Abstract
Background
Vitiligo is a chronic, common disease of unknown etiology, and oxidative stress is suggested to have a role in its etiopathogenesis.
Objective
Advanced oxidation protein products (AOPPs), prooxidant-antioxidant balance (PAB), and ferric-reducing antioxidant power (FRAP) were evaluated regarding their role in the pathogenesis of vitiligo as well as their relationship with clinical presentation and disease severity, and these parameters were compared with those of healthy controls.
Methods
The study included 53 patients with vitiligo and 20 healthy volunteers as the control group. AOPP level, PAB, and FRAP were determined by colorimetric methods.
Results
PAB and FRAP level were significantly higher in patients with vitiligo than in healthy controls (p<0.001). The AOPP levels in vitiligo patients were not statistically significantly higher than those in healthy controls. The Vitiligo Area Scoring Index positively correlated with disease duration (rs: 0.531, p<0.001).
Conclusion
To the best of our knowledge, this is the first report of AOPP and PAB status in vitiligo. PAB may be used as an indicator for oxidative stress in the etiopathogenesis of vitiligo. Our results show that these parameters may play a major role in the melanocyte damage observed in vitiligo. Further studies are required to confirm the mechanisms underlying this effect.
Keywords: Advanced oxidation protein products, Ferric-reducing antioxidant power, Prooxidant-antioxidant balance, Vitiligo
INTRODUCTION
Vitiligo is a chronic, asymptomatic, usually acquired pigmentation disorder characterized by depigmented white areas on the skin and mucosal surfaces1. Depigmentation is caused by melanocyte destruction that results in absence of pigment production in these areas2. It affects approximately 0.1%~8.8% of the population2. The etiology remains unclear, but the theories regarding its pathogenesis include genetic, neural, cytotoxic, viral, growth factor, autoimmune, and oxidant-antioxidant theories. However, none of these theories has been proven3.
Recently, oxidative stress has been extensively investigated in melanocyte destruction. This reaction is a result of overproduction of reactive oxygen species (ROS) or impaired clearance of these species and characterized by an imbalance in the prooxidant and antioxidant systems4. To evaluate prooxidant-antioxidant balance (PAB), the determination of both the oxidant status and the antioxidant status is often necessary. The majority of currently available methods that separately determine oxidative balance measure the total prooxidant and antioxidant capacities.
Consequently, these methods are difficult, time-consuming, expensive, and imprecise5. Here, we evaluated the PAB in vitiligo patients using a simple, rapid, and inexpensive PAB assay. Despite the well-documented role of oxidative stress in vitiligo, the determination of PAB is not a routine clinical laboratory test, mainly because of the lack of a universally accepted method6,7.
Some investigators have reported increased total antioxidant levels in serum, while others have reported normal levels or decreased levels of these biomarkers2,8,9,10. In recent years, a simple test measuring the ferric-reducing ability of plasma, the ferric-reducing antioxidant power (FRAP) assay, has been presented as a new method for evaluating antioxidant capacity11. The total antioxidant capacity (TAC) can be defined as the cumulative action of all antioxidants present in the plasma and body fluids, thus providing a composite parameter rather than the sum of measurable antioxidants12.
Clinically, the most important indicators of oxidative stress are lipid peroxidation and protein oxidation. Lipid peroxidation is particularly important in the vascular damage and melanocyte destruction in vitiligo. The addition of carbonyl groups, which are the result of proteolysis and breakage of protein side chains, are the most important components of protein oxidation13,14. Many of the published studies involve the detection of lipid peroxidation production and antioxidant levels in serum2,8,9,10. Studies regarding protein oxidation levels in patients with vitiligo are lacking. Recently, a new marker of protein oxidation, advanced oxidation protein products (AOPPs), has been considered for oxidant-mediated protein damage15. AOPPs are proteins that are damaged by oxidative stress, most notably albumin and its aggregates. AOPPs contain abundant dityrosines, which allow cross-linking, disulfide bridges, and carbonyl groups. AOPPs are formed primarily by chlorinated oxidants, including hypochloric acid and chloramines, which result from myeloperoxidase activity16. Although several studies show the importance of oxidative stress in the pathogenesis of vitiligo, we were unable to find data in the literature regarding the effects of immune-mediated oxidative stress on AOPPs and PAB in vitiligo patients. Therefore, PAB, AOPP levels, and FRAP were evaluated regarding their role in the pathogenesis of vitiligo as well as their relationship with clinical presentation and disease severity, and these parameters were compared with those of healthy controls.
