Abstract
Background
Eltrombopag is a thrombopoietin receptor agonist with excellent treatment outcomes in immune thrombocytopenia (ITP). Here, we analyzed the dose of eltrombopag required to achieve and maintain safe platelet counts in Korean ITP patients.
Methods
Adult refractory ITP patients (<30,000 platelets/µL) were enrolled. Eltrombopag doses were increased to achieve a target platelet count (≥50,000 cells/µL). After achieving the target platelet count, the dose of concomitant ITP medications and eltrombopag was reduced to identify the lowest effective dose required to maintain the platelet count.
Results
Among 18 patients, 66.7% achieved complete response, 5.6% achieved platelet counts between 50,000 and 100,000 cells/µL, and 27.8% failed to achieve the target platelet count. The median ITP duration was significantly shorter in patients who achieved the target platelet count. The initial dose required to achieve the target platelet count was 25 mg/d. The adjusted maintenance doses were 25 mg twice per week or 25 mg/d. After discontinuation, 83.3% relapsed, and the median relapse-free survival was 15 days. Two relapsed and 1 failed patient switched to romiplostim. The response to romiplostim was similar to eltrombopag. During eltrombopag treatment, 38.9% showed hepatobiliary laboratory anomalies. Among 9 follow-up bone marrow examinations, 1 revealed fibrosis after 1 year of treatment.
Conclusion
Eltrombopag was well tolerated with excellent treatment outcomes in refractory adult ITP patients. Low-dose eltrombopag effectively maintained the target platelet count. However, some patients required longer or higher-dose treatment to maintain the target platelet count, especially in heavily pretreated or longer ITP cases.
Keywords: Eltrombopag, Immune thrombocytopenic purpura, Platelets, Romiplostim
INTRODUCTION
Eltrombopag is a non-peptide thrombopoietin (TPO) receptor agonist that has been approved for second-line treatment of immune thrombocytopenia (ITP). It has excellent oral bioavailability, reaching a peak concentration 2-6 hours after oral administration, and a half-life of 21-32 hour [1]. Eltrombopag selectively binds to the transmembrane domain of the TPO receptor and stimulates megakaryocytopoiesis through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway [1]. In a phase 3, double-blind, placebo-controlled study of previously treated ITP patients (RAISE) [2], 79% of patients showed an optimal response to eltrombopag treatment compared with placebo treatment. A recently published long-term study of eltrombopag revealed sustained long-term efficacy and safety after 3 years of treatment [3].
For non-East Asian ITP patients, the standard starting and maximal doses of eltrombopag are 25 and 75 mg/d, respectively. However, the area under the curve after exposure to eltrombopag was 2-fold greater in healthy Japanese volunteers than in Caucasians [4], and 87% greater in ITP patients of East Asian descent than in those not of East Asian descent [5]. Thus, the recommended starting dose is 25 mg once daily, and the maximal dose is restricted to 50 mg/d for East Asian patients. A recent Japanese trial evaluated the efficacy and safety of eltrombopag with a lower starting dose (12.5 mg daily) and a maximum dose of 50 mg daily for Japanese ITP patients [6]. In that study, 22% (5/23) of patients responded to 12.5 mg of eltrombopag during the first 3 weeks of treatment, indicating the lower starting dose of eltrombopag may be effective in Japanese ITP patients.
Generally, to avoid an excessive increase in platelet counts, eltrombopag doses are carefully adjusted to maintain safe platelet counts. TPO receptor agonists, including eltrombopag, require continuous treatment to maintain the response. Notably, when TPO receptor agonists are abruptly discontinued, ITP recurs or is transiently worsened [7, 8]. In addition, adult ITP is usually a chronic disease, and the sensitivity to TPO receptor agonists may increase or decrease over time. Therefore, it is necessary to find an effective eltrombopag maintenance dose in every ITP patient.
Recent evidence suggests the absence of cross-resistance between 2 available TPO receptor agonists, eltrombopag and romiplostim [9, 10, 11]. However, the majority of clinical evidence is from case reports with limited sample sizes. The reasons for this phenomenon are currently unknown; however, one study suggested that the absence of cross-resistance between the 2 drugs is likely due to their different mechanisms of action [12].
In this study, we attempted to determine an effective dose of eltrombopag to achieve and maintain safe platelet counts and to evaluate the safety of eltrombopag in Korean adult ITP patients. Furthermore, we also evaluated the possibility of sequential treatment with romiplostim in patients who experienced relapse or were resistant to eltrombopag treatment.
