Abstract
Background:
The effect of exercise during hemodialysis has been a controversial issue, however, there are just few studies about the effect of active exercise during hemodialysis.
Objectives:
This study aimed to compare the effects of passive and active intradialytic pedaling exercises on dialysis efficacy, electrolytes, hemoglobin, hematocrit, blood pressure and health-related quality of life in hemodialysis patients.
Patients and Methods:
This quasi-experimental study was conducted on 16 hemodialysis patients in Akhavan hemodialysis center in Kashan from April to November 2013. Active or passive intradialytic pedaling exercise was performed using a Mini-Bike for 30 minutes during the first two hours of the dialysis sessions. The quality of life (QOL) was assessed before and after the intervention. Blood pressure was examined at the beginning and then hourly during the dialysis sessions. Dialysis efficacy, levels of phosphorus, calcium, sodium, potassium and Blood urea nitrogen (BUN) were measured at the end of the intervention. Kolmogorov-Smirnov test, paired t test, Wilcoxon signed rank and Friedman tests and repeated measure analysis of variancewere used to analyze the data.
Results:
No significant changes were observed in serum potassium, phosphorus and calcium levels at the end of the passive exercise program compared to the baseline. However, phosphorus levels were significantly decreased in the active exercise program (P < 0.05). Moreover, the mean diastolic blood pressure was significantly decreased after the passive exercise (P = 0.039). Passive exercise did not significantly change the dialysis efficacy, urea reduction rate, hemoglobin and calcium levels. The mean overall QOL was 63.78 ± 21.15 at the beginning of the study, which was increased to 77.07 ± 21.14 at the end of eight weeks of the intradialytic exercise (P = 0.007).
Conclusions:
The passive intradialytic exercise had a positive effect on blood pressure. The active exercise could decrease the serum phosphorus and potassium levels. Moreover, both exercise programs could significantly improve the QOL. Both active and passive intradialytic exercises can have some beneficial effects.
Keywords: Exercise, Hemodialysis, Quality of Life
1. Background
In end stage renal disease (ESRD) approximately 90% of renal function is lost, and body is not able to maintain fluid and electrolyte balance, adequate waste removal, and normal hormone function (1). Several types of renal replacement therapies are available; however, hemodialysis (HD) is the most common method (2). Nowadays, more than two million people with renal failure are treated by Hemodialysis worldwide (3). Hemodialysis, is a time-consuming procedure that takes at least 3 to 5 hours a day, two or three times a week (4). Dialysis induces notable metabolic changes including hypovolemia due to ultrafiltration, and rapid changes in electrolyte concentrations and systemic inflammation, which can all adversely affect physical function (3). Despite regular HD treatment to replace some of the kidney functions, HD patients suffer from a symptoms characterized by the "uremic" syndrome. These are typically manifested asautonomic and motor neuropathies, cardiac and skeletal muscle myopathies, peripheral vascular changes, anemia, dysfunction of bone metabolism, immunologic compromise, and an assortment of physiologic complaints such as nausea, vomiting, insomnia and fatigue (1). A high-quality dialysis improves the quality of life and increases the survival rate in patients with ESRD (5). An intradialytic exercise program can improve serum urea clearance and dialysis efficacy (1, 6, 7). Moreover, an intradialytic exercise increases muscle blood flow and open capillary surface area in working muscles that will result in a greater flux of urea and other toxins from the tissue to the vascular compartment for the subsequent removal at the dialyzer (1). Also, the exercise or regular physical activity in hemodialysis can improve the control of high-blood pressure and diabetes and also increase the health-related quality of life (8). Moreover, exercise can increase the blood-flow to the muscles that consequently reduce the pain in these patients (2). A recent study showed that moderate exercise had positive effects on physical and mental health. These include improvements in aerobic capacity, lower extremity muscle strength, systolic blood pressure, and lipid metabolism (9).
