication (r=0.679), and Life Activities (r=0.638) dimensions.21 A score of 5-9 on the GAD-7 indicates mild anxiety, 10-14 moderate, and 15-21 severe anxiety symptoms.19 In our sample of 546 participants, we categorized participants with GAD-7 scores of ≥5 as having anxiety symptoms and those with GAD-7 scores ≤1 as controls. Thus, there were 157 anxious and 179 control participants, and anxiety was included in all the analyses as a dichotomous variable.
2.3 Gene expression profiles
RNA was extracted from whole blood collected in Tempus tubes during the participants’ first visit to the CHDWB. All samples had RIN (Bioanalyzer RNA Integrity Number) > 8 indicating high quality. Complementary DNA was derived from RNA and then hybridized, and raw probe intensities were generated on Illumina HT12 v3 or v4 beadchip arrays. Probes with expression levels below background were filtered out, namely 33120 and 33220 probes respectively, leaving 14,111 probes for inclusion in the analysis. These 14,111 probes have been found to express consistently across multiple studies. We then performed log2 transformation and normalization, using the Supervised Normalization Method,22 modeling standardized average GAD-7 score as the biological variable and removing the effects of the adjustment variables batch, RIN, and self-reported ethnicity. The sample included 370 females and 176 males, 134 African American, 26 Asian American, 1 Native American, and 382 Caucasian American participants. The dataset has been submitted to GEO with the accession number GSE61672.
2.4 Nine major axes of covariance of blood gene expression
Each of the nine major axes of covariance is defined by 10 blood informative transcripts (BITs), and the derivation of these 10 BITs has been described in detail elsewhere.16 Briefly, these axes capture conserved patterns of co-regulation of gene expression as observed across multiple peripheral blood gene expression studies performed by different groups. Each axis includes hundreds to thousands of genes that suggest roles in specific aspects of immune and hematological functions. Each axis is represented by an Axis score, which is the first principal component of the 10 highly co-regulated BITs, and captures, on average, 79% of the variance of these 10 BITs.16 Associations between anxiety and the major axes were assessed with linear regression in which each axis score was the outcome, anxiety the independent variable, and gender, age, body mass index, race, and batch as the covariates. We used the 260 genes that constitute Axis 2, derived by Preininger and colleagues,16 for further gene set enrichment analysis of the Axis 2 genes.
2.5 Genome-wide differential gene expression
Genome-wide differential gene expression between anxiety and control groups was performed using analysis of covariance (ANCOVA) in which normalized gene expression for each probe was the outcome, anxiety the dichotomous independent variable, gender, age, body mass index, race, and batch as the covariates. Multiple hypothesis testing was accounted for using the false discovery rate of 0.1.23 We also examined differential gene expression in men and women separately. Analyses were performed using JMP Genomics version 7.
2.6 Gene set enrichment analysis
To gain insights into the biological processes involving the differentially expressed genes, we performed gene set enrichment analysis using the Pre-ranked Gene Set Enrichment Analysis (GSEA-P) analytical software provided by the Broad Institute.24,25 Genes that were differentially expressed between anxiety and control group at the false discovery rate ≤ 0.10 and having fold change of at least 0.6 in magnitude were pre-ranked using their t-statistics derived from the ANCOVA for differential expression between anxiety and control as described above. Gene sets were obtained from the Molecular Signature Database (MSigD) v4.0.24 We selected the gene sets from the Curated, Gene Ontology, Positional, Motif, Computational, Oncogenic, and Immunologic Signatures collections, particularly those with at least eight genes in common with our preranked gene list, and the weighted p option (p=1) for the running sum statistics for enrichment score. While an FDR cutoff of 0.25 can be used to define significance level in GSEA,24 we used a more conservative FDR cutoff of 0.15.
In addition, we performed pre-ranked GSEA on the genes constituting Axis 2 to identify enriched biological pathways. These genes were pre-ranked according to their differential expression between anxiety and control groups using the t statistics from linear regression models for association between Axis 2 genes and anxiety, controlling for gender, age, body mass index, race, and batch. We used similar gene sets from the MSigDB as described above.
3. Results
3.1. Characteristics of the anxiety vs. control group
A total of 336 participants, 157 anxious and 179 control, were included in the analysis (Table 1). GAD-7 scores in the anxious group ranged from 5-21, with a mean of 8, and in the control group 0-1, with a mean of 0.4. There were more men in the control group and this difference in sex was controlled for in the subsequent analyses. There was no difference in mean age, body mass index, or employment status between the control and anxious group. All participants were Emory University employees at the time of assessment.
Table 1.