MATERIALS AND METHODS
Patients and treatment protocol
Eighteen adult chronic ITP patients who were refractory to previous ITP treatments (platelet count <30,000 cells/µL) were enrolled in the study. Eligible patients were 18 years or older and had ITP for more than 3 months (persistent or chronic phases according to the new definitions of ITP [13, 14]). All patients were diagnosed with primary ITP according to the current definitions [14, 15]. Bone marrow (BM) examinations, including reticulin, Masson-trichrome, and collagen staining, were performed, and it was confirmed that none of the patients had BM fibrosis before eltrombopag treatment. Follow-up BM examinations were performed after 1 year of eltrombopag treatment or at the time of eltrombopag discontinuation. BM fibrosis grading was determined by current guidelines [16]. Approval was obtained from the institutional review boards for this study. All patients gave informed consent for the treatment and data analysis.
Treatment was started in all patients with 25 mg/d oral eltrombopag, except patients with a history of hepatobiliary laboratory abnormality (HBLA) occurring after administration of ITP medication in whom treatment was started with 12.5 mg/d oral eltrombopag. The dose was then adjusted every 1 or 2 weeks, based on the platelet counts, to maintain the target platelet count (≥ 50,000 cells/µL). When patients achieved the target platelet count, we first reduced the dose of ITP medicines being administered concomitantly with eltrombopag. We then reduced the eltrombopag dose to identify the lowest effective dose required to maintain platelet ≥ 50,000 cells/µL (maintenance dose). If the platelet count exceeded 2,500,000 cells/µL, eltrombopag administration was stopped. All patients were allowed to continue concomitant low-dose ITP medications at a stable dose, including rescue interventions.
In patients who experienced relapse or were refractory to eltrombopag, romiplostim was started at 1 µg/kg/wk, and the dose was then adjusted to achieve the target platelet count, increasing up to 10 µg/kg/wk based on the patient's platelet count.
Outcome measures and statistical analysis
The response to initial therapy was evaluated after induction or salvage chemotherapy. A complete response (CR) was defined as a platelet count of 100,000 cells/µL, following the recommended criteria [14]. Conversely, a target platelet response was defined as an increase in the platelet count ≥50,000 cells/µL, which was previously used in another large study of eltrombopag [2, 3, 17] and romiplostim [18, 19] to reduce the risk of bleeding.
