Abstract
Background
Cutaneous mucinoses are a heterogeneous group of disorders characterized by an abnormal amount of mucin in the skin. However, the pathomechanism of an excessive mucin deposition in the skin is still unknown. Eczematous dermatitis is sub-classified histologically into acute, subacute, and chronic variants. The characteristic histopathologic findings for chronic eczema are variable. However, periadnexal mucin deposition is not known as a feature of chronic eczema.
Objective
To evaluate the presence of periadnexal mucin deposition in chronic eczematous dermatitis.
Methods
We analyzed the skin biopsy specimens from 36 patients who were pathologically diagnosed with chronic eczematous dermatitis. Alcian blue, colloidal iron, and periodic acid-Schiff stains were used to evaluate the mucin deposition in histologic sections. Two dermatologists and two dermatopathologists evaluated the degree of mucin deposition using a 4-point scale.
Results
Various amounts of mucin deposition were observed in the periadnexal area of patients who were diagnosed with chronic eczema. Mucin deposition was more visible after staining with mucin-specific stains. Evaluation of the staining analysis scores revealed that the staining intensities were significantly higher in patients with chronic eczema than age- and site-matched controls (normal, acute to subacute eczema, and psoriasis vulgaris).
Conclusion
Periadnexal mucin (secondary mucinoses) may be an additional finding of chronic eczematous dermatitis.
Keywords: Eczema, Periadnexal mucin, Mucinoses
INTRODUCTION
Cutaneous mucinoses are a heterogeneous group of disorders characterized by an abnormal amount of mucin in the skin1. These mucinoses can be divided into two groups2: 1) primary cutaneous mucinosis, in which mucin deposition is the main histologic feature, resulting in clinically distinctive lesions; and 2) secondary mucinoses in which the deposition of mucin is an additional finding. The reason why excess mucin