Characteristics of the anxiety versus control group (n=336)
| Characteristics | Anxiety (n=157) | Control (n=179) | p-value |
|---|---|---|---|
| Age | 47.8 ± 10.9 | 49.3 ± 10.8 | 0.21 |
| Gender (% male) | 27.4% | 38.5% | 0.03 |
| Race | 0.0007 | ||
| White | 76.4% | 59.2% | |
| Others (African American, Asian, American Indian) | 23.6% | 40.8% | |
| Employment | 100% | 100% | 1.00 |
| Body mass index | 27.4 ± 6.0 | 28.3 ± 7.1 | 0.25 |
| GAD-7 score | 8.0 ± 3.7 | 0.4 ± 0.5 | <0.0001 |
3.2. Genome-wide differential gene expression between anxiety and control group
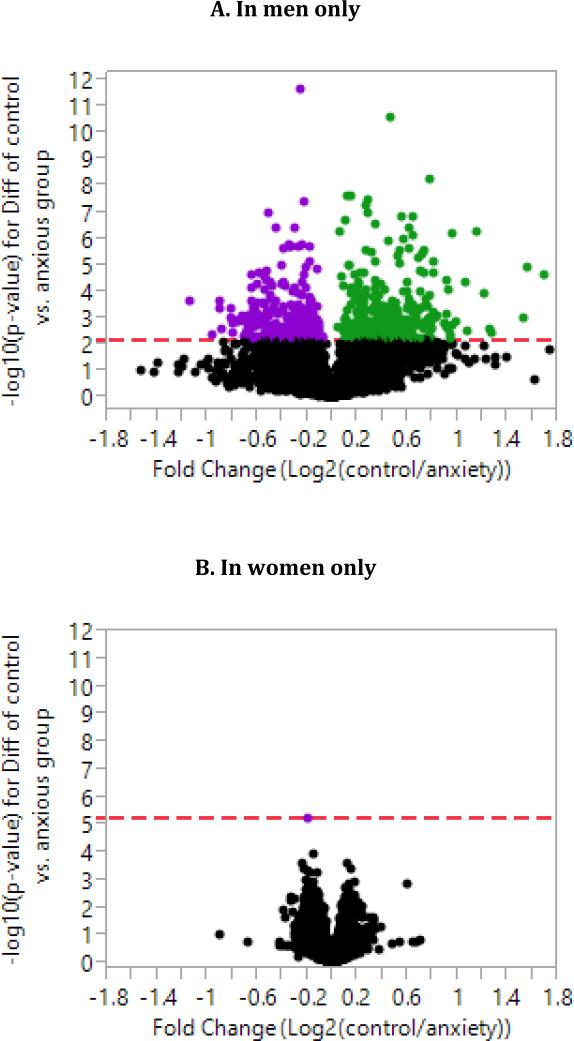
One gene, eukaryotic translation initiation factor 4E binding protein 3 (EIF4EBP3), was differentially expressed between the anxiety and control group at the false discovery rate (FDR) of 0.10 and with a decrease of 0.1 log2 units in anxious individuals. Among females only, one gene, opsin3 (OPN3), was significantly differentially expressed between anxious and control group at the FDR of 0.10, with an increase of 0.2 log2 units in anxious relative to control women (Figure 1B). Among men only, 631 probes were differentially expressed between the anxious and control group at FDR of 0.10 with fold changes ranging from −1.2 to 1.7 in both directions (Figure 1A).
Figure 1.
Volcano plots for genome-wide differential gene expression between anxiety and control groups based on analysis of covariance, controlling for age, body mass index, race, and batch, in men (A) and women (B). The colored dots in (A) are probes that were differentially expressed in men at FDR of 0.1. Green probes had higher expression levels in control vs. anxious group. Purple probes had higher expression levels in anxious vs. control group.
We examined whether these 631 genes were similarly regulated (up-regulated or down-regulated) in anxious women, albeit at a smaller magnitude, by correlating men and women's t-statistics derived from the genome-wide analyses for these genes. Among the 259 up-regulated genes in anxious men relative to controls (coded purple in Figure 1A), there was no significant correlation between the t-statistics between men and women (r=0.055; p=0.371), indicating that these genes were not similarly up-regulated in anxious women. Similarly, among the 372 down-regulated genes in anxious men relative to controls (coded green in Figure 1A), there was no significant correlation between men and women's t-statistics (r=0.04; p=0.468), also implying that these genes were not similarly down regulated in anxious women.
3.3. Gene set enrichment analysis (GSEA) for differentially expressed genes in anxious men
To gain insights into the biological processes involving the differentially expressed genes in anxious versus control men, we performed GSEA using the gene sets obtained from the MSigDB database. Seven relevant gene sets were significantly enriched at a FDR of 0.15 (Table 2). Of these, six gene sets, identified in three separate studies,26-28 are involved in response of various immune cells to acute infection or vaccination: a) response of human B cells and monocytes to influenza vaccination26; b) response of human monocytes and myeloid dendritic cells to influenza vaccination26; c) genes differentially expressed in activated neutrophils versus activated mast cells27; d) response of peripheral blood mononuclear cells to acute Staphylococcus aureus infection28; e) response of peripheral blood mononuclear cells to Streptococcus pneumonia infection28; f) response of peripheral blood mononuclear cells to acute Influenza infection.28 The seventh gene set contains genes that form the macrophage-enriched metabolic network that has been implicated in metabolic syndrome traits such as obesity, diabetes, and atherosclerosis.29
Table 2.