For between-group comparisons, Fisher exact test (categorical data) and the Mann-Whitney U test (continuous data) were used. Relapse-free survival (RFS) was analyzed using Kaplan-Meier survival curve estimates and the logrank test to compare differences in the distribution of survival in the 3 groups. A P value <0.05 was considered statistically significant. All statistical computations were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL).
RESULTS
Patient characteristics
Eighteen ITP patients were enrolled and treated with eltrombopag from September 2009 to July 2014. According to the current definitions of ITP [13, 14], 7 patients were in the persistent phase and 11 were in the chronic phase (Table 1). The median age was 55 years (range, 30-71 years), and approximately two-thirds of patients were women. The median platelet count at baseline was 14,000 cells/µL (range, 1,000-28,000 cells/µL). The median number of prior ITP treatments was 3 (range, 2-9), including 4 patients who had undergone a splenectomy. The median ITP duration before eltrombopag treatment was 28 months (range, 3.0-174.2 months). At baseline, all patients received concomitant ITP medications. Low-dose corticosteroids were the most common concomitant ITP medication (88.9%). Other therapies included cyclosporine, oxymetholone, and danazol. The median duration of eltrombopag treatment was 5.6 months (range, 1.2-42.3 months), and the median follow-up time was 40.5 months (range, 1.2-59.4 months).
Table 1. Baseline characteristics and treatment outcomes of eltrombopag treatment in adults with refractory chronic ITP (N=18).
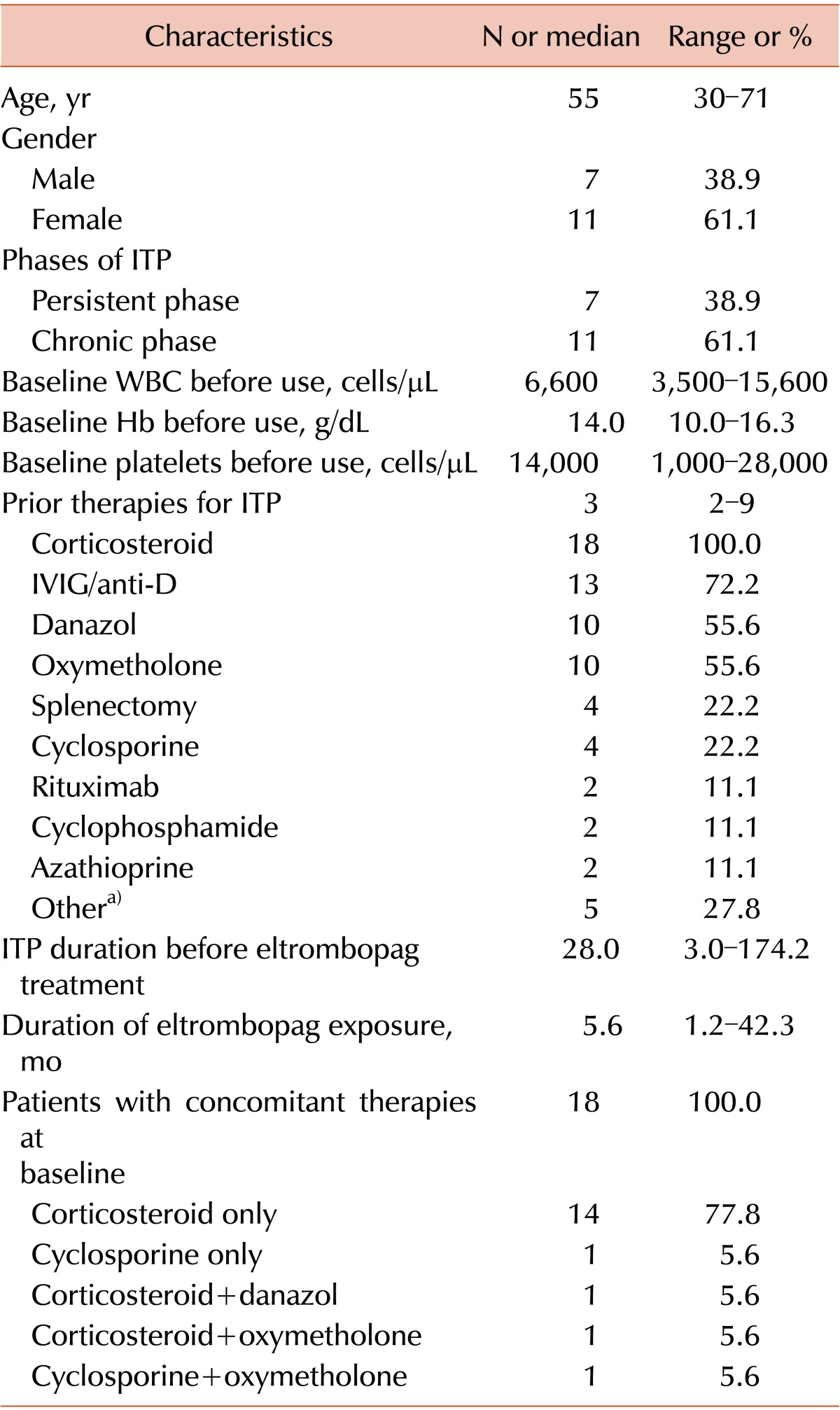
a)Other therapies for ITP include Helicobacter pylori eradication, azathioprine, etoposide, thalidomide, and dapsone.
Abbreviations: ITP, immune thrombocytopenia; IVIG, intravenous immunoglobulin; anti-D, anti-D immunoglobulin.
Efficacy of eltrombopag
Sixteen patients (88.9%) started eltrombopag at a dose of 25 mg/d, and 2 patients (11.1%) started at 25 mg every other day (EOD) because of previously detected hepatic problems (Table 2). The dose was increased every 1 or 2 weeks to achieve a target platelet count ≥50,000 cells/µL. Among the 18 patients, 12 (66.7%) achieved CR (platelet counts ≥100,000 cells/µL), 1 (5.6%) achieved a platelet between 50,000 and 100,000 cells/µL, and 5 (27.8%) failed to achieve the target platelet count (<50,000 cells/µL). Among patients who had previously received ≥4 ITP treatments, 57.1% achieved the target platelet count. Among non-splenectomized patients, 85.7% (12/14) achieved the target platelet count; however, only 25.0% (1/4) of splenectomized patients achieved more than 50,000 platelets/µL (P <0.05).
Table 2. Therapeutic responses to eltrombopag.