is produced in the skin is still unknown3. The clinical classification of eczema is not unified. However, it is generally classified as acute, subacute, or chronic eczema according to its etiology, skin manifestation, and the involved sites. However, the histopathologic classification of these three types is relatively precise. In particular, chronic eczema shows parakeratosis, hyperkeratosis, and acanthosis in the epidermis. In the dermis, there is fibrosis of the papillary dermis and numerous inflammatory cellular infiltrations. However, periadnexal mucin deposition is not known as an additional feature of chronic eczema.
We conducted this study to evaluate the periadnexal mucin deposition in chronic eczematous dermatitis.
MATERIALS AND METHODS
Case selection
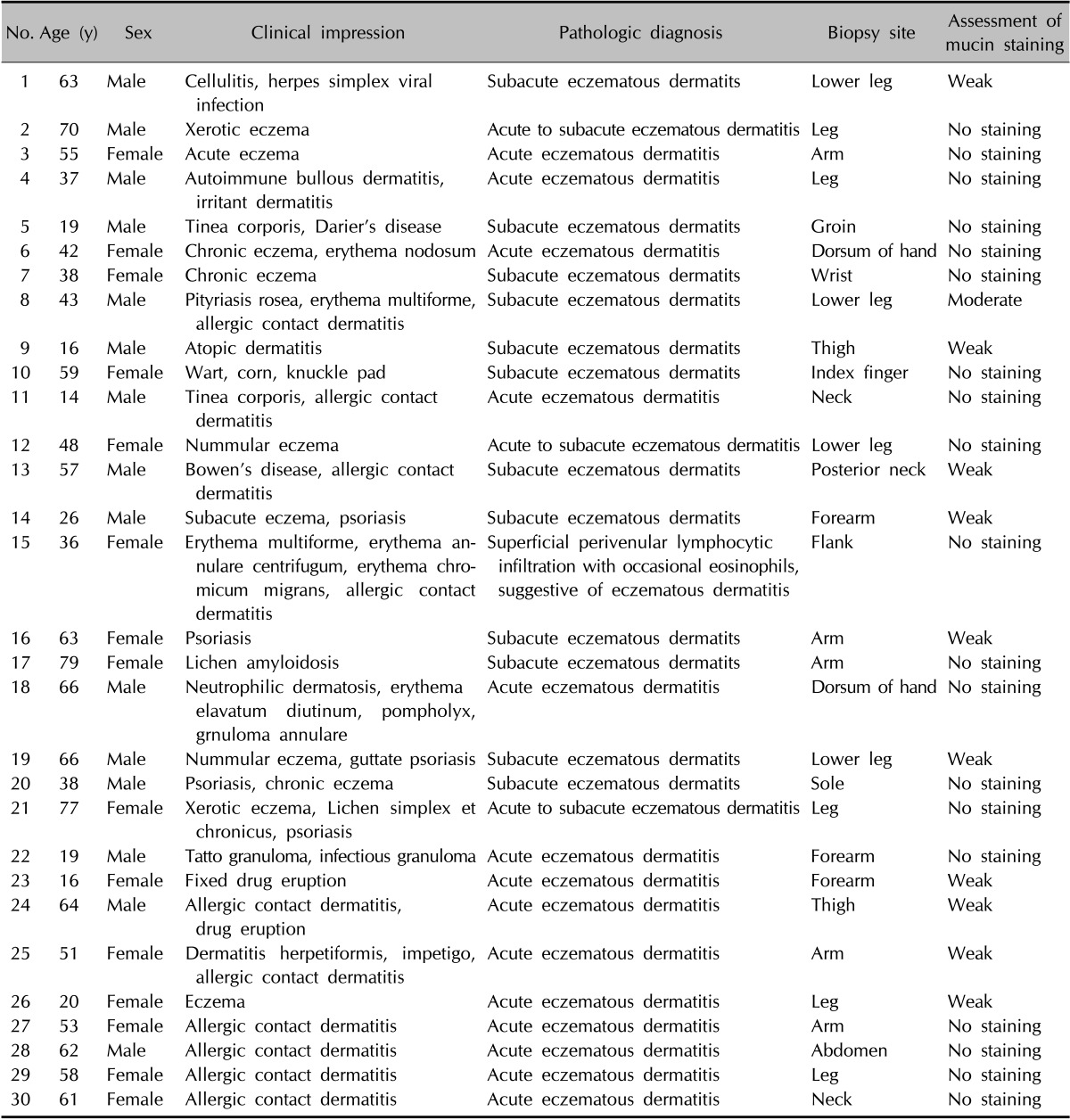
We retrospectively reviewed patients' files from the Department of Dermatology, Yonsei University Wonju Severance Christian Hospital from January 2007 to December 2013. Patients with clinical features, including scales, lichenification, pruritus, and pathological features that were equivalent to chronic eczematous dermatitis (i.e., parakeratosis, hyperkeratosis, marked proliferation of the epidermis by acanthosis, fibrosis of the papillary dermis, and inflammatory cell infiltration of the dermis but not of the epidermis) were selected. Thirty-six patients (male [M] : female [F], 1.1 : 1) with a median age of 51.5 years were included in this study. The characteristics of the patients are presented in Table 1. Age- and site-matched control samples were obtained from 30 patients who were diagnosed with acute eczema (M : F, 1 : 1; median age, 52 years) (Table 2) and from 20 patients with normal skin (M : F, 1 : 0.8; median age, 32 years). Twenty cases of psoriasis (M : F, 1 : 1; median age, 37 years) and other chronic inflammatory dermatoses were obtained to draw comparisons with the selected cases.
Table 1. Clinical data of patients with chronic eczematous dermatitis.