Description of gene sets significantly enriched at FDR≤0.15 among the differentially expressed genes in anxious versus control males from gene set enrichment analysis.
| Description of gene sets | NES | Nominal p-value | FDR | Core enrichment genes |
|---|---|---|---|---|
| Genes involved in response of B cells and monocytes to influenza vaccination in humans (genes upregulated) | −2.26 | 0.000 | 0.011 | CD68, CSF3R, PKM2, LILRA5, PLAUR, STXBP2, C5AR1 |
| Genes differentially expressed in activated neutrophils versus activated mast cells | −2.26 | 0.000 | 0.016 | MOSC1, FLOT1, NCF4, SPI1, CDKN2D, C5AR1, LSP1 |
| Genes involved in response of peripheral blood mononuclear cells to acute Staph aureus infection | −2.04 | 0.000 | 0.033 | NCF4, CD68, CSF3R, SPI1, CDKN2D, LILRA5, PLAUR, PGD |
| Genes involved in response of monocytes and myeloid dendritic cells to influenza vaccination in humans (genes down-regulated) | −1.95 | 0.000 | 0.055 | MOSC1, NCF4, CD68, CSF3R, LILRA5, PGD, C5AR1, SORL |
| Genes differentially expressed in peripheral blood mononuclear cells in patients with acute Strep pneumonia infection relative to healthy controls. | −1.67 | 0.022 | 0.133 | NCF4, ALDOA, CSF3R, SPI1, PLAUR, PGD |
| Genes differentially expressed in peripheral blood mononuclear cells of patients with acute influenza infection versus healthy controls | −1.68 | 0.041 | 0.137 | ALDOA, CD68, PKM2, SPI1, STXBP2, LSP1 |
| Genes forming the macrophage-enriched metabolic network affecting traits of metabolic syndrome | −1.66 | 0.028 | 0.127 | WDR1, F13A1, NCF4, APBB1IP, CD68, CSF3R, PKM2, SPI1, PLAUR, STXBP2 |
NES: normalized enrichment score
FDR: false discovery rate
3.4. Anxiety and nine major axes of co-regulated genes
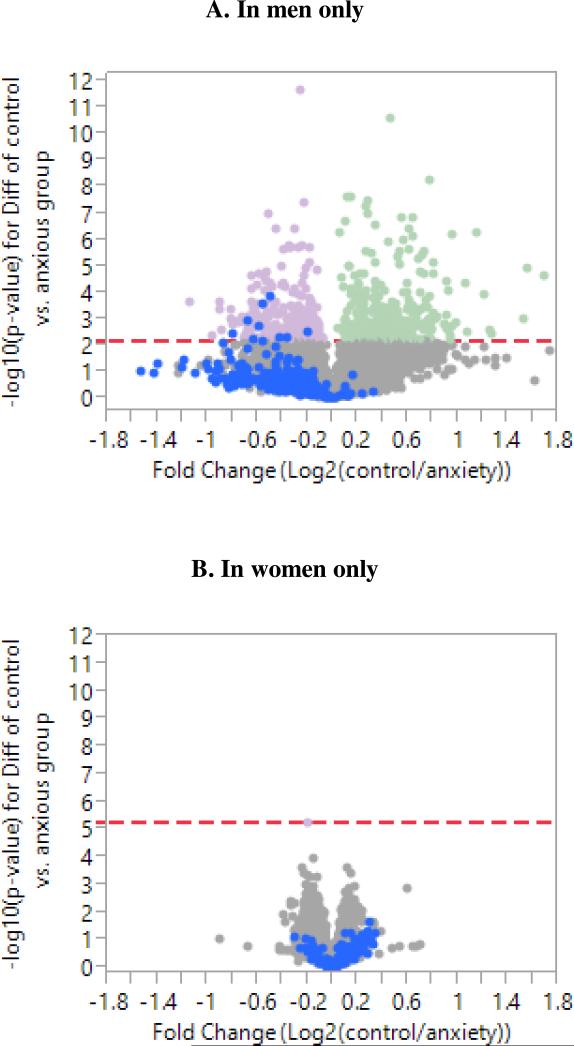
Among the nine axes of co-regulated genes, only Axis 2 was significantly associated with anxiety (β=0.67, p=0.0318), after age, gender, body mass index, race, and batch were controlled for. Anxious individuals had higher Axis 2 scores than control individuals. Also, in this model, age (β=0.047, p=0.0009) and body mass index (β=0.137, p<0.0001) were significantly associated with Axis 2 score. Notably, among men only, the association between Axis 2 and anxiety became stronger, as reflected by a larger beta coefficient and smaller p-value (β=1.33, p=0.019), while among women only this association was no longer significant (β=0.39, p=0.304), after age, body mass index, and batch were controlled for. To better visualize Axis 2 genes in relation to all other genes, Axis 2 genes were highlighted in blue in the volcano plots for anxiety in men and women separately (Figure 2). Figure 2A indicates that most of the Axis 2 genes were not part of the significantly differentially expressed genes in anxious men, but rather form a shelf that includes many genes that are more strongly up-regulated in anxious men, on average, than many of the significantly differentially expressed genes.