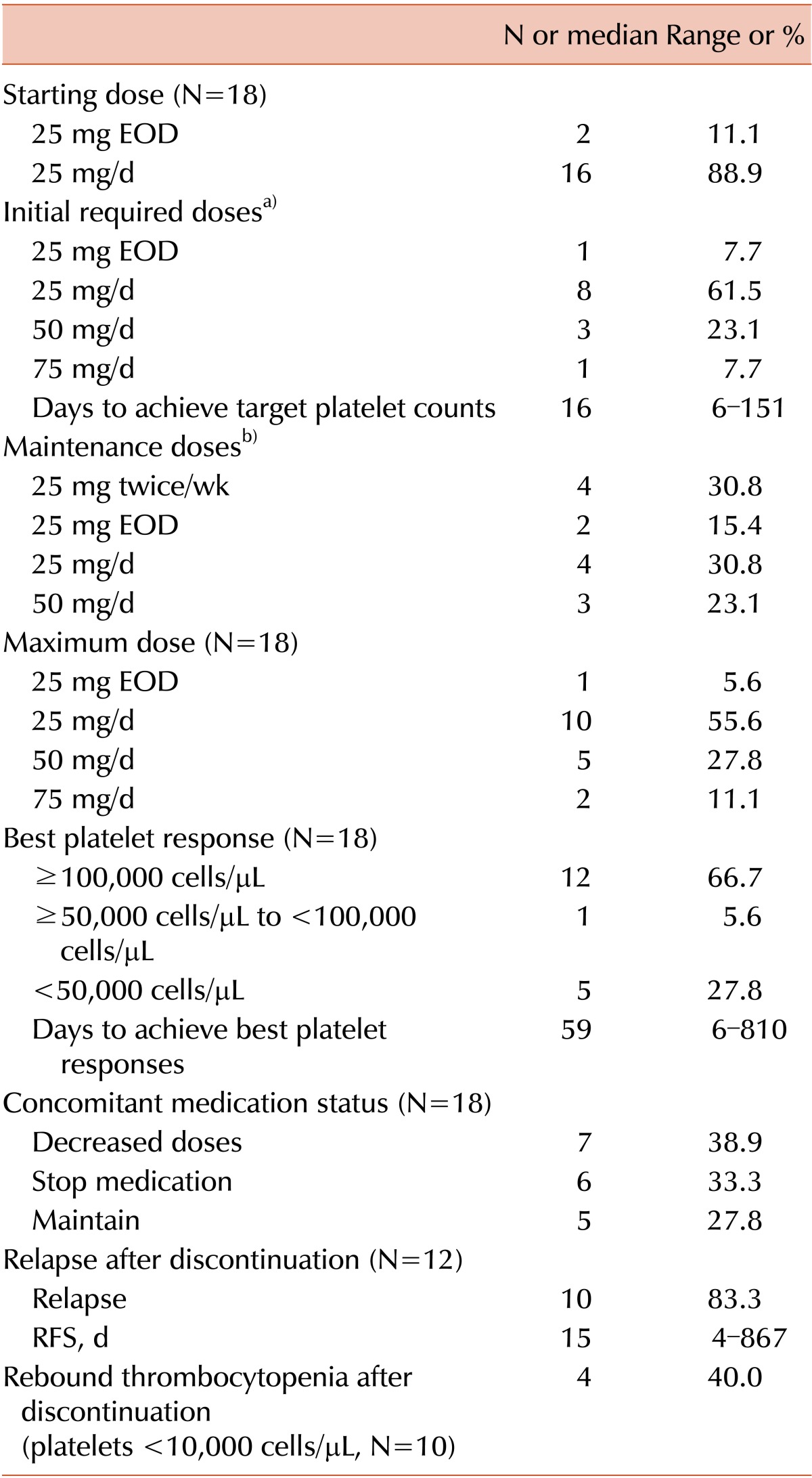
a)Initial required doses to achieve target platelet count (≥50,000 cells/mL, N=13). b)Doses to maintain target platelet count (≥ 50,000 cells/mL, N=13).
Abbreviations: EOD, every other day; RFS, relapse-free survival.
The most common initial dose required to achieve the target platelet count was 25 mg/d (61.5%); others were 25 mg EOD (7.7%), 50 mg/d (23.1%), and 75 mg/d (7.7%). Although the maximal dose recommended for East Asians is 50 mg/d, 1 patient required 75 mg/d to achieve the target platelet count. The patient was diagnosed with ITP 60.8 months previously and received 9 ITP treatments before starting eltrombopag.
The median time required to achieve the target platelet counts was 16 days (6-151 days). The median ITP duration before eltrombopag treatment was significantly shorter in patients who achieved the target platelet counts than in the failed patients (25.1 vs. 97.9 months, P <0.05). Six (33.3%) patients stopped and 7 (38.9%) reduced the dose of concomitant ITP medications.
In the 13 patients who responded, 12 transiently discontinued eltrombopag treatment. Among these patients, 10 (83.3%) relapsed, with a median RFS of 15 days (4-867 days). Severe rebound thrombocytopenia (platelet<10,000 cells/µL) was detected in 4 patients (40.0%). These 10 relapsed patients restarted eltrombopag and maintained the lowest dose required to maintain the target platelet count. After achieving the target count, the adjusted maintenance dose was 25 mg twice per week (30.8%) or 25 mg/d (30.8%), with others requiring 25 mg EOD (15.4%) and 50 mg/d (23.1%).
