Abstract
Hepatitis B virus (HBV) reactivation has previously occurred in hepatitis B surface antigen-negative patients with malignant lymphoma who received rituximab-based combination chemotherapy. However, few reports have described cases of HBV reactivation in patients with multiple myeloma thus far. We report a case of HBV reactivation in a patient with multiple myeloma treated with chemotherapy, autologous hematopoietic stem cell transplantation, and maintenance steroid therapy. For the HBV reactivation, the patient was treated with the antiviral agent entecavir. The clinical symptoms and laboratory findings improved after 3 months. Further studies should target the identification of patients at high risk of HBV reactivation in multiple myeloma treated with autologous hematopoietic stem cell transplantation and steroid therapy for maintenance and establish viral prophylaxis strategies, especially in Korea, in which HBV infection is endemic.
Keywords: HBV reactivation, Multiple myeloma, Transplantation, Prednisolone, Chemotherapy
INTRODUCTION
Hepatitis B virus (HBV) infection is endemic in South Korea. HBV reactivation in patients who are positive for hepatitis B surface antigen (HBsAg) is well established, and antiviral prophylaxis is recommended to HBsAg carriers before they undergo cytotoxic and immunosuppressive therapies [1].
Recently, HBV reactivation has been observed in patients with negative laboratory results for HBsAg after receiving cytotoxic or immunosuppressive therapy. This has been reported particularly in patients with malignant lymphoma who received rituximab-based combination chemotherapy [2, 3]. Clinical data on HBV reactivation in patients with multiple myeloma have not been frequently reported, and no appropriate strategies have been established for prophylaxis and surveillance of HBV reactivation in patients with multiple myeloma who are found either with or without HBsAg.
We report a case of HBV reactivation in a patient without HBsAg who received vincristine, doxorubicin, and dexamethasone (VAD) chemotherapy, autologous hematopoietic stem-cell transplantation (HSCT), and steroid therapy for the treatment of multiple myeloma.
CASE REPORT
A 55-year-old man was diagnosed with multiple myeloma (IgG-κ type; stage II in both the Durie-Salmon and international staging systems; serum M-protein level, 3.5 g/dL) in January 2009 and underwent chemotherapy with VAD as an induction chemotherapy. He had diabetes mellitus, hypertension, and no specific family history of any disease. When VAD chemotherapy was started, his laboratory results were negative for HBsAg but positive for the hepatitis B surface antibody (HBsAb; 1,000 IU/mL) and hepatitis-B core Ab (HBcAb [IgG]). After 6 cycles of VAD, he achieved partial response. Later, in June 2009, he received a high-dose cyclophosphamide regimen for peripheral hematopoietic stemcell mobilization and underwent autologous HSCT.
The conditioning regimen was high-dose melphalan (100 mg/m2, D-3 and D-2). Before undergoing autologous HSCT, his serological results were still positive for HBsAb and HBcAb, and negative for HBsAg, but the HBsAb level decreased to 27 IU/mL. Two weeks later, after autologous HSCT, he was discharged without any complications. Subsequently, he was treated with prednisolone (1 mg/kg/day for 4 days every month) as maintenance therapy from August 2009 to December 2009. In January 2010, his aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were found to have been elevated. His general condition was good, and he looked healthy.
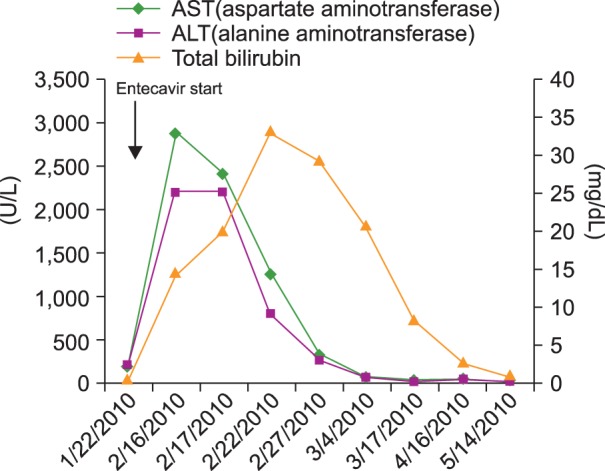
His laboratory results were as follows: AST, 186 U/L; ALT, 214 U/L; total bilirubin, 0.5 mg/dL; albumin, 4.4 g/dL; international normalized ratio (INR), 1.04. In addition, his serological test results were as follows: HBsAg (+), HBsAb (-), hepatitis B e antigen (-), hepatitis B e antibody (+), and anti-hepatitis C virus (HCV; -). The serum HBV DNA level was 4,105,000 IU/mL. The patient also showed partial response, and the serum M-protein level was 0.5 g/dL. Therefore, we concluded that the HBV reactivation caused liver damage. Thus, entecavir at a dose of 0.5 mg daily was started immediately.