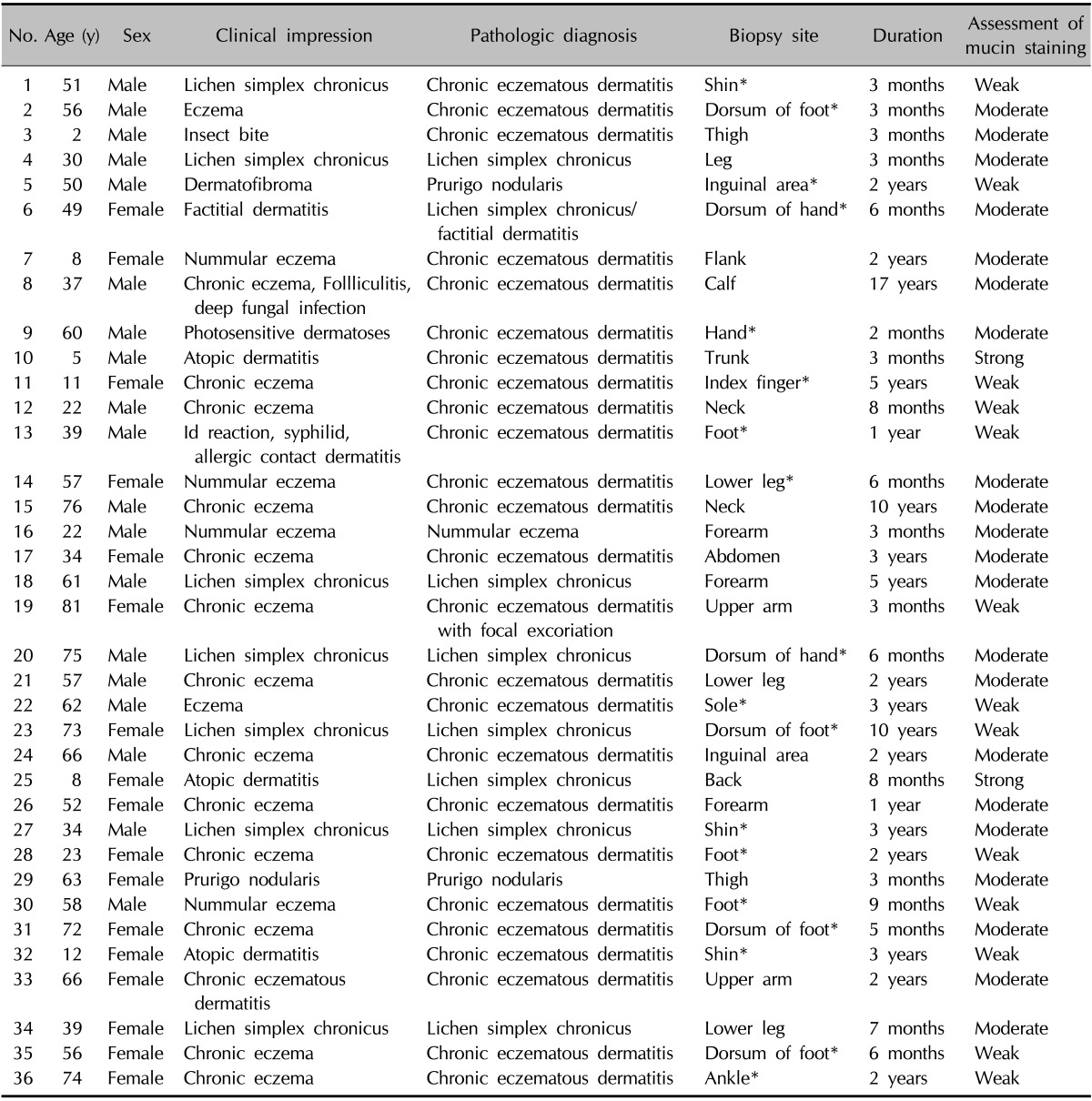
*Cases which do not contain hair follicle in the slide sections.
Table 2. Clinical data of patients with acute eczema.
Tissue and histopathology
In this study, biopsied specimens were collected for routine diagnostic procedures. Skin samples were obtained under local anesthesia with 2% lidocaine using a 3-mm punch biopsy or incision. Eczematous dermatitis is histopathologically sub-classified into acute, subacute, or chronic variants. In chronic eczema, there are variable degrees of hyperkeratosis with hypergranulosis and psoriasiform hyperplasia; additionally, there are patchy infiltrations of chronic inflammatory cells and a fibrosed papillary dermis4.
Histology
The tissues were fixed in 10% phosphate-buffered formaldehyde and were embedded in paraffin. According to standard procedures, the sections were stained with hematoxylin-eosin. Alcian blue, colloidal iron, and periodic acid-Schiff (PAS) were used as stains to evaluate the mucin deposition in the histologic sections.
Staining analysis
The mucin staining intensity was scored qualitatively on a 4-point scale: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The staining was evaluated by two dermatologists and two dermatopathologists.
Statistical analysis
PASW Statistics version 18.0 (IBM Co., Armonk, NY, USA) was used to perform all statistical analyses. We performed unpaired t-tests to compare the staining intensities among the chronic eczema, acute eczema, normal, and psoriasis groups. p-values<0.05 were considered statistically significant.