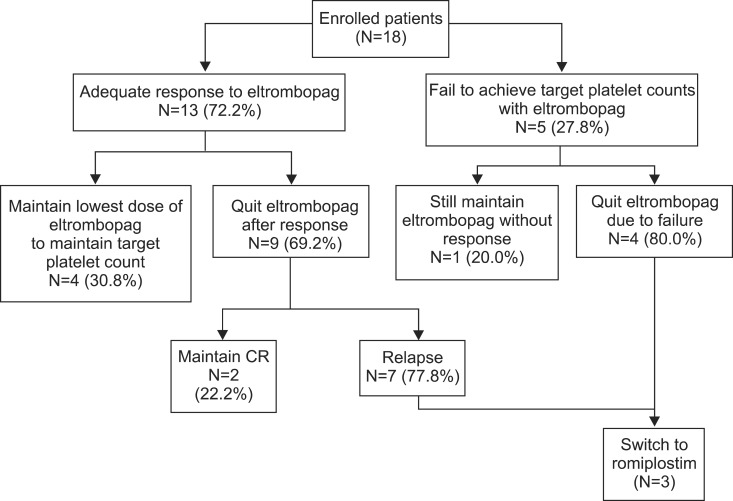
Of the 18 enrolled patients, 4 patients (22.2%) remained at the lowest eltrombopag dose required to maintain the target platelet count (Fig. 1). Among the 9 patients who stopped eltrombopag after a response, 2 (22.2%) maintained a CR without ITP treatment (CR duration was 35.9 and 1.2 months, respectively), and 7 (77.8%) relapsed after the response. Among the 5 patients who failed to achieve the target platelet count, 4 patients stopped eltrombopag and 1 patient was in plan to use a higher dose.
Fig. 1. Summary of patient disposition in eltrombopag-treated patients (N=18). Among 5 patients who failed to achieve the target platelet count, 4 patients quit eltrombopag treatment and 1 increased the eltrombopag dosage. Among 13 patients who responded, 4 required the lowest dose of eltrombopag to maintain the target platelet counts and 9 quit eltrombopag treatment. Two of these 9 patients maintained CR, and 7 experienced a relapse. Two eltrombopag-resistant patients and 1 relapse patient were treated with romiplostim. Abbreviation: CR, complete response.
Eltrombopag safety
Seven patients (38.9%) showed HBLA, including 2 grade 3 cases, after eltrombopag treatment. However, HBLA resolved after reduction or short-term discontinuation of eltrombopag, and there was no reappearance after treatment was restored. Six patients who were treated with eltrombopag for more than 1 year and 3 patients who stopped eltrombopag within 1 year underwent follow-up BM assessment. One patient treated with eltrombopag for 1 year revealed grade 1 BM fibrosis (BM cellularity, 40%-50%; blasts, <5%; cytogenetics, 46,XY); however, there was no significant hematologic abnormality or lactate dehydrogenase elevation in the peripheral blood and no definitive abnormal immature cell clusters in the BM specimen. The BM examination of this patient after 2 years of eltrombopag treatment revealed no significant change. There were no severe bleeding episodes during eltrombopag treatment (Table 3).
