Dear Editor:
Antinuclear antibody (ANA) was originally developed in an attempt to improve the sensitivity and specificity of tests for the diagnosis of systemic lupus erythematosus1. However, a positive ANA test result is also associated with a number of systemic rheumatic diseases including systemic sclerosis, rheumatic arthritis, polymyositis, dermatomyositis, localized scleroderma, various infectious diseases, malignancies, and miscellaneous other conditions1,2. Pattern hair loss (PHL) is the most common type of hair loss in men and women. It appears to result from androgen hyperactivity and a genetic predisposition3. However, pathomechanisms of PHL other than androgen-dependent traits have not been determined. Progressive hair miniaturization and hair follicle replacement with fibrosis might be induced by an autoimmune reaction4. Even with PHL, a relationship with ANA has not been reported. We sought to determine the frequency of ANA positivity and evaluate the relationship between ANA and clinical characteristics in patients with PHL.
We retrospectively reviewed patients with PHL who visited our hospital and had a qualitative analysis of serum ANA in 2009~2011. We analyzed data from medical records including age, sex, disease severity, and laboratory findings (hematologic tests, thyroid function tests, ANA, and rheumatoid factor [RF]). We evaluated the relationship between PHL severity and ANA positivity using data from hair phototrichogram and the basic and specific (BASP) classification3. Approval for this study was obtained from the institutional review board of St. Paul's Hospital, Catholic Medical Center (IRB No. PC13RISE0001).
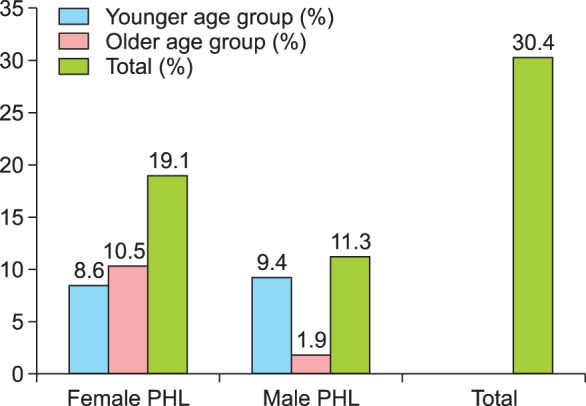
A total of 105 patients (49 women and 56 men) with PHL were included. Their median age was 41.2 years (men, 38.1 years; women, 42.1 years). ANA positivity was compared by age and sex. First, we divided patients with PHL into female and male groups. Then, we subdivided each group into younger (<40 years) and older (≥40 years) age groups. ANA positivity was present in 30.4% of the 105 patients (women, 19.1%; men, 11.3%). Briefly, the female group showed two times higher ANA positivity than the male group. However, the relationship with age was inconsistent (Fig. 1). The majority of patients (98%) with a positive ANA had a homogenous ANA pattern. Of the remaining patients, 1 patient showed a cytoplasmic pattern, and another patient showed a mixed pattern. Of the ANA-positive patients, 5 patients had abnormal thyroid stimulating hormone levels, and only 1 patient had a high RF titer.
Fig. 1. Antinuclear antibody positivity of patients with pattern hair loss (PHL) according to demographic information.
We examined the relationship between clinical characteristics and ANA positivity (Table 1). The ANA-positive group had a significantly higher female : male ratio than the ANA-negative group (p<0.05). However, there was no significant difference between the two groups in the mean age or proportion of patients with mild/moderate/severe grade using the BASP classification. Phototrichogram data also showed inconsistent results. Whereas the hair density of ANA-positive patients was lower than that of ANA-negative patients, the mean diameter of the hair shaft of ANA-positive patients was a little thicker than that of ANA-negative patients, although the difference was not statistically significant.
Table 1. Characteristics of patients with pattern hair loss by antinuclear antibody (ANA) positivity.
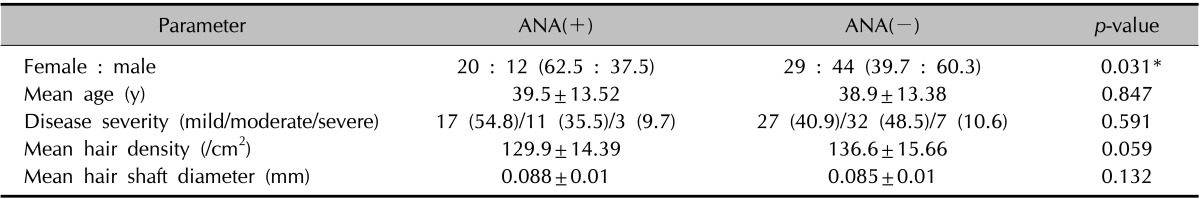
Values are presented as number (%) or mean±standard deviation. *p<0.05.