RESULTS
Histology
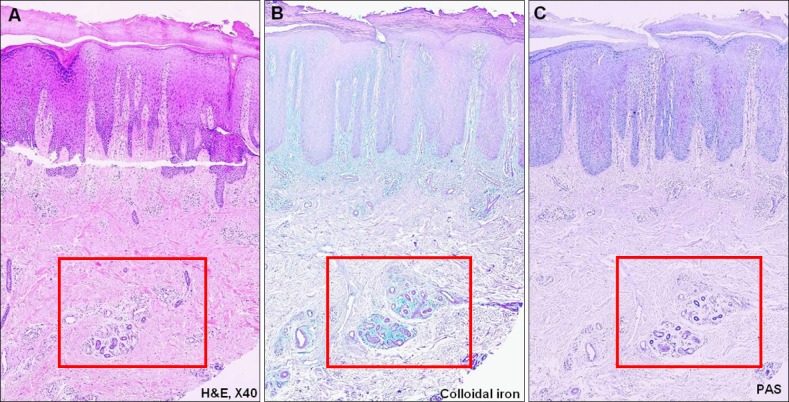
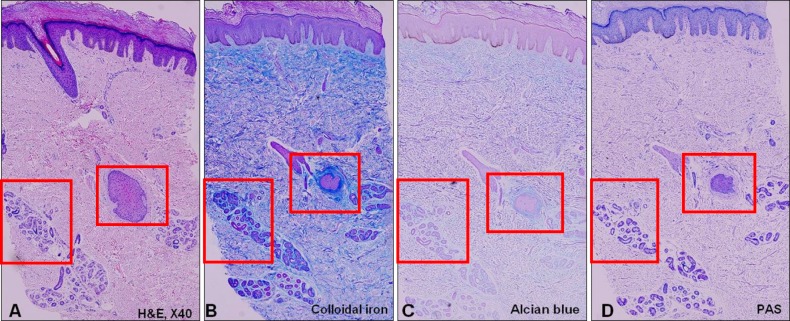
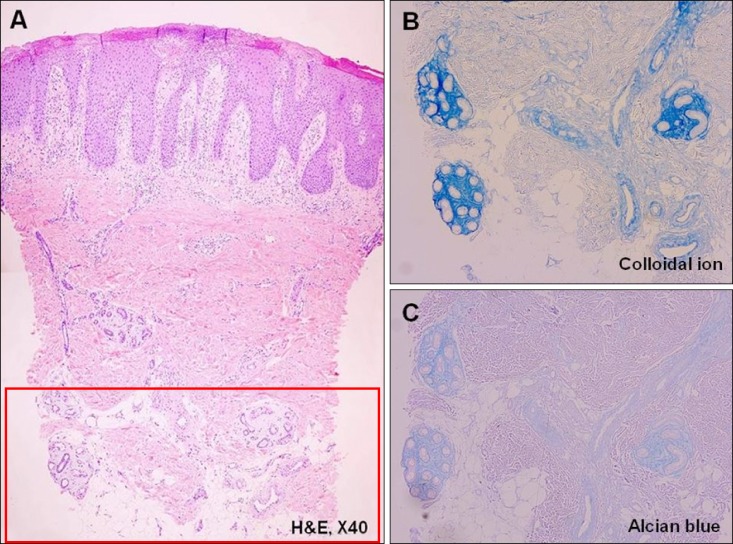
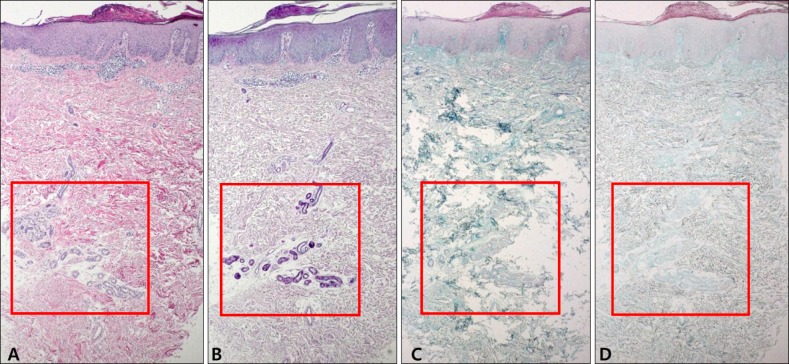
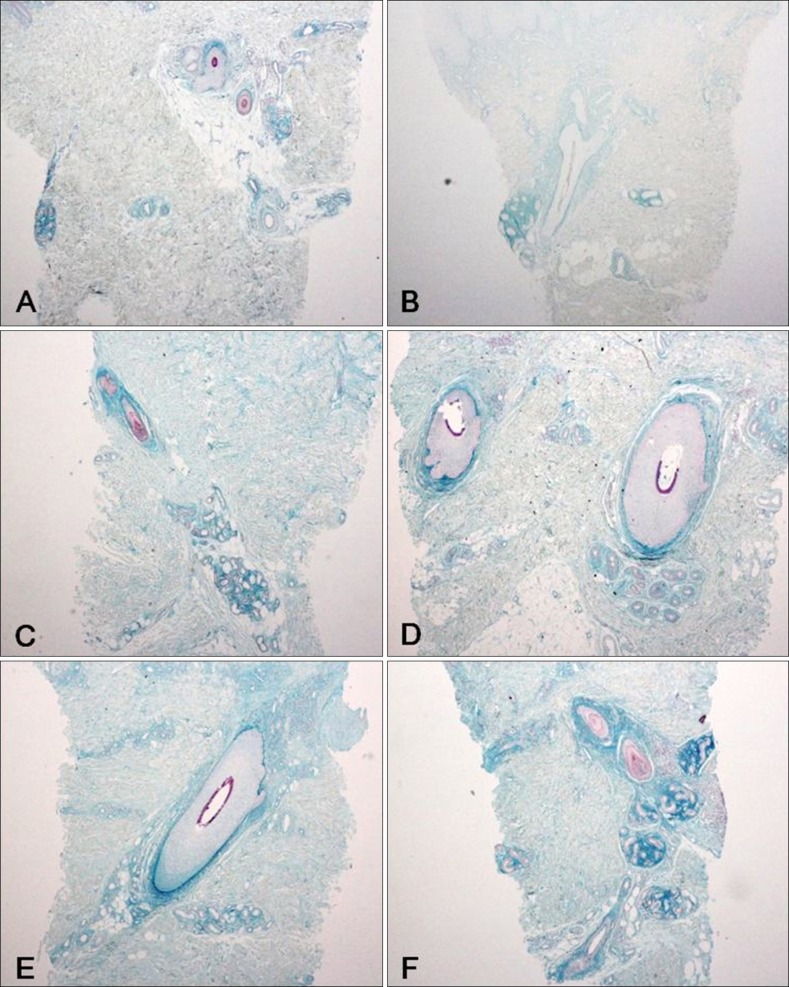
The histopathological examination of the specimens revealed variable degrees of mucin deposition in the periadnexal area in patients diagnosed with chronic eczema. Mucin deposition was more visible after staining with mucin-specific stains While the samples were stained positive with Alcian blue (pH 2.5) and colloidal iron, they were stained negative with PAS. Mucin deposition in the periadnexal area was significantly higher in patients with chronic eczema than in the age- and site-matched controls (Fig. 1,2,3,4). Among psoriasis patients, none of the samples were stained with PAS, colloidal iron, or Alcian blue (Fig. 5). Only three samples were stained weakly when Alcian blue and colloidal iron were used as the staining agent. Among 36 slides, 18 in which hair follicles existed in the tissue specimens were stained weakly to strongly with colloidal iron (Fig. 6).
Fig. 1. Pathology of a 56-year-old male who is diagnosed with chronic eczema. (A) Periadnexal mucin deposition (H&E, ×40), (B) mucin deposition is more visible when the slide is stained with colloidal iron (×40), and (C) the periodic acid-Schiff (PAS) staining is negative. These findings indicate that the mucin around the periadnexal area is dermal mucin, not epithelial mucin (PAS, ×40).
Fig. 2. Pathology of a 61-year-old male who is diagnosed with lichen simplex chronicus. (A) Chronic eczema showing periadnexal mucin deposition (H&E, ×40), (B) the mucin deposition is clearly visible after staining with colloidal iron and (C) Alcian blue (×40), and (D) the periodic acid-Schiff (PAS) staining is negative (×40).
Fig. 3. Pathology of a 5-year-old male who is diagnosed with atopic dermatitis. (A) Chronic eczema showing periadnexal mucin deposition (H&E, ×40), (B) the mucin deposition is more prominent after staining with colloidal iron, and (C) Alcian blue (×200).