Figure 2.
Volcano plots for differential gene expression between control and anxiety groups in men only (A) and women only (B), controlled for age, body mass index, race, and batch. Each plot shows the significance of differential gene expression on the y-axis and fold change on the x-axis. Axis 2 genes are highlighted in blue while significantly up- and down-regulated genes in anxious group (at FDR<0.1, indicated by dashed red line) are pale purple and pale green respectively.
This may stem from the heterogeneity of the effect of anxiety on expression of Axis 2 genes in men. An Axis 2 score of one or greater was seen in 44.2% of the anxious men, but only in 27.5% of the controls. Conversely, an Axis 2 score of 0 or less was seen in 46.5% of the anxious men and 53.6% of the controls. It can be inferred from these results that the up-regulation of Axis 2 score was observed in only a subset of anxious men. This difference was observed in four out of five batches of hybridizations. A closer examination of the anxious men with Axis 2 score ≥ 1 (n=19) and anxious men with Axis 2 score <1 (n=24) showed the mean, median, and range of the GAD-7 scores in each group to be 8.3±3.4; 7; [5-17], and 7.6±2.8; 7; [5-15] respectively. Further, there was no statistical difference between the two groups in age (Wilcoxon Rank Sum statistic (WRS)=448.0; p=0.47), race (chi-square=0.66; p=0.62), body mass index (WRS statistic=472.5; p=0.19), perceived stress measured with the Perceived Stress Scale30 (WRS statistic=433.0; p=0.72), GAD-7 scores (WRS statistic=447.5; p=0.48), and depression assessed with the Beck Depression Inventory31 (WRS statistic=422.5; p=0.92).
3.5. Gene set enrichment analysis (GSEA) for Axis 2 genes
GSEA of 260 genes constituting Axis 2 yielded one enriched gene set at the FDR of 0.04 and normalized effect size of 2.27. This gene set contains genes that are up-regulated in the maturation of dendritic cells upon their stimulation with Galetin-1, a protein known to regulate immune function.32 Since this cell type is a minor fraction of peripheral blood, and only four genes are involved, dendritic cell function alone does not explain the association. The core enrichment genes included PTGS1, VCL, CALD1, and ITGB3. All of these genes were down regulated in anxious versus control group.
3.6. Correlation of Axis 2 genes and reported gene sets in eudaimonia33
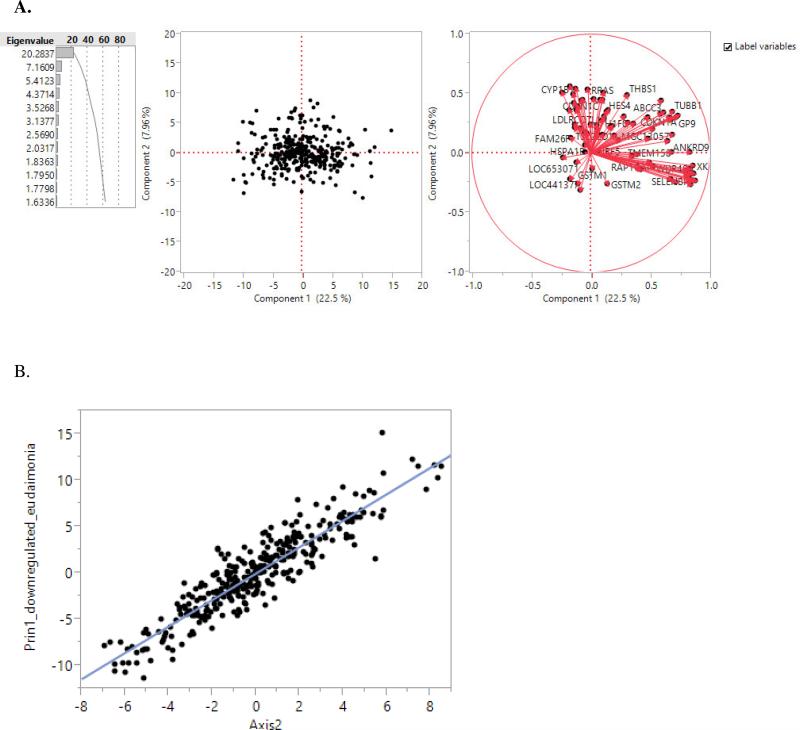
In a published study on gene expression in eudaimonia and hedonism,33 two gene sets were reported to have at least 1.5 fold difference in expression level in eudaimonic well-being. One consisted of 123 down regulated and the other 65 up regulated transcripts. We examined these two gene sets in relation to our Axis 2 genes using our data. To that end, we retrieved the gene symbols from the up and down-regulated gene sets from reference 31 to create the first principal components for them using the gene expression data from our study, and then examined their correlation with the Axis 2 scores. The first principal component of the down-regulated gene set explained 22.5% of the total variance of the gene set (Figure 4A) and was significantly and very strongly correlated with Axis 2 score at an adjusted R-square of 0.84 and p-value of 2.8×10−136 (Figure 4B). This result demonstrates that Axis 2 genes are co-regulated in the same manner as the down-regulated gene set in eudaimonia. Furthermore, visualization of the loadings on the first principal component of the down-regulated gene set (the circular plot on the far end of Figure 4A) indicates that the eudaimonia down-regulated genes are actually themselves divisible into two co-regulated subgroups. The first subgroup includes 31 genes with eigenvectors of >0.1 for the first principal component. These 31 genes include five of the 10 blood informative transcripts for Axis 2 (EPB42, IFIT1L, SELENBP1, SLC4A1, SNCA), and 31 of the 260 Axis-2 genes. Moreover, the first principal component of these 31 genes explained 56.8% of their variance and was almost identical (r>0.99; p<0.0001) to the first principal component of the full set of the 123 down-regulated genes in eudaimonia. For the up-regulated gene set, its first principal component explained only 15% of the total variance of the gene set, and was weakly and inversely correlated with Axis 2 (adjusted R-square=0.200; p<0.0001). This is as expected since the Axes are defined by positive co-variance.
Figure 4.