Similar to our results, healthy people also have positive ANA tests at lower titers, with 25%~30% of healthy people reported to have an ANA titer of 1 : 40, 10%~15% an ANA titer of 1 : 80, and 5% at least an ANA titer of 1 : 160. In particular, more female patients (21.4%) had a relationship with ANA than male patients (15.6%)2. With age, particularly in women, this frequency increases1.
The prevalence of ANA positivity in our patients with PHL was 30.4%. ANA positivity with PHL was similar in both the old and young age groups. This result indicates that PHL patients <40 years old have a relatively higher frequency of ANA than an age-matched normal population. A low ANA titer was not related to clinical characteristics such as disease duration or PHL severity. A positive ANA with PHL was significantly related to female sex. Sexual hormone levels or certain lifestyles that increase the susceptibility to skin damage might contribute to the development of ANA. Women more frequently style their hair, including dyeing and permanent hair curling. Consistent with this hypothesis, there is a report that exposure to hair dye may be associated with high ANA titers5.
It is uncertain whether a positive ANA in PHL is a causative agent or an effect of the PHL. The formation of ANA is suggested to play some roles in physiological immune homeostasis2. Certain drugs, infectious agents, and other environmental factors such as sunlight or silica dust may induce autoantibodies including ANA with or without development of autoimmune disease manifestations5,6. Additionally, the skin on the scalp of PHL patients is more exposed to daily sunlight due to balding, and the sun damage could destroy part of the skin structure. This may explain how ANA occurs in PHL patients. A relative increase in skin tissue autoantigens such as integrin-α6β4, desmoglein-4, and MMP-1 in healthy individuals with a positive ANA has been shown2. This supports the hypothesis that ANA positivity in PHL may result from the damage of skin organs and is associated with co-occurrence of other skin auto-antigens that can affect the immune environment of the hair follicle.
However, with increasing age, the older male group showed low positivity (1.9%), unlike the older female group. In a healthy population, the tendency of ANA positivity generally increases with aging6. We suggest that some physiologic role of male PHL might result in the decreased ANA positivity in the older male group but not the female group. Therefore, future comparisons should be conducted between older patients with and without PHL. It is also possible that the relatively small sample in the present study could have influenced the results, which might not be generalizable to the general population. ANA positivity did not correlate with PHL severity. Because our study was performed with a small sample that had a low ANA titer cut-off value (1 : 40), the significance of higher ANA titers in PHL patients and the correlation with disease severity should be studied further.
The majority of the patients showed a homogenous pattern of ANA. A homogenous pattern of ANA was previously observed in 70% of patients with morphea7. A homogeneous pattern might be associated with antibodies to native DNA or antibodies to histone and increased manifestation of autoimmunity7.
We also evaluated 17 patients with alopecia areata (AA), but we did not perform statistical analysis because of the limited number of patients. As preliminary data, AA patients showed similar results. A positive ANA test was present in 35.3% of the 17 AA patients. The female group (23.5%) had an ANA positivity that was two times higher than that of the male group (11.8%).
In conclusion, ANA positivity in our study showed similar results as those in healthy people, and the significantly higher ANA frequency in women with PHL was also similar to that in healthy people. However, ANA positivity in PHL patients did not increase with age as it does in healthy people. We suggest that the formation of ANA might result from skin immune homeostasis in PHL. The significance of ANA positivity and quantitative analysis of ANA should be performed in alopecia patients in the future.
References
- 1.Solomon DH, Kavanaugh AJ, Schur PH American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum. 2002;47:434–444. doi: 10.1002/art.10561. [DOI] [PubMed] [Google Scholar]
- 2.Li QZ, Karp DR, Quan J, Branch VK, Zhou J, Lian Y, et al. Risk factors for ANA positivity in healthy persons. Arthritis Res Ther. 2011;13:R38. doi: 10.1186/ar3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee WS, Ro BI, Hong SP, Bak H, Sim WY, Kim do W, et al. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37–46. doi: 10.1016/j.jaad.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Nyholt DR, Gillespie NA, Heath AC, Martin NG. Genetic basis of male pattern baldness. J Invest Dermatol. 2003;121:1561–1564. doi: 10.1111/j.1523-1747.2003.12615.x. [DOI] [PubMed] [Google Scholar]
- 5.Cooper GS, Parks CG, Schur PS, Fraser PA. Occupational and environmental associations with antinuclear antibodies in a general population sample. J Toxicol Environ Health A. 2006;69:2063–2069. doi: 10.1080/15287390600746165. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson BO, Skogh T, Ernerudh J, Johansson B, Löfgren S, Wikby A, et al. Antinuclear antibodies in the oldest-old women and men. J Autoimmun. 2006;27:281–288. doi: 10.1016/j.jaut.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Guevara-Gutiérrez E, Yinh-Lao J, García-Gutiérrez P, Tlacuilo-Parra A. Frequency of antinuclear antibodies in mestizo Mexican children with morphea. Clin Rheumatol. 2010;29:1055–1059. doi: 10.1007/s10067-010-1515-2. [DOI] [PubMed] [Google Scholar]