Table 3. Adverse events during eltrombopag treatment.
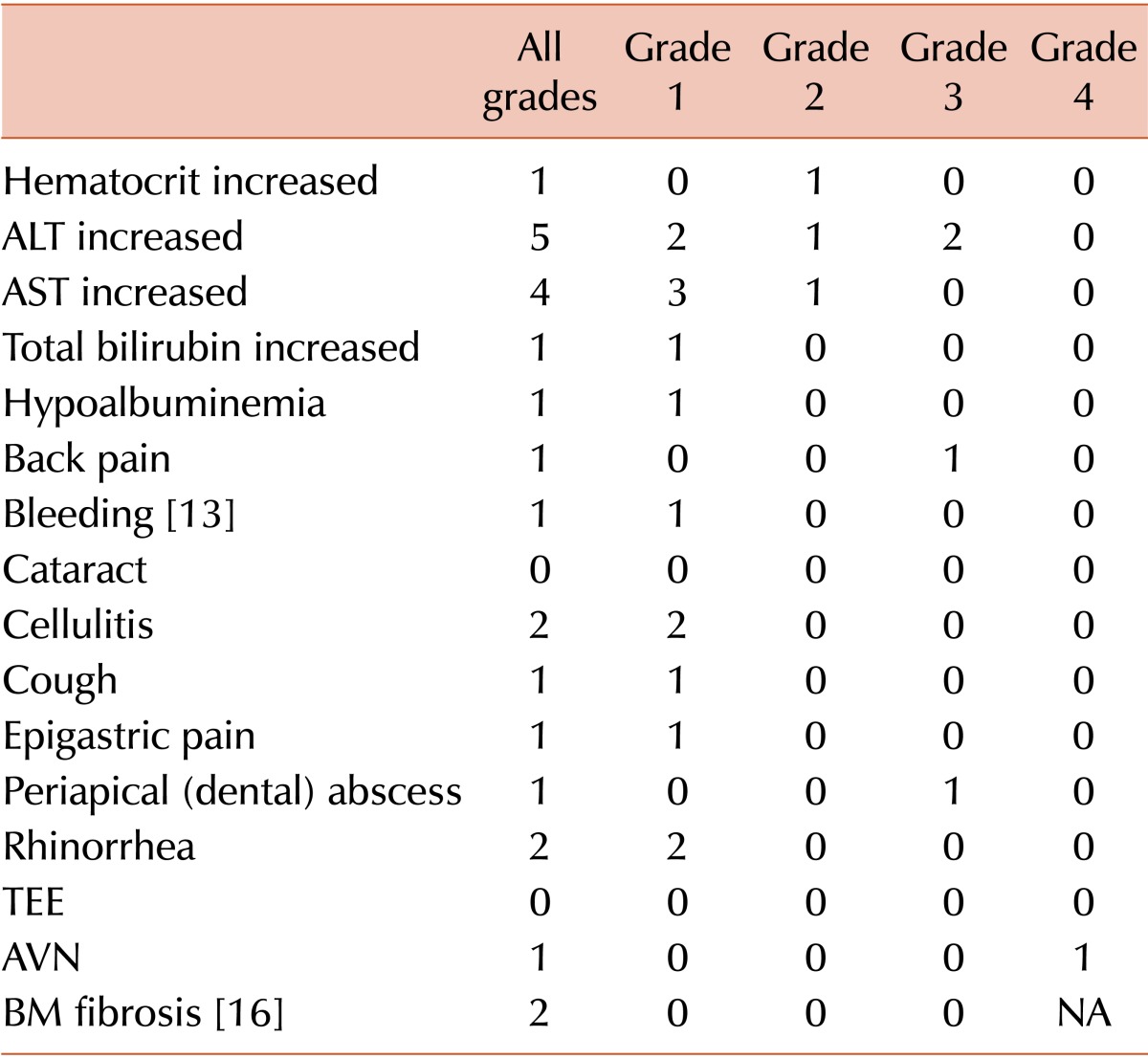
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TEE, thromboembolic event; AVN, avascular necrosis; BM, bone marrow; NA, not applicable.
Efficacy and safety of switching to romiplostim
Two relapsed and 1 failed patient received romiplostim as a salvage treatment (Table 4). Two patients who relapsed after an eltrombopag-induced CR also showed a CR with romiplostim treatment. One relapsed shortly after the discontinuation of romiplostim and the other maintained the CR (7.5 months). In contrast, 1 eltrombopag-resistant patient also showed a poor response with romiplostim. No grade 2 or higher adverse events were observed during romiplostim treatment.
Table 4. Summary of 3 patients switching to romiplostim from eltrombopag.
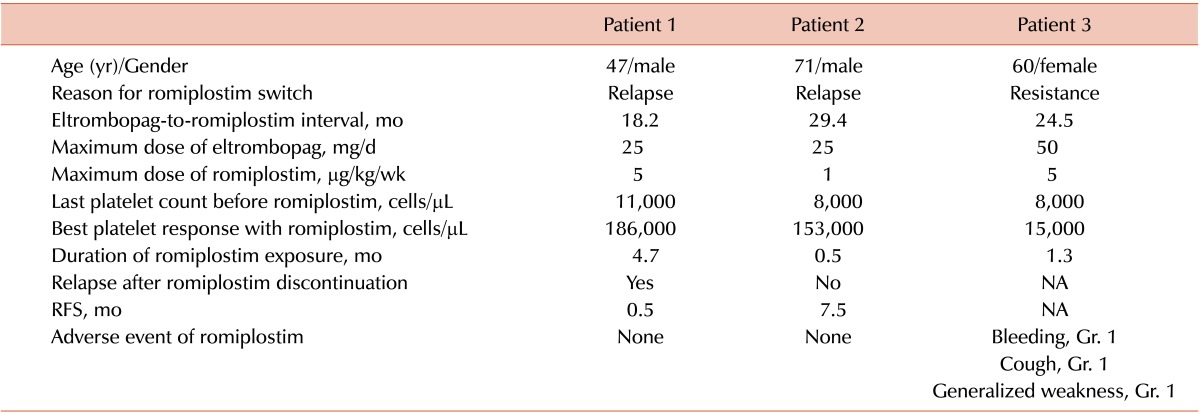
Abbreviations: CR, complete response; RFS, relapse-free survival; NA, not applicable; Gr, grade.
DISCUSSION
In this study, eltrombopag showed an excellent treatment outcome in heavily pretreated, refractory adult ITP patients. However, compared with the recently published Eltrombopag Extended Dosing Study (EXTEND), which evaluated eltrombopag for 3 years [3], the number of patients that achieved target platelet counts at any time during treatment in our study was slightly lower (85.0% vs. 72.2%). Furthermore, most large, randomized trials for TPO-receptor agonists, including eltrombopag, showed similar platelet responses regardless of splenectomy status [2, 3, 17, 18, 19]. However, although the number of splenectomized patients was small (N=4) in this study, a higher proportion of non-splenectomized patients achieved the target platelet count compared with splenectomized patients.
