Abstract
Background
Although deferasirox (DFX) is reported to have anti-tumor effects, its anti-leukemic activity remains unclear. We evaluated the effect of DFX treatment on two murine lymphoid leukemia cell lines, and clarified the mechanisms underlying its potential anti-leukemic activity.
Methods
L1210 and A20 murine lymphoid leukemia cell lines were treated with DFX. Cell viability and apoptosis were evaluated by the 3-(4,5-dimethylthaizol-2-yl)-5-(3-carboxymethylphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay and fluorescence-activated cell sorting (FACS) analysis, respectively. Immunoblotting was performed to detect the expression of key apoptotic proteins.
Results
In dose- and time-dependent manner, DFX decreased viability and increased apoptosis of murine leukemic cells. Fas expression was significantly higher in A20 cells than in L1210 cells at all DFX concentrations tested. Although both cell lines exhibited high caspase 3 and caspase 9 expression, a critical component of the intrinsic mitochondrial apoptotic pathway, expression was greater in L1210 cells. In contrast, caspase 8, a key factor in the extrinsic apoptotic pathway, showed greater expression in A20 cells. Cytochrome c expression was significantly higher in L1210 cells. In both cell lines, co-treatment with ferric chloride and DFX diminished the expression of these intracellular proteins, as compared to DFX treatment alone.
Conclusion
Treatment with DFX increased caspase-dependent apoptosis in two murine lymphoid leukemia cell lines, with differing apoptotic mechanisms in each cell line.
Keywords: Lymphoid leukemia, Deferasirox, Apoptosis, Caspase
INTRODUCTION
Hematological malignancies such as acute leukemia require frequent blood transfusions [1]. Multiple blood transfusions may lead to secondary iron overload (SIO), which promotes cancer cell growth and induces deleterious effects on the innate immune system [2,3,4]. An iron-chelating agent (ICA) is used to minimize the adverse effects of iron overload in patients with SIO who require repeated blood transfusions [5, 6].
Deferoxamine (DFO) and deferasirox (DFX) are ICAs with different iron-chelating mechanisms. DFO has a short half-life, and must be injected intravenously or subcutaneously, because it is a large and highly hydrophilic molecule [7, 8]. In contrast, DFX has relatively high lipophilicity and is an orally administered iron chelator that was developed to treat transfusional iron overload. Previous work indicates that DFX is safe and effective for the reduction of iron overload [8, 9].
Various malignant cells have been used to study the anti-tumor effect of ICAs [10, 11]. Messa et al. demonstrated that DFX exerts anti-leukemic effects in a myeloid leukemia cell line by inhibiting nuclear factor-κB (NF-κB) activity [12]. Upregulation of regulated in development and DNA damage responses 1 (REDD1) in the mammalian target of rapamycin (mTOR) pathway in DFX-treated myeloid leukemia cells may also have an anti-proliferative effect [13].
However, studies on the potential anti-leukemic effects of DFX in lymphoid malignancies remain limited. Previously, we reported that DFX has anti-leukemic effects in murine lymphoid leukemia cell lines [3]. In this study, we sought to validate the anti-leukemic activity of DFX in murine lymphoid leukemia cell lines, and clarify the intracellular apoptotic mechanisms that are responsible for the potential anti-leukemic effect.
MATERIALS AND METHODS
Cell lines, cell culture, and DFX treatment
The murine leukemia cell lines A20 (H-2d, BALB/c origin) and L1210 (H-2d, DBA origin) were purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured in RPMI 1640 (Gibco/Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% antibiotics (Invitrogen, Carlsbad, CA, USA). The cells were maintained in a humidified atmosphere containing 5% CO2 at 37℃. Cells were passaged 2-3 times per week by dilution to 1×105 cells/mL. DFX was provided by Novartis (Basel, Switzerland). A DFX stock solution was prepared in dimethyl sulfoxide (Sigma-Aldrich, Schmelldorf, Germany), 0.1M NaOH, and RPMI 1640. The stock solutions were further diluted in RPMI 1640 for cell culture experiments.