Fig. 4. Pathology of the control samples that are diagnosed with normal skin or acute eczema. Mucin deposition is less visible in the control samples. (A) Normal skin (H&E, ×40), (B) normal skin (colloidal iron, ×40), (C) acute eczema (H&E, ×40), and (D) acute eczema (colloidal iron, ×40).
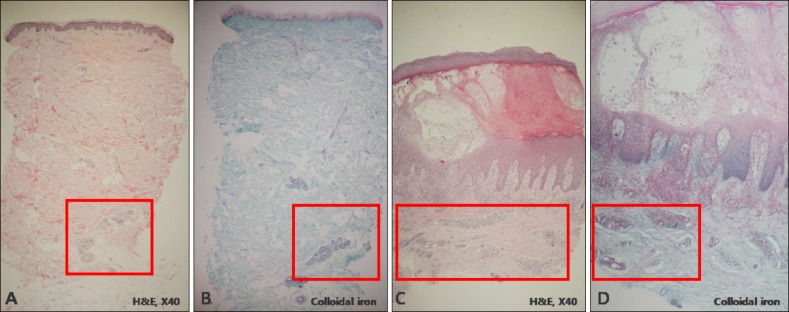
Fig. 5. Pathology of the control samples that are diagnosed with psoriasis vulgaris. (A) H&E (×40), (B) periodic acid-Schiff (×40), (C) Alcian blue (×40), and (D) colloidal iron (×40) staining are negative.
Fig. 6. Pathology of the patients diagnosed with chronic eczema with their hair follicles in the specimen (colloidal iron, ×100). A, B: weak, C, D: moderate, E, F: strong.
Staining analysis
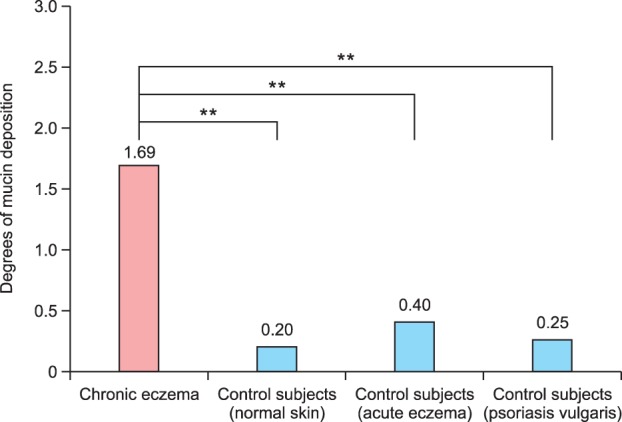
The staining intensity scores were significantly higher in patients with chronic eczema than in those with acute eczema, psoriasis, or normal skin (all, p<0.01) (Fig. 7).
Fig. 7. Physician assessment of the mucin deposition using a 4-point scale. The staining intensities are significantly higher in patients with chronic eczema compared to those with acute eczema, psoriasis, or normal skin (**p<0.01).
DISCUSSION
Cutaneous mucinoses are a heterogeneous group of diseases characterized by the abnormal deposition of mucin, an amorphous substance composed primarily of hyaluronic acid and sulfated glycosaminoglycans5. Although many dermatologic diseases show cutaneous mucinoses and have considerable overlap in their characteristics, the underlying cause of abnormal mucin deposition is still in dispute6.
Mucin, which is normally present in several organs, is sub-classified as epithelial and dermal mucin according to its composition. Epithelial and dermal mucins have different staining patterns. In the skin, epithelial mucin or sialomucin is present in the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains neutral and acid glycosaminoglycans and stains with Alcian blue (pH 2.5) similar to dermal mucin, but it is PAS-positive and resistant to hyaluronidase2. Conversely, dermal mucin, which is normally produced by dermal fibroblasts, is present in the ground substance of the dermis. Dermal mucin consists of acid glycosaminoglycans, which are repeating polysaccharides that form complex carbohydrates. It stains with Alcian blue at a pH of 2.5 but not at a pH of 0.4; in addition, it is PAS-negative, stains metachromatically purple with toluidine blue at a pH of 4.0 but not at a pH of 1.5, and is sensitive to hyaluronidase2. In this study, there were variable degrees of mucin deposition in the periadnexal area, especially in the perieccrine structure, in patients diagnosed with chronic eczema. Although they did not stain when PAS was used as a staining agent, the samples stained positively with Alcian blue (pH 2.5) and colloidal iron. These findings indicate that the periadnexal mucin present in the chronic eczematous patients was dermal mucin.