Based on estimates from a population-based pharmacokinetics model, systemic exposure to eltrombopag is increased in people of East Asian descent [4, 5]. Therefore, a lower starting dose (25 mg/d) and a maximal dose (50 mg/d) are generally recommended. One randomized Japanese trial also suggested that a lower starting dose (12.5 mg/d) may be efficacious in some Japanese patients [6]. In our study, 1 patient achieved the target platelet count, but the most common initial dose required to achieve the target platelet count was 25 mg/d (N=8, 61.5%). Moreover, some patients needed a higher dose (up to 75 mg/d) to achieve the target platelet count.
In our study, the median time to achieve the target platelet count was 16 days (6-151 days). Ten patients reached the target platelet count within 1 month; however, 3 patients required more than 1 month of treatment (48, 95, or 151 days). One required a dose elevation to 75 mg/d. Therefore, the maximum dose and duration of treatment should be personalized. There was no significant difference in the rate of achieving the target platelet count; however, our study showed that the median ITP duration before eltrombopag treatment was significantly shorter in patients who achieved the target platelet counts compared with failed patients (25.1 vs. 97.9 months, P <0.05).
After terminating TPO receptor agonist treatment, including eltrombopag, platelet counts usually return to baseline levels within 2 weeks. In one eltrombopag discontinuation trial, almost one-third of the patients who discontinued eltrombopag treatment maintained adequate platelet counts for at least 6 months [20]. In our study, 12 patients achieving CR transiently stopped eltrombopag treatment. However, 83.3% of the patients relapsed after eltrombopag discontinuation. The median RFS was 15 days, and 5 patients relapsed within 1 week. Although there were no grade 2 bleeding episodes, 4 of the 10 relapsed patients experienced severe rebound thrombocytopenia (<10,000 cells/µL). Thus, it is necessary to increase the platelet monitoring frequency to once or twice weekly after terminating eltrombopag treatment.
The sensitivity of patients to TPO receptor agonists may increase or decrease over time, and excess platelet elevation should be avoided. Therefore, the dose should be adjusted based on the patient's platelet count. A recently published case report suggests that 25 mg 2-3 times per week may be useful to maintain long-term CR [21]. We also tried to identify the lowest eltrombopag dose required to maintain the target platelet count. In our patients, 53.8% (N=7) required 25 mg/d or more to maintain the target platelet count; however, in 4 (30.8%) and 2 (15.4%) patients, 25 mg 2 times per week or 25 mg EOD, respectively, was enough to maintain the target platelet count.
The most common adverse event was HBLA. There were 2 grade 3 alanine aminotransferase (ALT) elevations that caused transient discontinuation of eltrombopag. However, in these patients, the elevated ALT returned to normal levels after treatment discontinuation, and there was no relapse after readministration. One patient underwent a total hip replacement due to avascular necrosis of the femoral head, which was diagnosed before eltrombopag treatment. The second patient experienced epigastric pain; however, there were no causal findings on endoscopic and abdominal computed tomographic examinations. In our study, no BM fibrosis was observed before eltrombopag treatment, but 1 patient showed grade 1 fibrosis after 1 year of eltrombopag treatment. However, there were no definitive abnormal immature cell clusters, and the peripheral blood smear revealed no significant abnormalities. A follow-up BM examination after 2 years of eltrombopag treatment showed a similar result.
Several reports have suggested that there is no cross-resistance between the TPO receptor agonists eltrombopag and romiplostim [9, 10, 11, 12, 22, 23, 24, 25]. The reason for this phenomenon is currently unknown, but it may be a result of different binding sites of the 2 TPO receptor agonists. Romiplostim competes with endogenous TPO for the extracellular binding site, whereas eltrombopag binds to the transmembrane domain [12, 26]. Thus, if the first TPO receptor agonist has no effect, switching to the other may be useful. In our study, 2 patients that relapsed after CR and 1 resistant patient switched to romiplostim. The 2 relapsed patients also showed a CR after romiplostim treatment. However, although we could not try the highest recommended dose (10 µg/kg/wk) of romiplostim, the eltrombopag-resistant patient also failed to achieve the target platelet count with romiplostim. To confirm these findings in our patients, a larger trial may be required.
In conclusion, eltrombopag is generally well tolerated and effectively achieves target platelet counts in refractory adult ITP patients. Low doses of eltrombopag were effective in maintaining safe platelet counts in most patients. However, some patients needed a longer time and higher dose to achieve and maintain the target platelet count, especially in heavily pretreated patients with a longer ITP duration before treatment.
Footnotes
This study was supported by a grant (CRI10014-1) from the Chonnam National University Hospital Biomedical Research Institute.