MTS cell proliferation assay
L1210 and A20 cell growth was examined by a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay, and the results are expressed as a percentage of control values. The MTS assay was conducted using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). Cells (105 cells/well) were seeded in flat-bottom 96-well plates and maintained in an incubator (5% CO2/95% air) at 37℃. After 24 hr incubation, cells were treated with 3.125, 6.25, 12.5, 25, 50, or 75 µM DFX, with or without 20 µM FeCl3, for 24 and 48 h. MTS was added, and the plates were returned to the incubator at 37℃ for 4 hr. The plates were read at various time intervals at a wavelength of 490 nm. Absorbance was recorded using a 96-well spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Color formation was proportional to the viable cell number.
Fluorescence-activated cell sorting (FACS) analysis
Flow cytometry was performed to confirm apoptosis and Fas expression. Cells (1×106 cells/mL) were seeded in 6-well plates and maintained in an incubator (5% CO2/95% air) at 37℃ for 24 h. The apoptosis assay was conducted using the FITC Annexin V/PI Apoptosis Detection Kit (BD Biosciences, San Diego, CA, USA). The cells were treated with 0, 12.5, 25, or 50 µM DFX, with or without 20 µM FeCl3, for 24 and 48 hr. The cells were collected and washed twice with cold phosphate-buffered saline (PBS) and resuspended in 1× Binding Buffer (1×106 cells/mL). An aliquot of the cell suspension (100 µL; 1×105 cells) was transferred to a FACS tube, and 5 µL fluorescein isothiocyanate-conjugated (FITC) Annexin V and 5 µL propidium iodide (PI) were added. The cells were vortexed and incubated for 10-15 min at room temperature in the dark. The cells were washed in 1× Binding Buffer and resuspended in 400 µL of 1× Binding Buffer. The cells were harvested 24 hr after DFX treatment to determine CD95 expression. Cells were washed once with PBS and Hanks' balanced salt solution (Gibco/Invitrogen) containing 5% FBS. The cells were stained with CD95 (Fas) (eBioscience, San Diego, CA, USA) for 20 min at 4℃. The cells were then washed and stained with FITC anti-mouse (BD Sciences). All data were analyzed by the FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
Immunoblotting analysis
L1210 and A20 cells were seeded in a 6-well plate (1×106 cells/mL) and maintained in an incubator (5% CO2/95% air) at 37℃ for 24 hr. The cells were treated with 50 µM DFX with or without 20 µM FeCl3 for 0, 6, 12, 24, and 48 hr. The cells were collected, washed twice with cold PBS, and lysed in radioimmunoprecipitation assay buffer (Thermo Scientific, Rockford, IL, USA) containing a mixture of protease inhibitors (Thermo Scientific) for 15 min on ice. After centrifugation, the supernatants underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA, USA). After electrophoresis, the gels were blotted onto a membrane (Whatman GmbH, Dassel, Germany) and blocked with 5% skim milk (BD Sciences) for 30 min at room temperature. The membranes were incubated overnight at 4℃ with primary antibodies. The proteins were analyzed by western blot using anti-caspase-3 (Abcam, Cambridge, MA, USA), anti-caspase-9 (Novus Biologicals, LLC., Wilmington, DE, USA), anti-caspase-8 (Abcam), anti-Bax (Abcam), anti-β-actin (Abcam), and anti-poly ADP ribose polymerase (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing with 1× Tris-buffered saline with Tween 20 (Cell Signaling Technology, Beverly, MA, USA) three times for 10 min, the proteins were probed at room temperature for 2 hr with anti-mouse and anti-rabbit secondary antibodies (Santa Cruz Biotechnology). Immunoreactive proteins were detected with the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and the Western Blotting Detection System (Biomics, Hayward, CA, USA). Mitochondria were isolated for the cytochrome assay using a Qproteome Mitochondria Isolation kit (Qiagen, Hilden, Germany). The two cell lines were probed with anti-cytochrome c (Abcam), and the Chemi-DOC system (Biomics) was used to detect signals.
Statistical analysis
Data are presented as the mean±standard deviation of three independent experiments. Statistical analyses were performed using GraphPad Prism ver. 5.0 software (GraphPad Software, San Diego, CA, USA). Comparisons among the groups were performed by analysis of variance with SPSS ver. 12.0 for Windows (SPSS Inc., Chicago, IL, USA). A P value<0.05 was considered significant.
