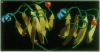Abstract
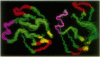
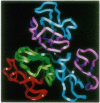
The solution structure of Ca(2+)-loaded protein S (M(r) 18,792) from the Gram-negative soil bacterium Myxococcus xanthus has been determined by multidimensional heteronuclear NMR spectroscopy. Protein S consists of four internally homologous motifs, arranged to produce two domains with a pseudo-twofold symmetry axis, overall resembling a triangular prism. Each domain consists of two topologically inequivalent "Greek keys": the second and fourth motifs form standard Greek keys, whereas the first and third motifs each contain a regular alpha-helix in addition to the usual four beta-strands. The structure of protein S is similar to those of the vertebrate eye lens beta gamma-crystallins, which are thought to be evolutionarily related to protein S. Both protein S and the beta gamma-crystallins function by forming stable multimolecular assemblies. However, protein S possesses distinctive motif organization and domain packing, indicating a different mode of oligomerization and a divergent evolutionary pathway from the beta gamma-crystallins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrowsmith C., Pachter R., Altman R., Jardetzky O. The solution structures of Escherichia coli trp repressor and trp aporepressor at an intermediate resolution. Eur J Biochem. 1991 Nov 15;202(1):53–66. doi: 10.1111/j.1432-1033.1991.tb16344.x. [DOI] [PubMed] [Google Scholar]
- Bagby S., Harvey T. S., Eagle S. G., Inouye S., Ikura M. NMR-derived three-dimensional solution structure of protein S complexed with calcium. Structure. 1994 Feb 15;2(2):107–122. doi: 10.1016/s0969-2126(00)00013-7. [DOI] [PubMed] [Google Scholar]
- Bagby S., Harvey T. S., Kay L. E., Eagle S. G., Inouye S., Ikura M. Unusual helix-containing greek keys in development-specific Ca(2+)-binding protein S. 1H, 15N, and 13C assignments and secondary structure determined with the use of multidimensional double and triple resonance heteronuclear NMR spectroscopy. Biochemistry. 1994 Mar 8;33(9):2409–2421. doi: 10.1021/bi00175a009. [DOI] [PubMed] [Google Scholar]
- Bax B., Lapatto R., Nalini V., Driessen H., Lindley P. F., Mahadevan D., Blundell T. L., Slingsby C. X-ray analysis of beta B2-crystallin and evolution of oligomeric lens proteins. Nature. 1990 Oct 25;347(6295):776–780. doi: 10.1038/347776a0. [DOI] [PubMed] [Google Scholar]
- Blundell T., Lindley P., Miller L., Moss D., Slingsby C., Tickle I., Turnell B., Wistow G. The molecular structure and stability of the eye lens: x-ray analysis of gamma-crystallin II. Nature. 1981 Feb 26;289(5800):771–777. doi: 10.1038/289771a0. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Wingfield P. T., Gronenborn A. M. High-resolution three-dimensional structure of interleukin 1 beta in solution by three- and four-dimensional nuclear magnetic resonance spectroscopy. Biochemistry. 1991 Mar 5;30(9):2315–2323. doi: 10.1021/bi00223a005. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Potter J. D., Horn M. J., Wilshire G., Jackman N. The amino acid sequence of rabbit skeletal muscle troponin C: gene replication and homology with calcium-binding proteins from carp and hake muscle. FEBS Lett. 1973 Nov 1;36(3):268–272. doi: 10.1016/0014-5793(73)80388-6. [DOI] [PubMed] [Google Scholar]
- Downard J. S., Kupfer D., Zusman D. R. Gene expression during development of Myxococcus xanthus. Analysis of the genes for protein S. J Mol Biol. 1984 Jun 5;175(4):469–492. doi: 10.1016/0022-2836(84)90180-3. [DOI] [PubMed] [Google Scholar]
- Dworkin M., Kaiser D. Cell interactions in myxobacterial growth and development. Science. 1985 Oct 4;230(4721):18–24. doi: 10.1126/science.3929384. [DOI] [PubMed] [Google Scholar]
- Fairbrother W. J., Gippert G. P., Reizer J., Saier M. H., Jr, Wright P. E. Low resolution solution structure of the Bacillus subtilis glucose permease IIA domain derived from heteronuclear three-dimensional NMR spectroscopy. FEBS Lett. 1992 Jan 20;296(2):148–152. doi: 10.1016/0014-5793(92)80367-p. [DOI] [PubMed] [Google Scholar]
- Grzesiek S., Döbeli H., Gentz R., Garotta G., Labhardt A. M., Bax A. 1H, 13C, and 15N NMR backbone assignments and secondary structure of human interferon-gamma. Biochemistry. 1992 Sep 8;31(35):8180–8190. doi: 10.1021/bi00150a009. [DOI] [PubMed] [Google Scholar]
- Ikura M., Clore G. M., Gronenborn A. M., Zhu G., Klee C. B., Bax A. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science. 1992 May 1;256(5057):632–638. doi: 10.1126/science.1585175. [DOI] [PubMed] [Google Scholar]
- Ikura M., Kay L. E., Bax A. A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry. 1990 May 15;29(19):4659–4667. doi: 10.1021/bi00471a022. [DOI] [PubMed] [Google Scholar]