Quality of life (QOL) is an important criterion for measuring the health care effectiveness, perceived health, sense of wellbeing and predicting the mortality and morbidity rates in patients. Physical activity can improve QOL (10). It has been confirmed that the physical activity reduces morbidity and mortality in patients with chronic renal failure (11). People who are physically inactive have a 20% to 30% increased risk of mortality (12). Although most exercise programs have been instituted between dialysis sessions, recent investigations have promoted the concept of intradialytic exercise as a convenient intervention to improve patients’ compliance (1). The exercises are usually performed during the early phase of hemodialysis because cardiovascular responses are more stable during the early phase of hemodialysis (13). The reduced activity may be attributed to several factors such as anemia, impaired blood-flow to the limbs, loss of the heart function and reduced daily physical activities (14). Despite the positive effects of exercise in hemodialysis patients in theory, studies had mixed results. Parsons et al. have reported that an eight-week exercise program during hemodialysis had no significant effects on dialysis efficacy, blood pressure, and QOL in patients with ESRD (15). However, in another study, a five-month program of 30 min exercise in the first two hours of the dialysis showed an increase in dialysis efficacy. However, no changes were observed in the levels of hemoglobin, potassium, albumin, and QOL (1). In another study, Makhlough et al. have reported that an eight-week intradialytic aerobic exercise in hemodialysis patients did not affect the dialysis efficacy but significantly reduced the phosphorus and potassium levels (16). Smart et al. also reported that exercise is useful and may improve dialysis efficiency (kt/v), serum potassium and depression in hemodialysis patients (6). Girija et al. have demonstrated that exercise in hemodialysis patient increases the quality of life through reducing the stress level (12). However, in a study by McMurray et al. no significant changes were observed in phosphorus levels and in systolic and diastolic blood pressures after the intradialytic pedaling exercise (9). Fallahi et al. also examined the effects of exercise during hemodialysis on dialysis efficacy, serum phosphorus level, hemoglobin and blood pressure. He found that an hour of exercise during hemodialysis could significantly reduce the patients’ systolic blood pressure. The exercise program also increased the dialysis efficacy and had slight positive effects on phosphorus and hemoglobin levels (2). Due to the progressive reduction in muscular activity of patients with ESRD, their muscular strength will gradually reduce. Most studies on intradialytic exercise have used passive exercise and few studies have examined the effects of active exercise during hemodialysis. Moreover, the patients have limited ability to perform active exercise during hemodialysis session.
2. Objectives
Due to the importance of exercise in hemodialysis patients, this study was conducted to compare the effects of passive and active intradialytic pedaling exercises on dialysis efficacy, electrolytes, hemoglobin, hematocrit, blood pressure and health-related QOL among hemodialysis patients.
3. Materials and Methods
3.1. Study Design and Participants
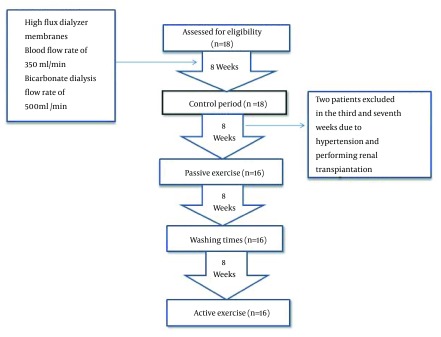
A quasi-experimental study was conducted on all eligible hemodialysis patients in the dialysis center of Akhavan Hospital in Kashan, Iran, from April to November 2013. In this center, 336 sessions of hemodialysis were performed for 120 patients with 28 dialysis apparatus every week. All participants received hemodialysis through arteriovenous (A-V) fistulas. The inclusion criteria included being under hemodialysis for at least three months, receiving hemodialysis three times a week, for at least 240 minutes (4 hours) per treatment, age between 15 to 80 years old. Patients with a history of cardiovascular diseases, myocardial ischemia in the last 6 months, cerebrovascular accidents, and pulmonary, musculoskeletal and immune disorders were excluded from the study. Other exclusion criteria included experiencing unstable angina, cardiac ischemia, severe persistent hypertension, severe hypotension (systolic blood pressure < 90 mmHg), severe uncontrolled diabetes, and fever (body temperature > 38°C) during the study period and not adhering to the exercise program.The study flow diagram is presented in Figure 1.