The first two principal components of the down-regulated gene set in eudaimonia (A). Correlation of the first principal component (Prin1) of the down-regulated gene set in eudaimonia with Axis 2 score (B) (R-square=0.84, F-statistic=1791.8, and p=2.8×10−136).
4. Discussion
We aimed to study physiological effects of generalized anxiety disorder symptoms at the molecular level by examining genome-wide blood gene expression profiles of a community sample of 336 men and women. We found that 631 genes were differentially expressed between anxious and control men at the FDR of 0.1 after we controlled for age, body mass index, race, and batch effect. Of these 631 genes, 123 had fold changes of at least 0.6 in magnitude. Gene set enrichment analysis of these 123 genes revealed that, compared to controls, anxious men had altered gene expression in biological pathways involving immune responses to acute viral or bacterial infection, as well as to influenza vaccination in a variety of immune cells, including monocytes, macrophages, myeloid dendritic cells, B cells, neutrophils, and mast cells. As mentioned earlier, clinical and epidemiological studies found that anxiety has a negative impact on immune functions11,12 as reflected by reduced immune response to vaccinations,7,8,34-36 reactivation of latent herpes virus,10 and increasing risk of infectious diseases.14,37 Our results are consistent with these epidemiological findings and shed some light on the molecular pathways in various immune cells by which anxiety can affect immune functioning.
Additionally, the above mentioned GSEA also revealed that anxious men had altered gene expression in the macrophage-enriched metabolic network that is implicated in traits of metabolic syndrome. Metabolic syndrome, defined by a cluster of traits or factors including abdominal obesity, elevated blood pressure, elevated fasting glucose, high triglyceride level, and low high-density cholesterol, is a well-known risk factor for developing cardiovascular disease.38 The higher the number of metabolic syndrome traits, the worse impact they have on cardiac function, cardiac remodeling, and carotid artery atherosclerosis.38 Our result suggests one of the molecular mechanisms underlying elevated risk of cardiovascular disease in individuals with generalized anxiety disorder observed in large prospective epidemiological studies.4-6
Intriguingly, the 631 genes differentially expressed in anxious men were not similarly expressed in anxious women in our sample. This sex difference is not unique to our study. For instance, obese men were shown to have an increased risk of acute myocardial infarction and cardiovascular death, while overweight women had a decreased risk of acute myocardial infarction in a prospective study of 4164 patients.39 In another instance, there was a sex difference in the risk of mental stress-induced myocardial ischemia in young survivors of acute myocardial infarction, with young women having twice the risk as men.40 In another study of approximately 2280 participants, elevated levels of C-reactive protein were found in anxious men but not in anxious women compared to controls.41
As gene expression profiles of human tissues typically reveal a complex and pervasive co-regulation of transcript abundance,15,16 we examined whether there is an association between the nine major axes of covariance of peripheral blood gene expression and anxiety. Interestingly, among the nine axes, only Axis 2 was significantly associated with anxiety, with higher Axis 2 scores linked to anxious state, after we adjusted for race, batch effect, gender, age, and body mass index (Figure 2). Furthermore, gene set enrichment analysis of the 260 co-regulated genes constituting Axis 2 revealed down regulation of genes involved in the maturation of dendritic cells, which provide an essential link between the innate and adaptive immune responses.42 This finding suggests an altered immune response in anxious men and women compared to healthy controls. Taken together, a common thread in our results from two analytical approaches, genome-wide differential gene expression and major axes of covariance analyses, is altered expression of genes in certain innate and adaptive immune responses in anxious persons compared to healthy controls, more prominent among men than women. Further, it is remarkable that even mild anxiety symptoms, which the majority of our participants had, were associated with discernable, and potentially detrimental, effects on the immune functions at the molecular level.