MATERIALS AND METHODS
Subjects
A total of 73 individuals (53 vitiligo patients, mean age: 36.87±11.09 years; 20 controls, mean age: 48.00±4.81 years) were included in this study. None of the patients had been treated for vitiligo. A general dermatological examination was performed for all patients. The location of vitiligo lesions was recorded. Clinical subtypes of vitiligo were defined as segmental or nonsegmental. The severity and extent of disease were calculated using the palmar unit measurement (one palmar unit represents the depigmented lesion size of the palm of the hand). According to the history provided by the patients, the Vitiligo Disease Activity Score (VIDA) was used to determine either active or stable disease. Active disease refers to an expansion of lesions and appearance of new lesions in the previous year. All patients and controls were of Turkish descent. All participants were informed about the survey and freely signed and dated the consent form. The protocol was approved by the ethics committee of the Cerrahpaşa Medical Faculty and was conducted in accordance with the Declaration of Helsinki.
Inclusion criteria
Eligibility was based on the following inclusion criteria: >18 years old; either sex; not pregnant; no smoking habit; no history of an autoimmune disease, concomitant dermatological disease, or thyroid dysfunction; and no history of smoking, alcohol intake, vitamin intake, or use of anti-inflammatory or other drugs. In addition, none of the patients had undergone any therapy for 3 months prior to enrolment.
Exclusion criteria
The control group consisted of 20 healthy subjects selected from the hospital staff with no personal or family history of diabetes or dyslipidemia, and had normal thyroid, hepatic, and renal function. The 2-h glucose tolerance test was used to rule out impaired glucose regulation in the healthy controls. Smokers and healthy subjects who were using drugs that were known to affect carbohydrate and lipid metabolism or oxidant and antioxidant status were also excluded from the control group.
Laboratory analysis
1) Sample collection and preparation
None of the patients and controls used any drugs for at least 24 h prior to blood collection. Clinical parameters, including routine biochemical parameters, were measured using standard protocols. Peripheral blood samples from patients with vitiligo were obtained from the Department of Dermatology in the Cerrahpaşa Medical School. Normal peripheral blood samples were obtained from the clinically healthy individuals in our medical school and in our departments. Blood samples were collected in EDTA-containing tubes and anticoagulant-free tubes after an overnight fast. After immediate centrifugation (3,000 g) for 10 min at 4℃, plasma and serum were separated in Eppendorf tubes and frozen immediately at -80℃ until analysis.
2) Measurements of plasma advanced protein oxidation product levels
Spectrophotometric determinations of AOPP levels were performed using a modification of the method by Gelisgen et al.17 Samples were prepared as follows: 200 µl of plasma was diluted 1 : 5 in PBS, followed by the addition of 10 µl of 1.16 M KI to each tube and, finally, 20 µl of absolute acetic acid 2 min later. The absorbance of the reaction mixture was immediately read at 340 nm against a blank containing 2,000 µl of PBS, 200 µl of acetic acid, and 100 µl of KI. The linear range of chloramine-T absorbance at 340 nm occurs between 0 and 100 µmol/L. AOPP concentrations are expressed in µmol/L of chloramine-T equivalents. The coefficients of intra- and inter-assay variation were 3.1% (n=15) and 3.6% (n=15), respectively.
3) Measurement of serum ferric-reducing antioxidant power
The FRAP assay was performed according to the protocol of Benzie and Strain11, with minor modifications. To prepare the FRAP solution, 10 ml of acetate buffer (300 mM, pH=3.6) was mixed with 1 ml of 20 mM FeCl3 dissolved in distilled water and 1 ml of 10 mM 2,4,6-tri (2-pyridyl)-s-triazine dissolved in 40 mM HCl. Next, 50 µl of serum was added to 1.5 ml of a freshly prepared FRAP solution and measured over a period of 4 min at 593 nm. Different concentrations of uric acid (0.09~9.00 mM) were used to obtain a calibration curve on each day of the experiment. Uric acid is the primary contributor of the antioxidant capacity of serum17 and was thus used as a standard. The coefficients of intra- and inter-assay variations were 4.2% (n=15) and 6.1% (n=15), respectively.