RESULTS
Effect of DFX on murine leukemia cell viability
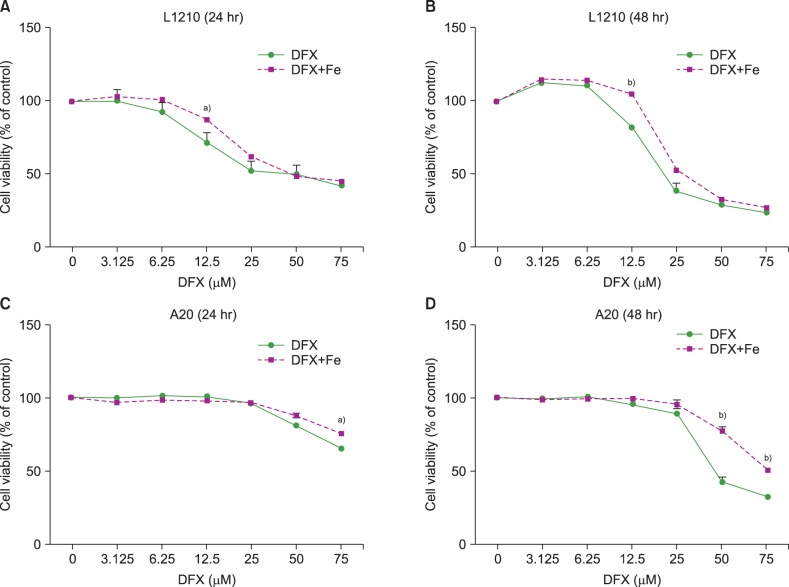
The viability of both L1210 and A20 cells decreased upon DFX exposure (Fig. 1). Longer treatment (48 hr vs. 24 hr) at higher DFX concentrations resulted in a greater decrease in cell viability. Of note, cell viability in L1210 cells decreased at lower DFX concentrations than A20 cells, in which a significant decrease in viability was only observed when treated with 50 µM DFX for 48 hr. The addition of FeCl3 preserved cell viability in the presence of 12.5 µM DFX for 24 and 48 hr in L1210 cells, and 50 µM DFX for 48 hr in A20 cells.
Fig. 1. Viability of L1210 (A, B) and A20 cells (C, D) after DFX treatment with or without FeCl3, according to treatment time (24 vs. 48 hr) and DFX concentration (3.125-75 µM). Experiments were performed in triplicate. All data are presented as the mean±standard error (a)P<0.05, b)P<0.01).
Effect of DFX on murine leukemia cell apoptosis
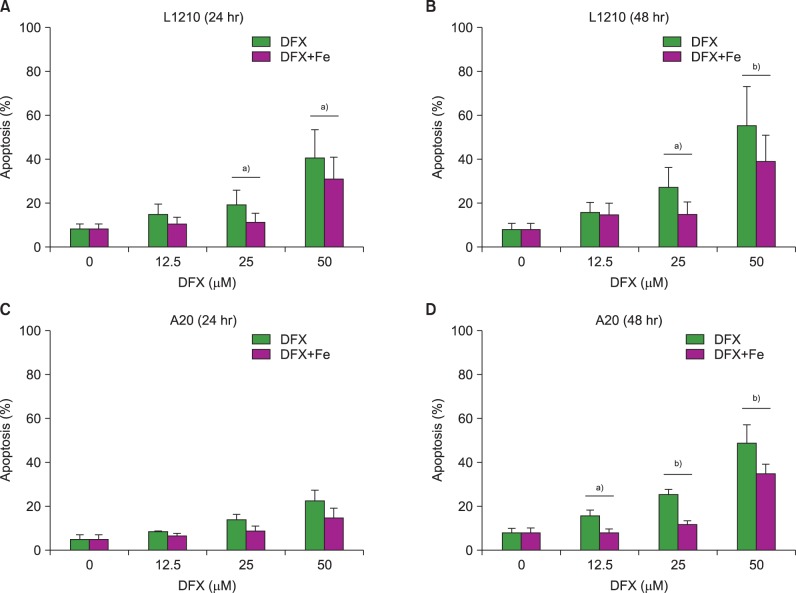
Apoptosis assays were performed to clarify whether increased cell apoptosis induced decreased cell viability following DFX treatment. The assays revealed an increase in apoptosis after DFX treatment in both murine leukemia cell lines (Fig. 2). In accordance with the proliferation assay results, lengthier treatment (48 hr vs. 24 hr) at higher DFX concentrations resulted in a greater percentage of apoptotic cells in both cell lines. As with the cell viability assay, the addition of FeCl3 significantly decreased apoptosis at certain DFX concentrations (Fig. 2).