Twenty days later, the patient was hospitalized with fatigue and jaundice for 1 month. On admission, his vital signs were stable, blood pressure was 140/80 mm Hg, pulse rate was 62 beats/min, respiratory rate was 18/min, and body temperature was 36.1℃. In addition, he was mentally alert but appeared acutely ill. His laboratory findings were as follows: hemoglobin, 12.7 g/dL; platelet count, 87,000/µL; white blood cell count, 5,260/µL; AST, 2,895 U/L; ALT, 2,196 U/L; total bilirubin, 17.2 mg/dL; albumin, 4.1 g/dL; and INR, 1.38.
Abdominal computed tomography revealed a gallstone, with diffuse wall thickening of the gallbladder and tiny stones in both kidneys. His HBV DNA level was reduced to 82,500 IU/mL, hepatitis A virus antibody [IgM] was absent, and HCV RNA was not detected. In our assessment, the acute hepatitis was caused by the HBV reactivation; thus, the entecavir therapy and conservative management were continued. The next day, his transaminase levels decreased, but his total bilirubin remained elevated. Ten days later, his total bilirubin level started to decrease. In February 2010, he was discharged and received continuous entecavir medication. All his liver function test results were normalized in 3 months. The changes in the laboratory results are presented in Fig. 1.
Fig. 1. Changes in the liver function panel after entecavir treatment.
In October 2010, his serum M-protein level increased to 1.2 g/dL. He received thalidomide, cyclophosphamide, and dexamethasone therapy from November 2010 to March 2011, and bortezomib and dexamethasone from May 2011 to November 2011. In February 2012, his serum M-protein level increased again. Thus, he was transferred to another hospital, and information on his disease status and serological data were not available thereafter. The last test for serum HBV DNA level was performed in October 2011 and yielded a negative result. Nonetheless, antiviral treatment was continued to suppress HBV replication.
DISCUSSION
HBV infection is endemic in South Korea. Although the HBsAg seropositivity has decreased from 4.61% in 1998 to 2.98% in 2010 since the advent of vaccination programs [4], the prevalence of HBsAg in South Korea is still much higher than in the United States (0.27%) [5].
HBV reactivation during chemotherapy is a major concern in HBV-endemic areas, as the mortality can be high if reactivation is complicated by fulminant hepatic failure [3]. HBV reactivation has been well documented as a complication in HBsAg carriers treated with chemotherapy or immunosuppressive therapy [6, 7]. Therefore, antiviral prophylaxis is recommended to HBsAg carriers before undergoing cytotoxic and immunosuppressive therapy [1]. Recently, HBV reactivation has been reported in patients without HBsAg and received cytotoxic or immunosuppressive therapy, especially in those with malignant lymphoma treated with rituximab-based combination chemotherapy [2, 3].