4) Measurement of serum prooxidant-antioxidant balance
The PAB assay was performed according to the method of Alamdari et al.7, with minor modifications. Tetramethylbenzidine 3, 3', 5.5' (TMB) and TMB cations were used as oxidation-reduction indicators, due to their optical and electrochemical properties. With this approach, it is possible to measure the PAB simultaneously in one experiment by two different reaction types: one enzymatic reaction where the chromogen TMB is oxidized to a color cation by peroxides and one chemical reaction where the TMB cation is reduced to a colorless compound by antioxidants. The absorbance was measured using an enzyme-linked immunosorbent assay plate reader (ELX 800 UV; BioTek Instruments, Winooski, VT, USA) at 450 nm, with a reference wavelength of 620 or 570 nm. A standard curve was determined using the values obtained from the standard samples. PAB are expressed in arbitrary units, which correspond to the percentage of H2O2 in the standard solution. The coefficients of intra- and inter-assay variation were 5.3% (n=15) and 6.6% (n=15), respectively.
5) Statistical analysis
Data analysis was conducted using IBM SPSS Statistics 21.0 (IBM Co., Armonk, NY, USA). The results are reported as mean±standard deviation or median. Sex distribution was compared between the groups using Pearson chi-square analysis. Because the data were not normally distributed (Shapiro-Wilk test, p<0.05), nonparametric statistics (Mann-Whitney U tests) were used to evaluate AOPP levels and FRAP. Parametric statistics (Student t-tests) were used to evaluate PAB and age. Spearman correlation tests were used to determine correlations. Statistical significance was determined using p<0.05.
RESULTS
The demographic and clinical characteristics of the groups are shown in Table 1. According to the Pearson chi-square analysis, the distribution of sex was significantly different between the groups (p=0.018).
Table 1. Demographic and clinical characteristics of vitiligo patients and controls.
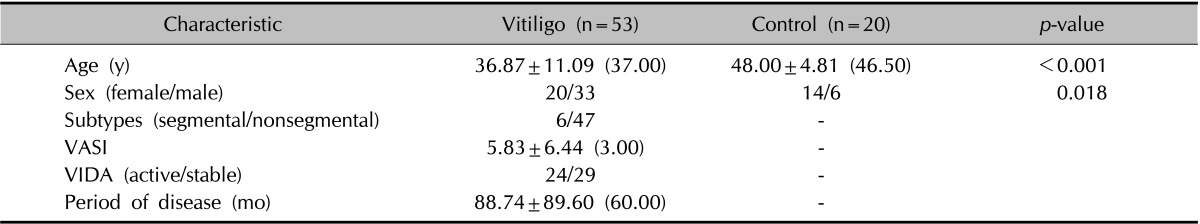
Values are presented as mean±standard deviation (median). VASI: Vitiligo Area Scoring Index, VIDA: Vitiligo Disease Activity Score.
The oxidative stress parameters in the groups are shown in Table 2. FRAP and PAB in patients with vitiligo were significantly higher than those in the control group (p<0.001). However, the AOPP levels in the patient group were not significantly higher than those in the control group because of the high median values in the control group.
Table 2. Oxidative stress parameters of vitiligo patients and controls.
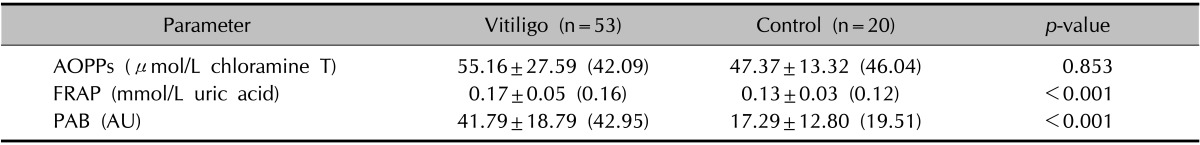
Values are presented as mean±standard deviation (median). AOPPs: advanced protein oxidation products, FRAP: ferric-reducing antioxidant power, PAB: prooxidant-antioxidant balance, AU: arbitrary units that correspond to the percentage of H2O2 in the standard solution.