Fig. 2. Measurement of apoptosis in L1210 (A, B) and A20 cells (C, D) after DFX treatment with or without FeCl3, according to treatment time (24 vs. 48 hr) and DFX concentration (12.5-50 µM). Experiments were performed in triplicate. All data are presented as the mean±standard error (a)P<0.05, b)P<0.01).
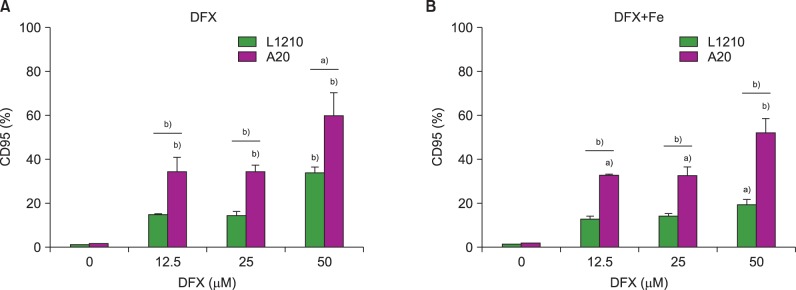
Effect of DFX on Fas expression in murine leukemia cells
Both leukemia cell lines were analyzed for CD95 (Fas) expression after DFX treatment to characterize the mechanisms regulating DFX-mediated cell apoptosis. CD95 expression increased in both L1210 and A20 cells upon treatment with DFX. Of note, A20 cells displayed a significantly greater percentage of CD95-expressing cells than the L1210 cell line for all DFX concentrations, regardless of FeCl3 addition (Fig. 3).
Fig. 3. Measurement of CD95 (Fas) expression in L1210 and A20 cells after 24 h DFX treatment with or without FeCl3, according to DFX concentration (12.5-50 µM). Experiments were performed in triplicate. All data are presented as the mean±standard error (a)P<0.05, b)P<0.01).
Expression of apoptotic proteins in DFX-treated murine leukemia cells
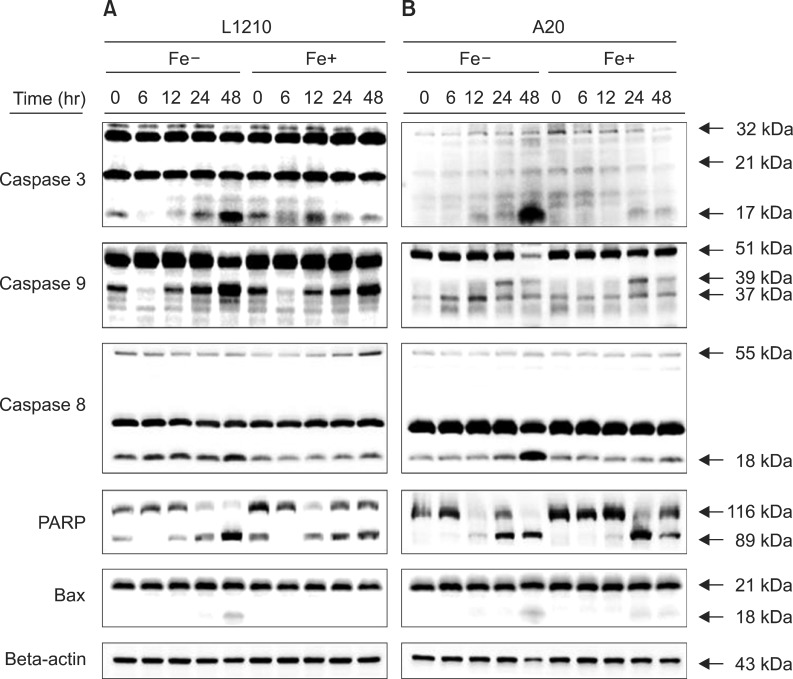
The expression of apoptotic proteins including caspases, poly ADP ribose polymerase (PARP), and Bax proteins was determined after treating the leukemia cell lines with 50 µM DFX at sequential time periods up to 48 hr treatment. Caspase 3 expression increased upon prolonged DFX treatment in both cell lines, with peak expression observed after 48 hr of treatment (Fig. 4). Caspase 9, which is predominantly expressed in the intrinsic mitochondrial apoptotic pathway, was significantly expressed only in the L1210 cell line, whereas caspase 8, a key protein in the extrinsic apoptotic pathway, was highly expressed in A20 cells. Expression of caspases 3, 8, and 9 was stronger in leukemia cells treated with DFX alone, as compared to cells treated with both DFX and FeCl3. PARP expression, which was measured to verify the apoptotic pathway, was confirmed in both leukemia cell lines, whereas Bax, a marker of the mitochondrial apoptotic pathway, was weakly expressed in both cell lines.