We report a case of HBV reactivation in a patient with multiple myeloma who had negative laboratory results for HBsAg before treatment. The patient's initial serum HBV-DNA titer was not assessed; therefore, whether he had occult infection or resolved HBV infection was unclear. During chemotherapy, he showed a dramatic reduction in HBsAb level, concomitant with seroreversion of HBsAg. Seven months later, after autologous HSCT and while on prednisolone maintenance therapy, his HBsAb test result was negative. Wands et al. suggested that antitumor chemotherapeutic agents can reduce the HBsAb level [6, 7]. A few reports described reverse seroconversion, appearance of HBsAg, and disappearance of HBsAb after allogeneic or autologous HSCT [8, 9]. Uhm et al. [10] analyzed HBV serological data in 141 patients after autologous HSCT. In their study, univariate analysis results showed that reverse seroconversions were observed more frequently with multiple myeloma than with other diseases (P=0.005; relative risk, 11.85; 95% confidence interval, 1.38-101.77). The median time for acute hepatitis in 5 patients was 10 months (range, 4-17 months). In the present case, the patient had acute hepatitis 7 months after autologous HSCT. Although Tur-Kaspa et al. [11] considered the use of corticosteroid to be responsible for reverse seroconversion, we did not find any report on HBV reactivation after steroid therapy in patients with multiple myeloma. We believe that the cause of HBV reactivation in this patient was multifactorial, specifically cytotoxic chemotherapy, autologous HSCT, corticosteroid use, and disease itself.
Although he showed worsening liver function after entecavir therapy was started, we continued the therapy. Virological breakthrough is usually followed by biochemical breakthrough, and biochemical parameters can worsen before complete viral suppression [1].
Yeo et al. evaluated 104 patients diagnosed with CD20+ diffuse large B-cell lymphoma [2]. Among the 46 patients without HBsAb and with HBcAb, 5 experienced HBV reactivation and 1 died because of fulminant hepatitis. The results showed that male sex (100% vs. 51%; P=0.0299), absence of anti-HBs (100% vs. 31.7%; P=0.0025), and use of rituximab (100% vs. 0%; P=0.05) were significantly associated with the development of HBV reactivation. They suggested that patients found with HBcAb, particularly those without HBsAb, should be closely monitored for HBV DNA and serum biochemistry during rituximab therapy and that the monitoring should be continued for at least 6 months after the completion of rituximab therapy.
The identification of patients at high risk of HBV reactivation in multiple myeloma treated with autologous HSCT and steroid therapy for maintenance should be investigated further, especially in Korea, as HBV infection is endemic in the area. Further studies should also establish viral prophylaxis strategies and the appropriate scheduling of follow-up testing for hepatic enzymes and HBV serology for such patients.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 2.Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605–611. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 3.Chung SM, Sohn JH, Kim TY, et al. Fulminant hepatic failure with hepatitis B virus reactivation after rituximab treatment in a patient with resolved hepatitis B. Korean J Gastroenterol. 2010;55:266–269. doi: 10.4166/kjg.2010.55.4.266. [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Shin AR, Chung HH, et al. Recent trends in hepatitis B virus infection in the general Korean population. Korean J Intern Med. 2013;28:413–419. doi: 10.3904/kjim.2013.28.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wasley A, Kruszon-Moran D, Kuhnert W, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 6.Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology. 1975;68:105–112. [PubMed] [Google Scholar]
- 7.Kim MK, Ahn JH, Kim SB, et al. Hepatitis B reactivation during adjuvant anthracycline-based chemotherapy in patients with breast cancer: a single institution's experience. Korean J Intern Med. 2007;22:237–243. doi: 10.3904/kjim.2007.22.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senecal D, Pichon E, Dubois F, Delain M, Linassier C, Colombat P. Acute hepatitis B after autologous stem cell transplantation in a man previously infected by hepatitis B virus. Bone Marrow Transplant. 1999;24:1243–1244. doi: 10.1038/sj.bmt.1702039. [DOI] [PubMed] [Google Scholar]
- 9.Goyama S, Kanda Y, Nannya Y, et al. Reverse seroconversion of hepatitis B virus after hematopoietic stem cell transplantation. Leuk Lymphoma. 2002;43:2159–2163. doi: 10.1080/1042819021000033042. [DOI] [PubMed] [Google Scholar]
- 10.Uhm JE, Kim K, Lim TK, et al. Changes in serologic markers of hepatitis B following autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:463–468. doi: 10.1016/j.bbmt.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Tur-Kaspa R, Shaul Y, Moore DD, et al. The glucocorticoid receptor recognizes a specific nucleotide sequence in hepatitis B virus DNA causing increased activity of the HBV enhancer. Virology. 1988;167:630–633. [PubMed] [Google Scholar]