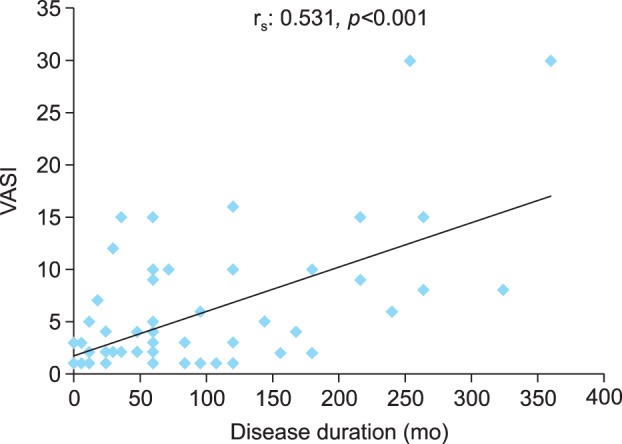
AOPP levels, FRAP, and PAB did not correlate with age, period of disease, vitiligo subtypes, the Vitiligo Area Scoring Index (VASI), or VIDA (Table 3) in the patient group. However, there was a significant positive correlation (rs: 0.531, p<0.001) between the disease duration and VASI (Fig. 1).
Table 3. Oxidative stress parameters of vitiligo patients with active or stable disease.
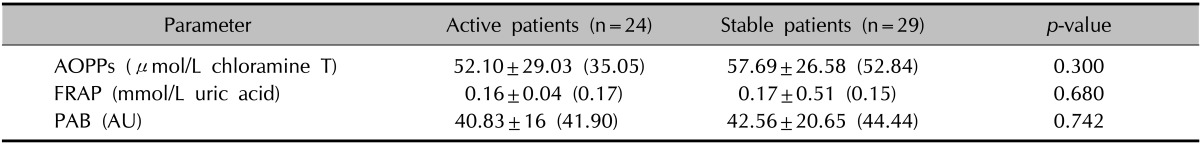
Values are presented as mean±standard deviation (median). AOPPs: advanced protein oxidation products, FRAP: ferric-reducing antioxidant power, PAB: prooxidant-antioxidant balance, AU: arbitrary units correspond to the percentage of H2O2 in the standard solution.
Fig. 1. Relationship between the Vitiligo Area Scoring Index (VASI) and disease duration in patients with vitiligo.
DISCUSSION
Human skin serves as an interface between the environment and the body. It is constantly exposed to a broad array of physical, chemical, and biological agents, many of which either are inherent oxidants or catalyze the generation of ROS. Oxidative stress is believed to be involved in the pathogenesis of inflammatory skin diseases18. The present study demonstrated that FRAP and PAB were significantly higher in patients with vitiligo than in healthy controls. Although the AOPP levels were higher in the patient group, this was not statistically significant. These results indicate that increased FRAP, PAB, and AOPP levels are likely associated with oxidative stress in vitiligo.
Melanocyte damage in vitiligo pathogenesis remains unclear. A single mechanism is not responsible for all cases of melanocyte damage in vitiligo, and oxidative stress is considered a possible pathogenic event in melanocyte loss6. PAB is important for establishing the health status of the body19. Decreased antioxidant levels and increased prooxidant levels may play important roles in the damage of melanocytes observed in vitiligo patients20. PAB was higher in vitiligo patients in the present study. One possible explanation for this might be impaired PAB. A shift in this equilibrium likely contributes to the pathogenesis of vitiligo. The PAB assay is a simple, rapid, and inexpensive method that can measure the prooxidant burden and the antioxidant capacity in one assay. This ratio can be used as an additive marker of oxidative stress in patients with vitiligo as in patients with Behçet disease (BD)19. Based on our results, PAB may be the initial pathogenic event in melanocyte degeneration. Nevertheless, we suggest that the PAB assay may be useful as a risk predictor in diseases such as vitiligo and could help to identify patients with high oxidative stress to facilitate early preventive interventions for melanocyte damage.