It is worth noting the implications of Axis 2 in mental and physical health. Among the nine highly conserved major axes of covariance in blood gene expression, Axis 2 was significantly associated with anxiety in our study, and with eudaimonia relative to hedonism in an independent study (Figure 4). In fact, we show here that Axis 2 captures much of the same transcriptome covariance as one component of the conserved transcriptional response to adversity (CTRA) profile identified by Cole and colleagues. The CTRA has previously been linked to social isolation, low socioeconomic status, being diagnosed with a life-threatening disease, and bereavement, as well as with psychological wellbeing.33,43,44 Our results are consistent with the idea that relatively low activity of this conserved aspect of gene expression reflects the psychological wellbeing associated with eudaimonia, while high activity with the psychological stress of anxiety. Interestingly, Axis 2 was also positively correlated with body mass index in this sample (adjusted R-square=0.06; p<0.0001), independent of anxiety, as it is in a Finnish and Australian cohort.16 In this study, Axis 2 was associated with anxiety after body mass index was adjusted for. Our earlier studies have suggested that Axis 2 may also be enriched for red blood cell and/or platelet function.16 Axis 2 has implications in mental and physical health since it is significantly associated with anxiety, eudaimonia, and body mass index. As higher body mass index has been linked to higher coronary heart disease and stroke,45 further study of Axis 2 genes in anxiety and cardiovascular risk is likely to be fruitful.
Our results should be interpreted in lights of its limitations. This is a cross-sectional study so we are unable to establish a causal relationship between the manifestation of anxiety symptoms and change in gene expression levels. Additionally, we do not have information on the duration of anxiety symptoms, which may play an important role in physical health burden. Second, participants in our study were recruited from the community, not a psychiatry or primary care clinics, and had mild to moderate anxiety symptoms. Hence our results may not be generalizable to patients with moderate to severe anxiety symptoms. Third, smoking and alcohol consumption may affect gene expression profiles, which may confound the association between differential gene expression and anxiety symptoms here. While we did not have information on smoking, history of alcoholism was one of the exclusion criteria in subject recruitment for this study.
5. Conclusions and future directions
Despite its limitations, this is the first study, to our knowledge, to examine genome-wide differential gene expression in anxiety, and associations between anxiety and the major axes of covariance in blood gene expression. We found that even mild anxiety symptoms were associated with altered gene expression levels related to innate and adaptive immune responses in men more than women. Our findings generate hypotheses for future studies focusing on biological mechanisms underlying physiological reverberations of anxiety disorders.
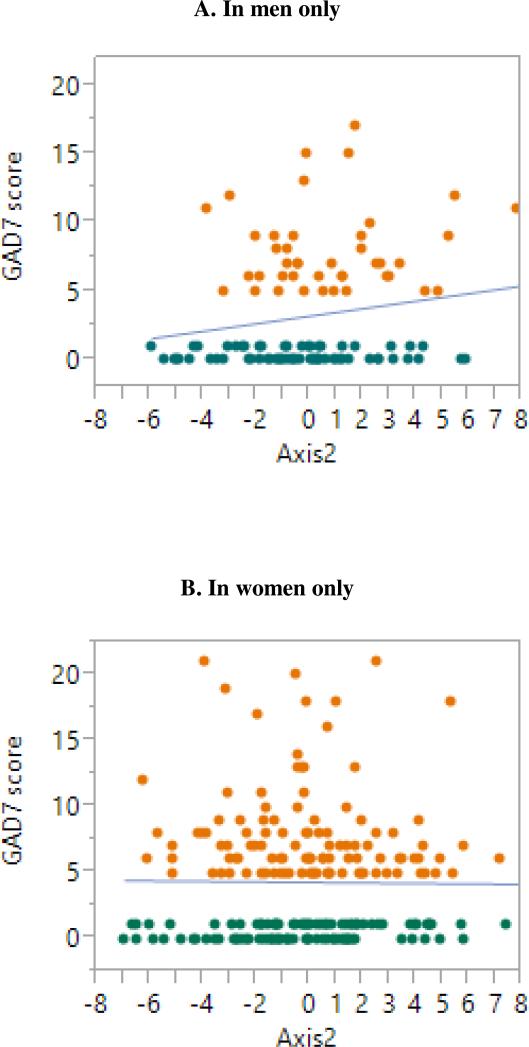
Figure 3.