Figure 1. The Study Flow Diagram.
3.2. Intervention
The patients were under study for 8 months. The first 8 weeks were considered as the control period. All patients were dialyzed using high-flux dialyzer membranes (made by SOHA Co. Iran), the blood-flow rate of 350 mL/min and bicarbonate-dialysate, flow rate of 500 mL/min by Gambro AK95 apparatus (made in Swiss) or Ferzinus apparatus (made in Germany). Afterwards, for eight weeks, the patients received the passive intradialytic exercise using the electrically powered Mini-Bike (Made in China) adapted to the patient's bed. All patients performed passive pedaling with a moderate speed for 30 minutes per session (performed as three 10-minute exercise bouts with a 20-minute recovery period between the bouts) during the first 2 hours of dialysis session. After 8 weeks of washout (17), the active intradialytic pedaling exercise was performed similarly for eight weeks, except that the Mini-Bike did not connect to the electrical power and patients pedaled the Mini-Bike actively. The type of the dialysis apparatus, dialyzer membrane and the blood and dialysate flow rates were kept unchanged for all patients during the study.
3.3. Data Collection Instruments
The study instruments consisted of three parts including a demographic questionnaire, a short-form36 (SF-36) questionnaire for measuring the QOL among the patients and a checklist for recording the blood pressure and other biochemistry tests and dialysis efficacy. The demographic questionnaire consisted of five questions regarding the patients’ age and gender, and duration of hemodialysis, and level of education,medical diagnosis. The SF-36 questionnaire has been translated to Farsi and validated by Montazeri et al. (Cronbach’s alpha 0.65). This questionnaire has 36 items in eight domains (general health, physical functioning, and limitations due to physical problems, bodily pain, social functioning, and limitations due to emotional problems, vitality and mental health). The patients can get a score between 0 to 100 from every domain and the whole SF-36 questionnaire. The demographic questionnaire was completed at the beginning of the study. The SF-36 questionnaire was completed through interviews, before and after the completion of the exercise programs. The patient's blood pressure was examined using a mercury sphygmomanometer at the beginning and then hourly during the dialysis sessions. Moreover, the dialysis efficacy and levels of phosphorus, calcium, sodium, potassium and Blood Urea Nitrogen (BUN) were measured at the end of the 8th week of the intradialytic exercise. For this purpose, 4 mL of blood was drawn from the arterial line at the start of dialysis. For measuring the BUN, an additional sample of two mL of blood was drawn similarly at the end of the dialysis, while the blood flow rate was 80 - 100 mL/min for two minutes and the dialysis pump was turned off for 20 seconds. The BUN level was determined by Biotechnical BT1500 model and lotno 90007 apparatus. The patient’s pre- and post-dialysis weight (in kilograms) was measured accurately using a digital scale at entering and leaving the hemodialysis ward. Then, the ultrafiltration (UF) and urea reduction ratio (URR) were calculated to determine the dialysis efficacy. Then, the dialysis efficacy was calculated using the existing data and standard formulas for Kt⁄V. Furthermore, the phosphorus, calcium, sodium and potassium levels were measured using standard solutions (i.e. Elton 20010630921: x1-A4, lotno 20010630 and E, 921B:x1). All tests were repeated at the beginning, the fourth and eighth weeks of passive and active exercises.
3.4. Statistical Analysis
Data were analyzed using SPSS 13.0 software (SPSS Inc, Chicago, Illinois, USA). Mean and standard deviation were calculated for quantitative variables. Kolmogorov-Smirnov test was used to determine if the data were normally distributed. Paired t-test and the Wilcoxon signed rank and Friedman tests were used to compare the variables. To analyze the difference between the variables in different time intervals, the repeated measure analysis of variance was used. In all tests, the level of significance was considered to be less than 0.05.