Several investigators have assessed the capacity of antioxidative defense systems in vitiligo21,22. However, the results are controversial. TAC has been reported to be lower21 or higher22, depending on the study. Akoglu et al.21 reported that patients with vitiligo had significantly lower TAC than controls. Boisseau-Garsaud et al.22 reported the blood antioxidant status of African American patients with active generalized vitiligo. The total blood antioxidant level was significantly higher in vitiligo patients than in sex- and age-matched controls. The FRAP in patients with vitiligo was also significantly higher than that in the control group. Our results are similar to those of Boisseau-Garsaud et al.22 Perhaps, antioxidant molecules cannot be used actively within the white areas. Clinically, it is also difficult to determine the active period of vitiligo. In contrast, we found that both PAB and FRAP were higher, but not correlated, in vitiligo patients. It is unclear whether high FRAP indicates a resource of unused antioxidants or up-regulated antioxidant production as a response to oxidative stress. We consider oxidative stress as a causative factor in patients with vitiligo. However, further studies are required to confirm the mechanisms underlying this effect.
AOPPs have been proposed as a possible marker of oxidative injury that originate as a result of the action of free radicals on proteins. Although AOPP levels were higher, this was not statistically significant. AOPP levels have been reported to be significantly increased in active periods of patients with BD compared with those in healthy controls and in the remission periods of patients with BD19. Previous studies (both human and experimental) have demonstrated that older people have higher AOPP levels19. Despite an older age in the control group, AOPP levels were higher (statistically insignificant) in patients with vitiligo in the present study. This might partially support the role of oxidative stress in vitiligo pathogenesis. In addition, high AOPP levels are indicative of an increase in oxidative stress and can cause direct oxidative damage to proteins in vitiligo patients. These oxidized proteins may partially contribute to the development of melanocyte loss. As a disease characterized with oxidative stress-autoimmunity-mediated melanocyte loss, the pathogenesis of vitiligo is unclear. Our results indicate that oxidative stress might play a role in the etiopathogenesis of vitiligo, because of the increased serum PAB and AOPP. PAB may be especially useful in this regard because of its measurement is rapid, easy, and cost-effective in laboratories. Thus, we recommend that PAB measurement be more widely used in clinical practice. FRAP should be reevaluated in future clinical trials in the early stage of the disease. Also, FRAP is important in treated patients with vitiligo due to changes in the antioxidant capacity as a result of treatment.
References
- 1.Denli Y, Acar MA, Sönmezoğlu Maraklı S, Yücel A. Vitiligo. Disorders of pigmentation. In: Tüzün Y, Gürer MA, Serdaroğlu S, Oğuz O, Aksungur V, editors. Dermatology. 3rd ed. İstanbul: Nobel Publishing; 2008. pp. 1465–1490. [Google Scholar]
- 2.Kutlubay Z, Uzuncakmak TK, Engin B, Tüzün Y. Vitiligo and oxidative stress. J Turk Acad Dermatol. 2011;5:1154r1. [Google Scholar]
- 3.Schallreuter KU, Bahadoran P, Picardo M, Slominski A, Elassiuty YE, Kemp EH, et al. Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol. 2008;17:139–140. doi: 10.1111/j.1600-0625.2007.00666_1.x. discussion 141-160. [DOI] [PubMed] [Google Scholar]
- 4.Karaca Ş, Güder H. Antioxidant system in dermatology. Turk J Dermatol. 2009;3:32–39. [Google Scholar]
- 5.Parizadeh SM, Azarpazhooh MR, Moohebati M, Nematy M, Ghayour-Mobarhan M, Tavallaie S, et al. Simvastatin therapy reduces prooxidant-antioxidant balance: results of a placebo-controlled cross-over trial. Lipids. 2011;46:333–340. doi: 10.1007/s11745-010-3517-x. [DOI] [PubMed] [Google Scholar]