GAD-7 scores versus Axis 2 in males only (A), and females only (B). Orange dots indicate participants with anxiety symptoms and green dots participants without anxiety symptoms.
Highlights.
We examined genome-wide differential gene expression in anxious versus control groups.
Anxious men had altered expression of genes involving immune response to vaccination.
Anxious men had altered expression of genes involving immune response to infection.
A set of 260 co-regulated genes was significantly associated with anxiety in men.
Even mild anxiety showed observable changes in immune-related gene expression.
Acknowledgement
Information upon which this work is based is from the Emory Predictive Health Participant Database and supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We are most grateful to all of the participants in the CHDWB for their willingness to trial this health program and to consent to analysis of their personal data. Jennifer Vazquez provided excellent logistic support and project management, ably assisted by Ashley Teal. Funding for the Predictive Health Institute was provided by Emory University, and Georgia Tech University. GG is supported in part by Project 3 of NIGMS P01 GM099568 (B. Weir, U. Washington, PI).
Funding: This study was supported in part by the Department of Veterans Affairs Career Development Award number IK2CX000601 and the NARSAD Young Investigator Award (to APW). The contents do not represent the views of the Department of Veterans Affairs or the United States Government. Gene expression data was generated with start-up funds to GG from the Georgia Tech Research Institute, and phenotypic data for the CHDWB was generated with support from the Emory University School of Medicine. GG is also supported by the NIH award P01GM099568 (Project 3).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torpy JM, Burke AE, Golub RM. JAMA patient page. Generalized anxiety disorder. Jama. 2011 Feb 2;305(5):522. doi: 10.1001/jama.305.5.522. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005 Jun 1;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. 2005. [DOI] [PubMed] [Google Scholar]
- 3.Dimsdale JE. What Does Heart Disease Have to Do With Anxiety? Journal of the American College of Cardiology. 2010;56(1):47–48. doi: 10.1016/j.jacc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Martens EJ, de Jonge P, Na B, Cohen BE, Lett H, Whooley MA. Scared to death? Generalized anxiety disorder and cardiovascular events in patients with stable coronary heart disease:The Heart and Soul Study. Arch Gen Psychiatry. 2010;67(7):750–758. doi: 10.1001/archgenpsychiatry.2010.74. [DOI] [PubMed] [Google Scholar]
- 5.Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-onset depression, anxiety, and risk of subsequent coronary heart disease: 37-year follow-up of 49,321 young Swedish men. J Am Coll Cardiol. 2010;56(1):31–37. doi: 10.1016/j.jacc.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56(1):38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Miller GE, Cohen S, Pressman S, Barkin A, Rabin BS, Treanor JJ. Psychological stress and antibody response to influenza vaccination: when is the critical period for stress, and how does it get inside the body? Psychosom Med. 2004 Mar-Apr;66(2):215–223. doi: 10.1097/01.psy.0000116718.54414.9e. [DOI] [PubMed] [Google Scholar]
- 8.Vedhara K, Cox NK, Wilcock GK, et al. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet. 1999 Feb 20;353(9153):627–631. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- 9.Glaser R, Sheridan J, Malarkey WB, MacCallum RC, Kiecolt-Glaser JK. Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosom Med. 2000 Nov-Dec;62(6):804–807. doi: 10.1097/00006842-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Cohen F, Kemeny ME, Kearney KA, Zegans LS, Neuhaus JM, Conant MA. Persistent stress as a predictor of genital herpes recurrence. Arch Intern Med. 1999 Nov 8;159(20):2430–2436. doi: 10.1001/archinte.159.20.2430. [DOI] [PubMed] [Google Scholar]
- 11.Godbout J, Glaser R. Stress-Induced Immune Dysregulation: Implications for Wound Healing, Infectious Disease and Cancer. Jrnl Neuroimmune Pharm. 2006;1(4):421–427. doi: 10.1007/s11481-006-9036-0. 2006/12/01. [DOI] [PubMed] [Google Scholar]
- 12.Arranz L, Guayerbas N, De la Fuente M. Impairment of several immune functions in anxious women. J Psychosom Res. 2007;62(1):1–8. doi: 10.1016/j.jpsychores.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Stone AA, Bovbjerg DH, Neale JM, et al. Development of common cold symptoms following experimental rhinovirus infection is related to prior stressful life events. Behavioral medicine (Washington, D.C.) 1992;18(3):115–120. doi: 10.1080/08964289.1992.9936961. Fall. [DOI] [PubMed] [Google Scholar]
- 14.Sareen J, Cox BJ, Clara I, Asmundson GJG. The relationship between anxiety disorders and physical disorders in the U.S. National Comorbidity Survey. Depression and Anxiety. 2005;21(4):193–202. doi: 10.1002/da.20072. [DOI] [PubMed] [Google Scholar]