As previously mentioned, cutaneous mucinoses can be divided into primary cutaneous mucinoses and secondary mucinoses. Primary mucinosis consists of diffuse form and focal form. The former includes pretibial myxedema, generalized myxedema, scleredema, and reticular erythematous mucinosis. There are cutaneous focal mucinosis, myxoid cysts, follicular mucinosis, and acral persistent papular mucinosis in the latter. We believe that our cases were secondary dermal mucinoses, because the mucin deposition was not a main histologic feature of the chronic eczema.
The reason why mucin is abnormally produced in chronic eczema is unclear. Some studies have proposed possible pathomechanisms for the abnormal mucin production. Pandya et al.3 reported that certain types of serum factors such as immunoglobulins or cytokines can stimulate glycosaminoglycan synthesis. Serum immunoglobulin levels or circulating autoantibodies are elevated in various dermatologic diseases such as lichen myxedematosus, Graves' disease-associated pretibial myxedema, and papulonodular mucinosis associated with lupus erythematosus7,8. In addition, some cytokines are known to stimulate glycosaminoglycan synthesis in the skin. Interleukin (IL)-1, the tumor necrosis factors (TNF), and transforming growth factor are examples of these kinds of cytokines that can play a major role in glycosaminoglycan synthesis9,10. There are some differences in cytokine expression according to the course of the disease. For example, expression of the cytokines IL-10, IL-6, interferon (IFN)-γ, IL-4, and TNF-α is significantly higher in the chronic state of atopic dermatitis than in the acute state11,12,13. Although there are many mechanisms involved in the pathogenesis of psoriasis, from an immunologic point, the proliferation of keratinocytes is stimulated by diverse cytokines produced by T cells (IFN-γ, IL-2, and TNF-α) and keratinocytes (IL-6, IL-8, TFG-α, and β). IL-6, TNF-α, and IFN-γ are overlapping cytokines in eczema and psoriasis. However, there are some differences in the pathogenesis between the two diseases; that is, there are other factors that contribute to the pathomechanism. Therefore, we hypothesize that inflammatory cytokines associated with chronic eczema may stimulate glycosaminoglycan synthesis in the skin.
Physician-assessed staining intensities of the mucin deposition were much higher in patients with chronic eczema than in a control group with acute eczema, psoriasis, and normal skin, and the differences were statistically significant. Mucin deposition in chronic eczema is mainly located in the periadnexal area, not the entire dermis. In addition, some degree of mucin deposition was also observed in the papillary dermis. There was hardly any mucin deposition around the hair follicles in the tissues of the normal or acute eczema group, which included hair follicles on the slide. Of 36 specimens from the chronic eczema group, 18 slides in which hair follicle existed showed a weak to strong mucin deposition in the perifollicular area. The periadnexal area or adventitial dermis has different properties compared to the other reticular dermis and represents the effects of the epidermis14.
To clarify the histologic significance, we compared samples of chronic eczema with samples of psoriasis to other chronic inflammatory dermatoses. Compared to chronic eczema, none of the psoriatic samples stained well with colloidal iron or Alcian blue, while only three of 20 samples stained weakly with Alcian blue and colloidal iron. The intensity of mucin staining for psoriasis was lower than for acute and chronic eczema. Based on these findings, we think that periadnexal mucin deposition can be one of the differential points between chronic eczematous dermatitis and chronic papulosquamous dermatoses such as psoriasis.
In summary, we used a histological approach to demonstrate the presence of periadnexal mucin deposition in chronic eczematous dermatitis biopsy samples. These findings were consistent with secondary mucinoses, because the mucin deposition was not a pathognomic sign of chronic eczematous dermatitis. In addition, these findings were more prominent in chronic eczematous dermatitis than in normal skin, acute eczematous dermatitis, and psoriasis. Therefore, we propose that periadnexal mucin may be an unusual and additional histological finding of chronic eczematous dermatitis.
ACKNOWLEDGMENT
This work was supported by a research grant from the Yonsei University Wonju College of Medicine (YUWCM_2012_54).