3.5. Ethical Considerations
This study was approved by the Institutional Review Board and the Research Ethics Committee of Kashan University of Medical Sciences with a grant number 9143. The objectives of the study were explained to all participants and all of them signed a written informed consent and were assured of the confidentiality of their individual information as well as the voluntary nature of participating in the study. In all stages the researchers were committed to observe the ethical issues in accordance to the Helsinki ethical declaration.
4. Results
This study was performed on 18 patients. Two patients excluded from passive exercise in the third and seventh weeks due to hypertension and performing renal transplantation, respectively. Of the 16 patients studied, 13 (81.2%) were males and 3 (18.8%) were females. The mean age of the patients was 51.98 ± 1.57 years, ranging from 24 to 75 years. Diabetes mellitus was the most common underlying causes of ESRD in the participants. Demographic characteristics of the participants are presented in Table 1. Compared to the baseline, no significant changes were observed in serum potassium, phosphorus and calcium levels at the end of the fourth and eighth weeks of the passive intradialytic exercise; however, the phosphorus levels were negligibly reduced and calcium levels were slightly increased. Moreover, the dialysis efficacy and urea uptake were slightly decreased at the fourth week and then slightly increased at the end of the eighth week of passive exercise. However, these changes were not statistically significant. Furthermore, hemoglobin and hematocrit levels were higher than baseline at the end of the eighth week of passive exercise. However, these changes were not statistically significant (Table 2). Compared to the baseline, the potassium and phosphorus levels were decreased while the calcium level was increased at the end of eighth week in the active intradialytic exercise group. Moreover, the dialysis efficacy and urea uptake were decreased and the levels of hemoglobin and hematocrit were increased at the end of the eighth week; however, these changes were not statistically significant (Table 3).
Table 1. Demographic Characteristics of the Participants a.
| Variables | Frequency |
|---|---|
| Gender | |
| Male | 13 (81.2) |
| Female | 3 (18.8) |
| Marital status | |
| Single | 3 (18.8) |
| Married | 13 (81.2) |
| Level of education | |
| Illiterate | 5 (32.2) |
| Elementary school | 6 (37.5) |
| High school | 4 (25) |
| University education | 1 (6.2) |
| Medical diagnosis | |
| Diabetes mellitus | 9 (56.2) |
| Hypertension | 2 (12.5) |
| Polycystic disorders | 2 (12.5) |
| Unknown | 2 (12.5) |
| Genetic | 1 (6.2) |
| Duration of dialysis | 2.27 ± 2.09 |
| Age | 51.98 ± 1.57 |
aData are presented as No. (%) or Mean ± SD.
Table 2. Mean and Standard Deviation of Dialysis Efficacy and Blood Parameters After Performing Passive Exercise a.
| Intervention Variables | Before Passive Exercise | 4 Weeks After passive Exercise | Statistical Values | 8 Weeks After Passive Exercise | Statistical Values |
|---|---|---|---|---|---|
| Dialysis efficacy | 1.32 ± 0.30 | 1.27 ± 0.29 | T = 0.058, P value = 0.954 | 1.43 ± 0.49 | T = 1.446, P value = 0.169 |
| URR | 65.99 ± 7.28 | 65.93 ± 8.67 | T = 0.328, P value = 0.747 | 67.3 ± 22.26 | T = 0.435, P value = 0.670 |
| Hemoglobin | 11.25 ± 1.62 | 11.46 ± 1.61 | T = 0.673, P value = 0.511 | 11.87 ± 2.02 | T = 1.178, P value = 0.257 |
| Hematocrit | 34.08 ± 4.36 | 35.26 ± 3.90 | T = 2.078, P value = 0.055 | 36.21 ± 4.89 | T = 2.015, P value = 0.062 |
| Potassium | 4.32 ± 0.75 | 4.33 ± 0.41 | Z = 1.371, P value = 0.170 | 4.32 ± 0.41 | Z = 0.155, P value = 0.877 |
| Calcium | 8.44 ± 1.00 | 8.85 ± 0.88 | T = 1.443 P value = 0.169 | 8.89 ± 0.99 | T = 1.437, P value = 0.171 |
| phosphorus | 4.82 ± 0.76 | 4.79 ± 1.14 | T = 0.171 P value = 0.867 | 4.80 ± 0.83 | T = 0.211, P value = 0.836 |
aAbbreviations: URR, Urea Reduction Ratio.