- 15.Chaussabel D, Quinn C, Shen J, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008 Jul 18;29(1):150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preininger M, Arafat D, Kim J, et al. Blood-informative transcripts define nine common axes of peripheral blood gene expression. PLoS Genet. 2013;9(3):e1003362. doi: 10.1371/journal.pgen.1003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath AP, Arafat D, Gibson G. Using blood informative transcripts in geographical genomics: impact of lifestyle on gene expression in fijians. Frontiers in genetics. 2012;3:243. doi: 10.3389/fgene.2012.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferranti EP, Dunbar SB, Higgins M, et al. Psychosocial factors associated with diet quality in a working adult population. Research in nursing & health. 2013 Jun;36(3):242–256. doi: 10.1002/nur.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer RL, Kroenke K, Williams JBW, Lowe B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch Intern Med. 2006 May 22;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. 2006. [DOI] [PubMed] [Google Scholar]
- 20.Lowe B, Decker O, Muller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Medical care. 2008;46(3):266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz MA, Zamorano E, Garcia-Campayo J, Pardo A, Freire O, Rejas J. Validity of the GAD-7 scale as an outcome measure of disability in patients with generalized anxiety disorders in primary care. J Affect Disord. 2011;128(3):277–286. doi: 10.1016/j.jad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Mecham BH, Nelson PS, Storey JD. Supervised normalization of microarrays. Bioinformatics. 2010 May 15;26(10):1308–1315. doi: 10.1093/bioinformatics/btq118. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19(3):368–375. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 26.Nakaya HI, Wrammert J, Lee EK, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12(8):786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeffrey KL, Brummer T, Rolph MS, et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nat Immunol. 2006 Mar;7(3):274–283. doi: 10.1038/ni1310. [DOI] [PubMed] [Google Scholar]
- 28.Ramilo O, Allman W, Chung W, et al. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood. 2007 Mar 1;109(5):2066–2077. doi: 10.1182/blood-2006-02-002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Zhu J, Lum PY, et al. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008 Mar 27;452(7186):429–435. doi: 10.1038/nature06757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983 Dec;24(4):385–396. [PubMed] [Google Scholar]
- 31.Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 32.Fulcher JA, Hashimi ST, Levroney EL, et al. Galectin-1-matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J Immunol. 2006 Jul 1;177(1):216–226. doi: 10.4049/jimmunol.177.1.216. [DOI] [PubMed] [Google Scholar]
- 33.Fredrickson BL, Grewen KM, Coffey KA, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci U S A. 2013;110(33):13684–13689. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glaser R, Kiecolt-Glaser JK, Bonneau RH, Malarkey W, Kennedy S, Hughes J. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom Med. 1992 Jan-Feb;54(1):22–29. doi: 10.1097/00006842-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Morag M, Morag A, Reichenberg A, Lerer B, Yirmiya R. Psychological variables as predictors of rubella antibody titers and fatigue--a prospective, double blind study. J Psychiatr Res. 1999 Sep-Oct;33(5):389–395. doi: 10.1016/s0022-3956(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 36.Burns VE, Drayson M, Ring C, Carroll D. Perceived stress and psychological well-being are associated with antibody status after meningitis C conjugate vaccination. Psychosom Med. 2002 Nov-Dec;64(6):963–970. doi: 10.1097/01.psy.0000038936.67401.28. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 38.Antonini-Canterin F, Mateescu AD, Vriz O, et al. Impact of metabolic syndrome traits on cardiovascular function: should the Adult Treatment Panel III definition be further stratified? Journal of cardiovascular medicine (Hagerstown, Md.) 2014 Oct;15(10):752–758. doi: 10.2459/JCM.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 39.Borgeraas H, Hertel JK, Svingen GF, et al. Association of body mass index with risk of acute myocardial infarction and mortality in Norwegian male and female patients with suspected stable angina pectoris: a prospective cohort study. BMC cardiovascular disorders. 2014 May 21;14(1):68. doi: 10.1186/1471-2261-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaccarino V, Shah AJ, Rooks C, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 2014 Apr;76(3):171–180. doi: 10.1097/PSY.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Translational psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palucka K, Banchereau J. Human dendritic cell subsets in vaccination. Current opinion in immunology. 2013 Jun;25(3):396–402. doi: 10.1016/j.coi.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole SW, Conti G, Arevalo JM, Ruggiero AM, Heckman JJ, Suomi SJ. Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20578–20583. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole SW. Human Social Genomics. PLoS Genet. Aug. 2014;10(8):e1004601. doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014 Mar 15;383(9921):970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]