References
- 1.Wintroub B, Arndt K, Robinson J, Leboit P. The cutaneous mucinosis. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, editors. Cutaneous medicine and surgery: an integrated program in dermatology. Philadelphia: WB Saunders Co.; 1996. pp. 1832–1840. [Google Scholar]
- 2.Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257–267. doi: 10.1097/00000372-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Pandya AG, Sontheimer RD, Cockerell CJ, Takashima A, Piepkorn M. Papulonodular mucinosis associated with systemic lupus erythematosus: possible mechanisms of increased glycosaminoglycan accumulation. J Am Acad Dermatol. 1995;32:199–205. doi: 10.1016/0190-9622(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 4.Wu H, Brandling-Bennett HA, Harrist TJ. Noninfectious vesiculobullous and vesiculopustular diseases. In: Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Lever's histopathology of the skin. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 237. [Google Scholar]
- 5.Rebora A, Rongioletti F. Mucinosis. In: Bolognia JL, Jorrizo JL, Rapini RR, editors. Dermatology. Mosby: Elsevier Limited; 2003. p. 647. [Google Scholar]
- 6.Edward M, Fitzgerald L, Thind C, Leman J, Burden AD. Cutaneous mucinosis associated with dermatomyositis and nephrogenic fibrosing dermopathy: fibroblast hyaluronan synthesis and the effect of patient serum. Br J Dermatol. 2007;156:473–479. doi: 10.1111/j.1365-2133.2006.07652.x. [DOI] [PubMed] [Google Scholar]
- 7.Harper RA, Rispler J. Lichen myxedematosus serum stimulates human skin fibroblast proliferation. Science. 1978;199:545–547. doi: 10.1126/science.622555. [DOI] [PubMed] [Google Scholar]
- 8.Cheung HS, Nicoloff JT, Kamiel MB, Spolter L, Nimni ME. Stimulation of fibroblast biosynthetic activity by serum of patients with pretibial myxedema. J Invest Dermatol. 1978;71:12–17. doi: 10.1111/1523-1747.ep12543646. [DOI] [PubMed] [Google Scholar]
- 9.Duncan MR, Berman B. Differential regulation of collagen, glycosaminoglycan, fibronectin, and collagenase activity production in cultured human adult dermal fibroblasts by interleukin 1-alpha and beta and tumor necrosis factoralpha and beta. J Invest Dermatol. 1989;92:699–706. doi: 10.1111/1523-1747.ep12696891. [DOI] [PubMed] [Google Scholar]
- 10.Falanga V, Tiegs SL, Alstadt SP, Roberts AB, Sporn MB. Transforming growth factor-beta: selective increase in glycosaminoglycan synthesis by cultures of fibroblasts from patients with progressive systemic sclerosis. J Invest Dermatol. 1987;89:100–104. doi: 10.1111/1523-1747.ep12580445. [DOI] [PubMed] [Google Scholar]
- 11.Vakirlis E, Lazaridou E, Tzellos TG, Gerou S, Chatzidimitriou D, Ioannides D. Investigation of cytokine levels and their association with SCORAD index in adults with acute atopic dermatitis. J Eur Acad Dermatol Venereol. 2011;25:409–416. doi: 10.1111/j.1468-3083.2010.03800.x. [DOI] [PubMed] [Google Scholar]
- 12.Villagomez MT, Bae SJ, Ogawa I, Takenaka M, Katayama I. Tumour necrosis factor-alpha but not interferon-gamma is the main inducer of inducible protein-10 in skin fibroblasts from patients with atopic dermatitis. Br J Dermatol. 2004;150:910–916. doi: 10.1111/j.1365-2133.2004.05937.x. [DOI] [PubMed] [Google Scholar]
- 13.Ackermann L, Harvima IT. Mast cells of psoriatic and atopic dermatitis skin are positive for TNF-alpha and their degranulation is associated with expression of ICAM-1 in the epidermis. Arch Dermatol Res. 1998;290:353–359. doi: 10.1007/s004030050317. [DOI] [PubMed] [Google Scholar]
- 14.Junqueira LC, Montes GS, Martins JE, Joazeiro PP. Dermal collagen distribution. A histochemical and ultrastructural study. Histochemistry. 1983;79:397–403. doi: 10.1007/BF00491775. [DOI] [PubMed] [Google Scholar]