Fig. 4. Western blot measurement of caspase 3, caspase 9, caspase 8, PARP, and BAX expression in L1210 (A) and A20 cells (B) after treatment with 50 µM DFX with or without FeCl3, according to treatment time (6-48 hr).
Cytochrome c release in murine leukemia cells treated with DFX
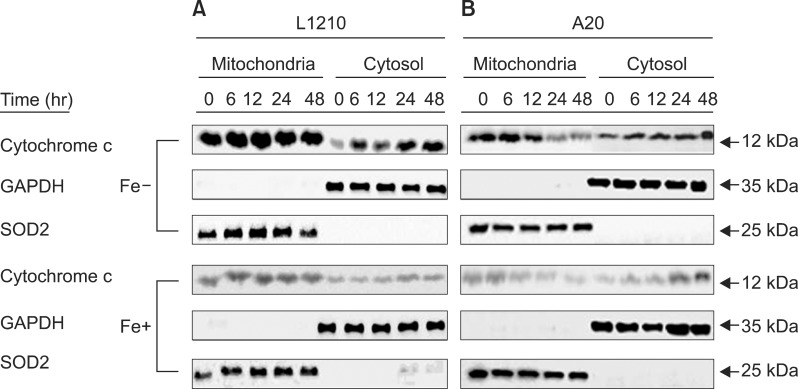
Expression of cytochrome c was measured in both leukemia cell lines after treatment with 50 µM DFX to further clarify whether the intrinsic mitochondrial pathway is the predominant apoptotic mechanism. L1210 cells displayed significantly greater cytochrome c expression than A20 cells (Fig. 5). As with the expression of caspase proteins, expression of cytochrome c was higher in both cell lines when treated with DFX alone, as compared to treatment with both DFX and FeCl3.
Fig. 5. Western blot measurement of cytochrome c expression in L1210 (A) and A20 cells (B) after treatment with 50 µM DFX with or without FeCl3, according to treatment time (6-48 hr).
DISCUSSION
SIO due to repeated blood transfusion affects heart, liver, and other vital organ function [14]. ICAs have been used to treat transfusional iron overload and overcome the complications of SIO, and are considered potential anti-cancer agents [15, 16]. Several studies support the anti-leukemic effects of ICAs [3, 12, 13, 17, 18, 19, 20]. This was based on evidence of anti-tumor effects, including the inhibition of proliferation and differentiation via inhibited DNA synthesis and apoptosis induction through caspase 3 and 9 [18, 19, 20]. The effects of DFX on human esophageal cancer and hepatoma cell line viability (OE33, OE19, and OE21) have been reported [21, 22]. Ford et al. demonstrated that DFX decreases esophageal cancer cell viability and proliferation [21]. In addition, Gaboriau et al. reported increased hepatoma cell viability in the presence of iron after ICA treatment [22].
Our results showed that the cell viability of two murine lymphoid leukemia cell lines, L1210 and A20, decreased in a concentration- and time-dependent manner. Of note, the L1210 cells showed decreased viability at lower DFX concentrations when compared to A20 cells, possibly indicating greater sensitivity to the effects of DFX.
The apoptosis assay also revealed a time- and concentration-dependent increase in apoptosis following DFX treatment, indicating that DFX-mediated apoptosis was responsible for decreased cell viability. Our results are similar to those of ICA-treated Jurkat cells and malignant lymphoma cell lines undergoing apoptosis [23, 24].