- 6.Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, Ramachandran AV, et al. Vitiligo: interplay between oxidative stress and immune system. Exp Dermatol. 2013;22:245–250. doi: 10.1111/exd.12103. [DOI] [PubMed] [Google Scholar]
- 7.Alamdari DH, Ghayour-Mobarhan M, Tavallaie S, Parizadeh MR, Moohebati M, Ghafoori F, et al. Prooxidant-antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin Biochem. 2008;41:375–380. doi: 10.1016/j.clinbiochem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Arican O, Kurutas EB. Oxidative stress in the blood of patients with active localized vitiligo. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:12–16. [PubMed] [Google Scholar]
- 9.Jain A, Mal J, Mehndiratta V, Chander R, Patra SK. Study of oxidative stress in vitiligo. Indian J Clin Biochem. 2011;26:78–81. doi: 10.1007/s12291-010-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koca R, Armutcu F, Altinyazar HC, Gürel A. Oxidant-antioxidant enzymes and lipid peroxidation in generalized vitiligo. Clin Exp Dermatol. 2004;29:406–409. doi: 10.1111/j.1365-2230.2004.01524.x. [DOI] [PubMed] [Google Scholar]
- 11.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 12.Karacay O, Sepici-Dincel A, Karcaaltincaba D, Sahin D, Yalvaç S, Akyol M, et al. A quantitative evaluation of total antioxidant status and oxidative stress markers in preeclampsia and gestational diabetic patients in 24-36 weeks of gestation. Diabetes Res Clin Pract. 2010;89:231–238. doi: 10.1016/j.diabres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Breusing N, Grune T. Biomarkers of protein oxidation from a chemical, biological and medical point of view. Exp Gerontol. 2010;45:733–737. doi: 10.1016/j.exger.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Q, Wu S, Jiang J, Tian J, Chen J, Yu X, et al. Accumulation of circulating advanced oxidation protein products is an independent risk factor for ischaemic heart disease in maintenance haemodialysis patients. Nephrology (Carlton) 2012;17:642–649. doi: 10.1111/j.1440-1797.2012.01640.x. [DOI] [PubMed] [Google Scholar]
- 16.Cakatay U, Kayali R, Uzun H. Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population. Clin Exp Med. 2008;8:51–57. doi: 10.1007/s10238-008-0156-0. [DOI] [PubMed] [Google Scholar]
- 17.Gelisgen R, Genc H, Kayali R, Oncul M, Benian A, Guralp O, et al. Protein oxidation markers in women with and without gestational diabetes mellitus: a possible relation with paraoxonase activity. Diabetes Res Clin Pract. 2011;94:404–409. doi: 10.1016/j.diabres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Glassman SJ. Vitiligo, reactive oxygen species and T-cells. Clin Sci (Lond) 2011;120:99–120. doi: 10.1042/CS20090603. [DOI] [PubMed] [Google Scholar]
- 19.Ozyazgan S, Andican G, Erman H, Tuzcu A, Uzun H, Onal B, et al. Relation of protein oxidation parameters and disease activity in patients with Behçet's disease. Clin Lab. 2013;59:819–825. doi: 10.7754/clin.lab.2012.120722. [DOI] [PubMed] [Google Scholar]
- 20.Schallreuter KU. Successful treatment of oxidative stress in vitiligo. Skin Pharmacol Appl Skin Physiol. 1999;12:132–138. doi: 10.1159/000029867. [DOI] [PubMed] [Google Scholar]
- 21.Akoglu G, Emre S, Metin A, Akbas A, Yorulmaz A, Isikoglu S, et al. Evaluation of total oxidant and antioxidant status in localized and generalized vitiligo. Clin Exp Dermatol. 2013;38:701–706. doi: 10.1111/ced.12054. [DOI] [PubMed] [Google Scholar]
- 22.Boisseau-Garsaud AM, Garsaud P, Lejoly-Boisseau H, Robert M, Quist D, Arveiler B. Increase in total blood antioxidant status and selenium levels in black patients with active vitiligo. Int J Dermatol. 2002;41:640–642. doi: 10.1046/j.1365-4362.2002.01472.x. [DOI] [PubMed] [Google Scholar]