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Cheng G. Eltrombopag, a thrombopoietin-receptor agonist in the treatment of adult chronic immune thrombocytopenia: a review of the efficacy and safety profile. Ther Adv Hematol. 2012;3:155–164. doi: 10.1177/2040620712442525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377:393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 3.Saleh MN, Bussel JB, Cheng G, et al. EXTEND Study Group. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121:537–545. doi: 10.1182/blood-2012-04-425512. [DOI] [PubMed] [Google Scholar]
- 4.Shida Y, Takahashi N, Nohda S, Hirama T. Pharmacokinetics and pharmacodynamics of eltrombopag in healthy Japanese males. Jpn J Clin Pharmacol Ther. 2011;42:11–20. [Google Scholar]
- 5.Gibiansky E, Zhang J, Williams D, Wang Z, Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011;51:842–856. doi: 10.1177/0091270010375427. [DOI] [PubMed] [Google Scholar]
- 6.Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012;10:799–806. doi: 10.1111/j.1538-7836.2012.04695.x. [DOI] [PubMed] [Google Scholar]
- 7.Siegal D, Crowther M, Cuker A. Thrombopoietin receptor agonists in primary immune thrombocytopenia. Semin Hematol. 2013;50(Suppl 1):S18–S21. doi: 10.1053/j.seminhematol.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghadaki B, Nazi I, Kelton JG, Arnold DM. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion. 2013;53:2807–2812. doi: 10.1111/trf.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki T, Harada Y, Matsubara E, et al. Thrombopoietin receptor agonists in refractory immune thrombocytopenia: differential responses to eltrombopag and romiplostim: a case report and possible explanations. J Clin Pharm Ther. 2012;37:729–732. doi: 10.1111/j.1365-2710.2012.01353.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsukamoto S, Nakaseko C, Takeuchi M, et al. Safety and efficacy of romiplostim in patients with eltrombopag-resistant or -intolerant immune thrombocytopenia. Br J Haematol. 2013;163:286–289. doi: 10.1111/bjh.12483. [DOI] [PubMed] [Google Scholar]
- 11.Sartori R, Candiotto L, Ruggeri M, Tagariello G. Immune thrombocytopenia successfully treated with eltrombopag following multiple therapies including romiplostim. Blood Transfus. 2014;12(Suppl 1):s151–s152. doi: 10.2450/2013.0135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polverelli N, Palandri F, Iacobucci I, Catani L, Martinelli G, Vianelli N. Absence of bi-directional cross-resistance of thrombopoietin receptor agonists in chronic refractory immune thrombocytopenia: possible role of MPL polymorphisms. Br J Haematol. 2013;161:142–144. doi: 10.1111/bjh.12186. [DOI] [PubMed] [Google Scholar]
- 13.Rodeghiero F, Michel M, Gernsheimer T, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121:2596–2606. doi: 10.1182/blood-2012-07-442392. [DOI] [PubMed] [Google Scholar]
- 14.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 15.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 16.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90:1128–1132. [PubMed] [Google Scholar]
- 17.Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:641–648. doi: 10.1016/S0140-6736(09)60402-5. [DOI] [PubMed] [Google Scholar]
- 18.Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 19.Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161:411–423. doi: 10.1111/bjh.12260. [DOI] [PubMed] [Google Scholar]
- 20.Leven E, Miller A, Boulad N, Haider A, Bussel JB. Successful discontinuation of eltrombopag treatment in patients with chronic ITP. Blood. 2012;120(ASH Annual Meeting):abst 1085. [Google Scholar]
- 21.Vlachaki E, Sousos N, Perifanis V, et al. Is there a role for low-dose eltrombopag as maintenance therapy in the treatment of immune thrombocytopenia? Acta Haematol. 2015;133:78–82. doi: 10.1159/000361075. [DOI] [PubMed] [Google Scholar]
- 22.Khellaf M, Viallard JF, Hamidou M, et al. A retrospective pilot evaluation of switching thrombopoietic receptor-agonists in immune thrombocytopenia. Haematologica. 2013;98:881–887. doi: 10.3324/haematol.2012.074633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scaramucci L, Giovannini M, Niscola P, Tendas A, Perrotti A, De Fabritiis P. Reciprocal absence of cross-resistance between eltrombopag and romiplostim in two patients with refractory immune thrombocytopenic purpura. Blood Transfus. 2014;12:605–607. doi: 10.2450/2014.0246.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccin A, Amaddii G, Natolino F, Billio A, Cortelazzo S. Idiopathic thrombocytopenic purpura resistant to eltrombopag, but cured with romiplostim. Blood Transfus. 2014;12(Suppl 1):s149–s150. doi: 10.2450/2013.0289-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Arena G, Guariglia R, Mansueto G, et al. No cross-resistance after sequential use of romiplostim and eltrombopag in chronic immune thrombocytopenic purpura. Blood. 2013;121:1240–1242. doi: 10.1182/blood-2012-11-465575. [DOI] [PubMed] [Google Scholar]
- 26.Garnock-Jones KP, Keam SJ. Eltrombopag. Drugs. 2009;69:567–576. doi: 10.2165/00003495-200969050-00005. [DOI] [PubMed] [Google Scholar]