Whether the anti-tumor activity of DFX is linked to iron depletion via iron chelation is not clear, as several previous studies concluded that this activity is either independent of the effects of iron chelation or clearly dependent on iron chelation [12, 25]. In our study, we found that the addition of FeCl3 significantly lowered DFX-mediated apoptosis in certain scenarios, particularly with high-dose, prolonged DFX treatment. However, the cell salvaging potential of FeCl3 was not uniform through all experiments, indicating that iron chelation may have a partial role in cell death, but does not account for the entire mechanism of action.
Expression of CD95 (Fas) was significantly higher in both leukemia cell lines upon DFX treatment. However, CD95 expression was significantly higher in A20 cells than L1210 cells at all DFX concentrations tested, indicating that CD95-mediated apoptosis may play a greater role in A20 cells.
We determined the expression of critical apoptotic proteins to clarify the mechanisms regulating apoptosis in each cell line. Caspase 3 expression was increased in both cell lines following prolonged DFX exposure, indicating that apoptosis occurred through a caspase-dependent pathway. However, caspase 9 and 8 expression differed between the cell lines, with L1210 cells displaying significantly higher caspase 9 expression and A20 cells showing higher caspase 8 expression. As caspase 9 is a key component of the intrinsic mitochondrial apoptotic pathway, our results indicate that this pathway may be the predominant mode of L1210 cell apoptosis. In contrast, caspase 8 is a critical protein in the extrinsic apoptotic pathway, which may be the key mechanism of A20 cell apoptosis. Supporting these results, expression of cytochrome c, a mitochondrial protein regulating the intrinsic apoptotic pathway, was significantly higher in L1210 cells. Mechanisms of DFX-mediated apoptosis have previously been reported for other malignancies. Caspase-dependent apoptosis was also observed in DFX-treated myeloid leukemia cells [19]. Caspase 3/7-, caspase 9-, and Bax-induced apoptosis was observed in a malignant lymphoma cell line [24]. Messa et al. demonstrated that oral iron chelation therapy has anti-tumor effects via NF-κB inhibition in patients with myelodysplastic syndrome [12].
Importantly, we found that the addition of FeCl3 decreased the expression of caspases and cytochrome c in both cell lines, underscoring the notion that DFX-mediated cell apoptosis occurs in the context of iron deprivation. However, the lack of a uniform effect of FeCl3 dictates that further studies are necessary to conclude whether the anti-tumor activity of DFX is dependent on its iron-chelating ability.
In conclusion, treatment with DFX increased apoptosis in two murine lymphoid leukemia cell lines through caspase-dependent pathways. The dominant apoptotic pathway, however, differed between cell lines, with the intrinsic mitochondrial pathway having a key role in L1210 cells, while the extrinsic pathway was more significant in A20 cells. Thus, the exact apoptotic mechanism may differ between lymphoid leukemia cell lines, although caspase-dependent apoptosis may be a common feature. Our results add to previous literature on the anti-tumor activity of DFX and underscore the mechanism of action. Whether this apoptotic activity is dependent on iron chelation requires further study.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Ali S, Pimentel JD, Munoz J, et al. Iron overload in allogeneic hematopoietic stem cell transplant recipients. Arch Pathol Lab Med. 2012;136:532–538. doi: 10.5858/arpa.2011-0190-OA. [DOI] [PubMed] [Google Scholar]
- 2.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 3.Lee DH, Jang PS, Chung NG, Cho B, Jeong DC, Kim HK. Deferasirox shows in vitro and in vivo antileukemic effects on murine leukemic cell lines regardless of iron status. Exp Hematol. 2013;41:539–546. doi: 10.1016/j.exphem.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham-Rundles S, Giardina PJ, Grady RW, Califano C, McKenzie P, De Sousa M. Effect of transfusional iron overload on immune response. J Infect Dis. 2000;182(Suppl 1):S115–S121. doi: 10.1086/315919. [DOI] [PubMed] [Google Scholar]