- Ikura M., Kay L. E., Krinks M., Bax A. Triple-resonance multidimensional NMR study of calmodulin complexed with the binding domain of skeletal muscle myosin light-chain kinase: indication of a conformational change in the central helix. Biochemistry. 1991 Jun 4;30(22):5498–5504. doi: 10.1021/bi00236a024. [DOI] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Biosynthesis and self-assembly of protein S, a development-specific protein of Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):209–213. doi: 10.1073/pnas.76.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Gene expression during development of Myxococcus xanthus: pattern of protein synthesis. Dev Biol. 1979 Feb;68(2):579–591. doi: 10.1016/0012-1606(79)90228-8. [DOI] [PubMed] [Google Scholar]
- Inouye S., Franceschini T., Inouye M. Structural similarities between the development-specific protein S from a gram-negative bacterium, Myxococcus xanthus, and calmodulin. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6829–6833. doi: 10.1073/pnas.80.22.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Franceschini T., Inouye M. Structural similarities between the development-specific protein S from a gram-negative bacterium, Myxococcus xanthus, and calmodulin. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6829–6833. doi: 10.1073/pnas.80.22.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Harada W., Zusman D., Inouye M. Development-specific protein S of Myxococcus xanthus: purification and characterization. J Bacteriol. 1981 Nov;148(2):678–683. doi: 10.1128/jb.148.2.678-683.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Ike Y., Inouye M. Tandem repeat of the genes for protein S, a development-specific protein of Myxococcus xanthus. J Biol Chem. 1983 Jan 10;258(1):38–40. [PubMed] [Google Scholar]
- Inouye S., Inouye M., McKeever B., Sarma R. Preliminary crystallographic data for protein S, a development-specific protein of Myxococcus xanthus. J Biol Chem. 1980 Apr 25;255(8):3713–3714. [PubMed] [Google Scholar]
- Kaiser D., Manoil C., Dworkin M. Myxobacteria: cell interactions, genetics, and development. Annu Rev Microbiol. 1979;33:595–639. doi: 10.1146/annurev.mi.33.100179.003115. [DOI] [PubMed] [Google Scholar]
- Lapatto R., Nalini V., Bax B., Driessen H., Lindley P. F., Blundell T. L., Slingsby C. High resolution structure of an oligomeric eye lens beta-crystallin. Loops, arches, linkers and interfaces in beta B2 dimer compared to a monomeric gamma-crystallin. J Mol Biol. 1991 Dec 20;222(4):1067–1083. doi: 10.1016/0022-2836(91)90594-v. [DOI] [PubMed] [Google Scholar]
- Palmer A. G., 3rd, Fairbrother W. J., Cavanagh J., Wright P. E., Rance M. Improved resolution in three-dimensional constant-time triple resonance NMR spectroscopy of proteins. J Biomol NMR. 1992 Jan;2(1):103–108. doi: 10.1007/BF02192804. [DOI] [PubMed] [Google Scholar]
- Powers R., Garrett D. S., March C. J., Frieden E. A., Gronenborn A. M., Clore G. M. 1H, 15N, 13C, and 13CO assignments of human interleukin-4 using three-dimensional double- and triple-resonance heteronuclear magnetic resonance spectroscopy. Biochemistry. 1992 May 5;31(17):4334–4346. doi: 10.1021/bi00132a026. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J. Control of morphogenesis in myxobacteria. Crit Rev Microbiol. 1987;14(3):195–227. doi: 10.3109/10408418709104439. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990 Dec;54(4):473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Teintze M., Inouye M., Inouye S. Characterization of calcium-binding sites in development-specific protein S of Myxococcus xanthus using site-specific mutagenesis. J Biol Chem. 1988 Jan 25;263(3):1199–1203. [PubMed] [Google Scholar]
- Teintze M., Thomas R., Furuichi T., Inouye M., Inouye S. Two homologous genes coding for spore-specific proteins are expressed at different times during development of Myxococcus xanthus. J Bacteriol. 1985 Jul;163(1):121–125. doi: 10.1128/jb.163.1.121-125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G. Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J Mol Evol. 1990 Feb;30(2):140–145. doi: 10.1007/BF02099940. [DOI] [PubMed] [Google Scholar]
- Wistow G. Lens crystallins: gene recruitment and evolutionary dynamism. Trends Biochem Sci. 1993 Aug;18(8):301–306. doi: 10.1016/0968-0004(93)90041-k. [DOI] [PubMed] [Google Scholar]
- Wistow G., Piatigorsky J. Recruitment of enzymes as lens structural proteins. Science. 1987 Jun 19;236(4808):1554–1556. doi: 10.1126/science.3589669. [DOI] [PubMed] [Google Scholar]
- Wistow G., Summers L., Blundell T. Myxococcus xanthus spore coat protein S may have a similar structure to vertebrate lens beta gamma-crystallins. 1985 Jun 27-Jul 3Nature. 315(6022):771–773. doi: 10.1038/315771a0. [DOI] [PubMed] [Google Scholar]
- Wistow G., Turnell B., Summers L., Slingsby C., Moss D., Miller L., Lindley P., Blundell T. X-ray analysis of the eye lens protein gamma-II crystallin at 1.9 A resolution. J Mol Biol. 1983 Oct 15;170(1):175–202. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]
- Zhao D., Arrowsmith C. H., Jia X., Jardetzky O. Refined solution structures of the Escherichia coli trp holo- and aporepressor. J Mol Biol. 1993 Feb 5;229(3):735–746. doi: 10.1006/jmbi.1993.1076. [DOI] [PubMed] [Google Scholar]
- Zuiderweg E. R., Fesik S. W. Heteronuclear three-dimensional NMR spectroscopy of the inflammatory protein C5a. Biochemistry. 1989 Mar 21;28(6):2387–2391. doi: 10.1021/bi00432a008. [DOI] [PubMed] [Google Scholar]