TO THE EDITOR: t(16;21)(p11.2;q22) is a non-random chromosomal translocation that occurs in acute myeloid leukemia (AML), myelodysplastic syndrome that evolves to AML, blast crisis of chronic myelogenous leukemia, and rare cases of Ewing's tumors [1]. AML cases harboring t(16;21)(p11.2; q22) are associated with poor prognosis and a high relapse rate. This translocation produces a fusion gene between the FUS (fused in sarcoma) gene on chromosome 16 and the ERG (erythroblast transformation-specific related) gene on chromosome 21. The FUS gene is highly related to the EWSR1 (Ewing sarcoma breakpoint region 1) gene, and the FUS protein is an RNA binding protein. On the other hand, the ERG gene encodes an external transcribed spacer (ETS) family transcription factor. In the chimeric protein, the N-terminal FUS transactivation domain fuses to the C-terminal DNA binding ETS domain of ERG. The fusion protein seems to function as a transcriptional activator [2].
However, the occurrence of t(16;21) in acute lymphoblastic leukemia (ALL) is very rare. To our knowledge, it has been reported in only 13 ALL patients, including 10 adults and three children [3, 4, 5, 6].
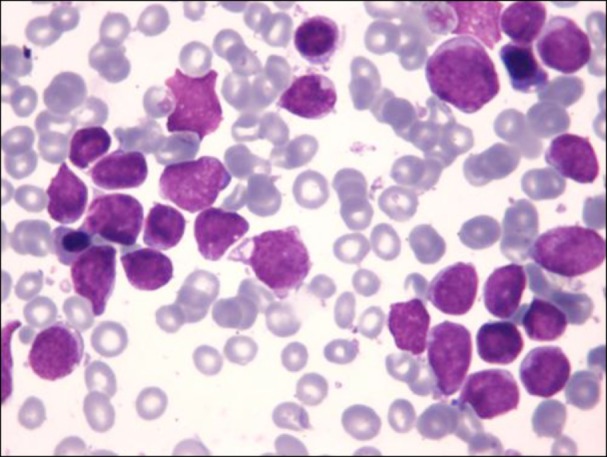
We herein describe the case of a 6-year-old boy who was admitted to our hospital owing to parotid enlargement and cranial nerve VII palsy. Physical examination revealed liver enlargement, and blood evaluation showed a white blood cell count of 6.1×109/L with 13% blasts, hemoglobin level of 10.9 g/dL, and platelet count of 190×109/L. A bone marrow aspirate showed that 81% of the cellularity was replaced by L1/L2 French-American-British morphology lymphoblasts (Fig. 1). Immunophenotyping of the blasts with flow cytometry using a FacSort instrument (Becton Dickinson, San José, CA, USA) indicated a common ALL (B II) according to the European Group for the Immunological Classification of Leukemias. The leukemic cells expressed CD79a, CD22, CD19, CD10, HLA-DR, and were partially positive for TdT, CD34, and CD117 while negative for cytoplasmic µ-chain.
Fig. 1. Morphology of leukemic cells at diagnosis showing L1/L2 blasts.