- 5.Malcovati L. Red blood cell transfusion therapy and iron chelation in patients with myelodysplastic syndromes. Clin Lymphoma Myeloma. 2009;9(Suppl 3):S305–S311. doi: 10.3816/CLM.2009.s.029. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Whitley K, Thompson AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59:358–364. doi: 10.1002/pbc.24179. [DOI] [PubMed] [Google Scholar]
- 7.Neufeld EJ. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood. 2006;107:3436–3441. doi: 10.1182/blood-2006-02-002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balooch FD, Fatemi SJ, Iranmanesh M, Hosseinkhani B. Combined chelation therapy with deferasirox and desferrioxamine in removing lead from rats. Am J Pharmacol Toxicol. 2013;8:134–140. [Google Scholar]
- 9.Peccatori J, Ciceri F. Allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2010;95:857–859. doi: 10.3324/haematol.2010.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dayani PN, Bishop MC, Black K, Zeltzer PM. Desferoxamine (DFO)-mediated iron chelation: rationale for a novel approach to therapy for brain cancer. J Neurooncol. 2004;67:367–377. doi: 10.1023/b:neon.0000024238.21349.37. [DOI] [PubMed] [Google Scholar]
- 11.Marx JJ. Iron and infection: competition between host and microbes for a precious element. Best Pract Res Clin Haematol. 2002;15:411–426. [PubMed] [Google Scholar]
- 12.Messa E, Carturan S, Maffè C, et al. Deferasirox is a powerful NF-kappaB inhibitor in myelodysplastic cells and in leukemia cell lines acting independently from cell iron deprivation by chelation and reactive oxygen species scavenging. Haematologica. 2010;95:1308–1316. doi: 10.3324/haematol.2009.016824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohyashiki JH, Kobayashi C, Hamamura R, Okabe S, Tauchi T, Ohyashiki K. The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD1. Cancer Sci. 2009;100:970–977. doi: 10.1111/j.1349-7006.2009.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology Am Soc Hematol Educ Program. 2001:47–61. doi: 10.1182/asheducation-2001.1.47. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg SL. Novel treatment options for transfusional iron overload in patients with myelodysplastic syndromes. Leuk Res. 2007;31(Suppl 3):S16–S22. doi: 10.1016/S0145-2126(07)70462-9. [DOI] [PubMed] [Google Scholar]
- 16.Bedford MR, Ford SJ, Horniblow RD, Iqbal TH, Tselepis C. Iron chelation in the treatment of cancer: a new role for deferasirox? J Clin Pharmacol. 2013;53:885–891. doi: 10.1002/jcph.113. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima T, Kawabata H, Nakamura T, et al. Iron chelation therapy with deferasirox induced complete remission in a patient with chemotherapy-resistant acute monocytic leukemia. Anticancer Res. 2011;31:1741–1744. [PubMed] [Google Scholar]
- 18.Becton DL, Roberts B. Antileukemic effects of deferoxamine on human myeloid leukemia cell lines. Cancer Res. 1989;49:4809–4812. [PubMed] [Google Scholar]
- 19.Kim JL, Kang HN, Kang MH, Yoo YA, Kim JS, Choi CW. The oral iron chelator deferasirox induces apoptosis in myeloid leukemia cells by targeting caspase. Acta Haematol. 2011;126:241–245. doi: 10.1159/000330608. [DOI] [PubMed] [Google Scholar]
- 20.Estrov Z, Tawa A, Wang XH, et al. In vitro and in vivo effects of deferoxamine in neonatal acute leukemia. Blood. 1987;69:757–761. [PubMed] [Google Scholar]
- 21.Ford SJ, Obeidy P, Lovejoy DB, et al. Deferasirox (ICL670A) effectively inhibits oesophageal cancer growth in vitro and in vivo. Br J Pharmacol. 2013;168:1316–1328. doi: 10.1111/bph.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaboriau F, Leray AM, Ropert M, et al. Effects of deferasirox and deferiprone on cellular iron load in the human hepatoma cell line HepaRG. Biometals. 2010;23:231–245. doi: 10.1007/s10534-009-9281-9. [DOI] [PubMed] [Google Scholar]
- 23.Gharagozloo M, Khoshdel Z, Amirghofran Z. The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: a comparison with desferrioxamine. Eur J Pharmacol. 2008;589:1–7. doi: 10.1016/j.ejphar.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 24.Choi JG, Kim JL, Park J, et al. Effects of oral iron chelator deferasirox on human malignant lymphoma cells. Korean J Hematol. 2012;47:194–201. doi: 10.5045/kjh.2012.47.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vazana-Barad L, Granot G, Mor-Tzuntz R, et al. Mechanism of the antitumoral activity of deferasirox, an iron chelation agent, on mantle cell lymphoma. Leuk Lymphoma. 2013;54:851–859. doi: 10.3109/10428194.2012.734614. [DOI] [PubMed] [Google Scholar]