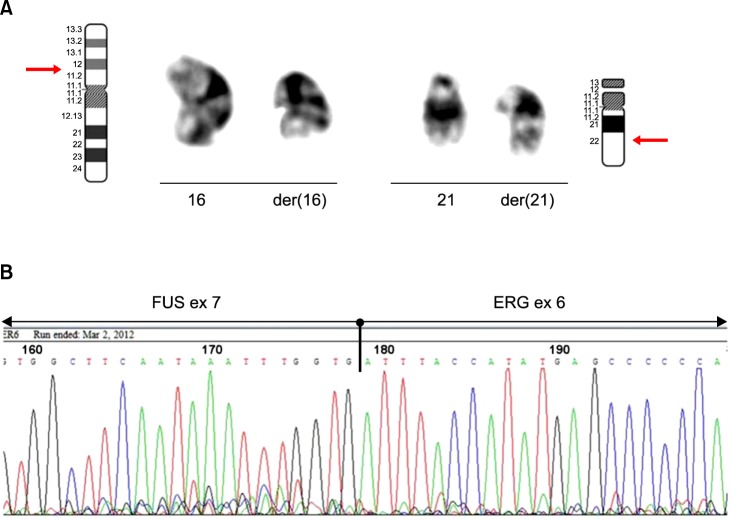
Conventional cytogenetic analysis of the bone marrow cell culture revealed the following karyotype: 46,XY,t(16;21) (p11.2;q22)[10]/46,XY[10] (Fig. 2A). Subsequently, reverse transcriptase polymerase chain reaction (RT-PCR) and direct sequencing were performed to assess the presence of FUS-ERG fusion transcript according to standard procedures [7, 8]. RT-PCR showed a 554-bp specific fragment, and sequencing analysis confirmed the presence of a chimeric transcript consisting of FUS exon 7 at the 5' end fused with ERG exon 6 at the 3' end (Fig. 2B).
Fig. 2. (A) Partial karyotype showing a reciprocal translocation between 16p11.2 and 21q22; (B) Sequencing analysis of the RT-PCR product identifying a fusion between exon 7 of FUS and exon 6 of ERG.
The patient was enrolled in our current protocol for ALL-BFM ALL-IC 2009. His response to the prednisone pre-phase was good. He then underwent induction chemotherapy consisting of vincristine, daunorubicin, and L-asparaginase. The minimal residual disease (MRD), detected by flow cytometry of the bone marrow aspirate, at day 15 of treatment was 0.19%. The patient achieved complete remission after the induction phase and was stratified as an intermediate risk patient, with central nervous system involvement owing to facial palsy. However, blast cells were not detected in the cerebrospinal fluid at diagnosis. He received consolidation chemotherapy with cytarabine, cyclophosphamide, and mercaptopurine, followed by high-dose methotrexate. Subsequently, he received late re-induction therapy with dexamethasone, vincristine, doxorubicin, L-asparaginase, cyclophosphamide, cytarabine, and thiopurine, as well as maintenance therapy with mercaptopurine and methotrexate. He remained free of leukemia 31 months after diagnosis. Flow cytometry analysis of MRD was negative on day 33 (after the induction phase) and on week 12 (prior to the administration of the consolidation phase with high-dose methotrexate). MRD was also confirmed to be negative by quantitative real-time PCR at these time points (day 33 and week 12).
According to the Mitelman database and the literature, about 71 cases of t(16;21) have been reported to date, most of them in AML [9]. The rearrangement of the two chromosomes involved in such a translocation forms the FUS-ERG fusion gene.
In AML, four types of chimeric transcripts have been described, designated as types A, B, C, and D, which correspond to the 255-, 211-, 176-, and 349-bp chimeric products, respectively [2, 8]. These transcripts consist of FUS exons 1 to 6, 1 to 7, or 1 to 8 fused to ERG exons 7 to 17 or 9 to 17. Types A and C are out-of-frame fusion transcripts and likely to have been produced by alternative splicing. The other two in-frame transcripts, types B and D, have been shown to result from the fusion of FUS and ERG genes at different breakpoint positions in t(16;21). These two types of fusion were predicted to produce the FUS-ERG chimeric protein, which may function as the transcriptional activator responsible for the neoplastic process of several t(16;21)-AML cases. The prognosis of AML patients with this translocation is usually very poor owing to resistance to chemotherapy and a high rate of early relapse.
On the other hand, in ALL, a recent study of 256 adult patients reported that FUS-ERG fusion transcript was found in 14 cases, including 10 with t(16;21) identified by cytogenetic analysis. Unfortunately, no information regarding the sequencing of the FUS-ERG fusion gene and the follow-up of these patients was provided [3]. Furthermore, three pediatric cases of t(16;21) have been reported, but only two of them provide clinical information and results from RT-PCR and sequencing of the FUS-ERG fusion gene [4, 5, 6]. The case reported by Kanazawa et al. involved a 1-year-old boy with precursor B cell ALL that did not initially respond to ALL-oriented therapy, but the patient achieved complete remission with an AML treatment protocol. Moreover, Oh et al. described the case of an 8-month-old infant with precursor B cell ALL who achieved complete remission but relapsed 4 months after diagnosis. These patients and the one in our case had the same unusual chimeric transcript that consisted of FUS exon 7 at the 5' end fused with ERG exon 6 at the 3' end, which is different from those described in AML [5, 6]. It is worthy of note that despite having the same molecular rearrangement, the three patients had different responses to ALL-oriented treatment. Our patient achieved complete remission with a chemotherapy schedule for ALL, and he remained free of leukemia 31 months after diagnosis. Therefore, it is challenging to establish the prognostic value of t(16;21) in ALL patients, mostly owing to the small number of reported cases with this unusual abnormality, differences in patient age, treatment heterogeneity, and short follow-up periods.
In conclusion, the present case supports the possible association between ALL cases with t(16;21) and a specific type of FUS-ERG fusion transcript first suggested by Oh et al. However, the role of this transcript in the leukemogenesis of ALL and its prognostic value remain uncertain. Therefore, an analysis of additional ALL cases with t(16;21)(p11.2;q22) and the FUS-ERG fusion chimeric transcript are needed to determine the significance of these findings.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Huret JL. t(16;21)(p11;q22) Atlas Genet Cytogenet Oncol Haematol. 2005;9:36–38. [Google Scholar]
- 2.Ichikawa H, Shimizu K, Hayashi Y, Ohki M. An RNA-binding protein gene, TLS/FUS, is fused to ERG in human myeloid leukemia with t(16;21) chromosomal translocation. Cancer Res. 1994;54:2865–2868. [PubMed] [Google Scholar]
- 3.Liu F, Gao L, Jing Y, et al. Detection and clinical significance of gene rearrangements in Chinese patients with adult acute lymphoblastic leukemia. Leuk Lymphoma. 2013;54:1521–1526. doi: 10.3109/10428194.2012.754888. [DOI] [PubMed] [Google Scholar]
- 4.Heller A, Loncarevic IF, Glaser M, et al. Breakpoint differentiation in chromosomal aberrations of hematological malignancies: Identification of 33 previously unrecorded breakpoints. Int J Oncol. 2004;24:127–136. [PubMed] [Google Scholar]
- 5.Kanazawa T, Ogawa C, Taketani T, Taki T, Hayashi Y, Morikawa A. TLS/FUS-ERG fusion gene in acute lymphoblastic leukemia with t(16;21)(p11;q22) and monitoring of minimal residual disease. Leuk Lymphoma. 2005;46:1833–1835. doi: 10.1080/10428190500162203. [DOI] [PubMed] [Google Scholar]
- 6.Oh SH, Park TS, Choi JR, et al. Two childhood cases of acute leukemia with t(16;21)(p11.2;q22): second case report of infantile acute lymphoblastic leukemia with unusual type of FUS-ERG chimeric transcript. Cancer Genet Cytogenet. 2010;200:180–183. doi: 10.1016/j.cancergencyto.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 7.van Dongen JJ, Macintyre EA, Gabert JA, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:1901–1928. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- 8.Kong XT, Ida K, Ichikawa H, et al. Consistent detection of TLS/FUS-ERG chimeric transcripts in acute myeloid leukemia with t(16;21)(p11;q22) and identification of a novel transcript. Blood. 1997;90:1192–1199. [PubMed] [Google Scholar]
- 9.Mitelman F, Johansson B, Mertens F. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. Bethesda, MD: National Cancer Institute; 2013. [Assessed May 20, 2014]. at http://cgap.nci.nih.gov/Chromosomes/Mitelman. [Google Scholar]