Table 3. Mean and Standard Deviation of Dialysis Efficacy and Blood Parameters After Performing the Active Exercise a.
| Intervention Variables | Before Active Exercise | 4 Weeks After Active Exercise | Statistical Values | 8 Weeks After Active Exercise | Statistical Values |
|---|---|---|---|---|---|
| Dialysis efficacy | 1.39 ± 0.26 | 1.38 ± 0.43 | T = 0.531, P value = 0.603 | 1.33 ± 0.28 | T = 1.000, P value = 0.335 |
| URR | 68.48 ± 6.91 | 66.75 ± 9.36 | T = 1.092, P value = 0.292 | 66.20 ± 7.09 | T = 1.364, P value = 0.196 |
| Hemoglobin | 10.59 ± 1.82 | 10.61 ± 1.78 | T = 0.237, P value = 0.816 | 10.69 ± 1.87 | T = 0.355, P value = 0.729 |
| Hematocrit | 33.18 ± 6.13 | 34.00 ± 4.79 | T = 0.848, P value = 0.410 | 30.86 ± 5.26 | T = 1.905, P value = 0.079 |
| Potassium | 5.03 ± 1.07 | 4.77 ± 0.96 | Z = 0.827, P value = 0.408 | 4.54 ± 0.50 | Z = 1.350, P value = 0.177 |
| Calcium | 8.77 ± 0.96 | 8.67 ± 1.15 | T = 0.393 P value = 0.700 | 9.09 ± 0.76 | T = 1.187, P value = 0.257 |
| phosphorus | 4.85 ± 0.96 | 4.12 ± 0.75 | T = 5.626, P value = 0.000 | 4.21 ± 0.64 | T = 3.441, P value = 0.004 |
aAbbreviations: URR, Urea Reduction Ratio.
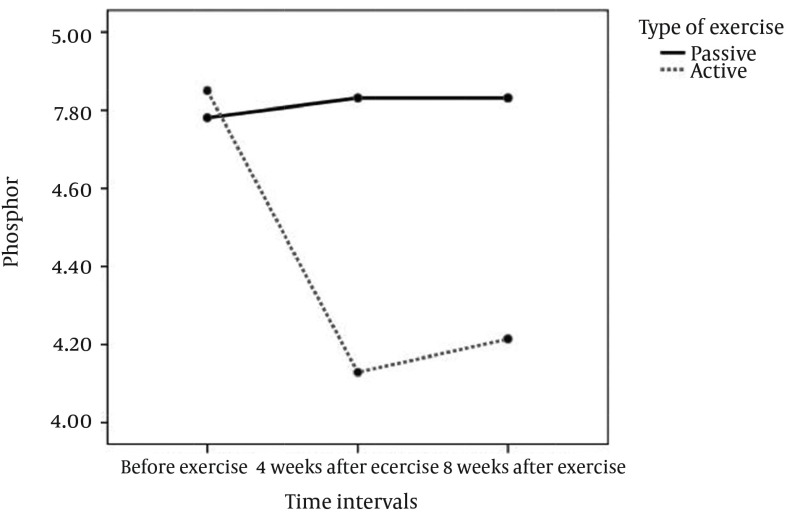
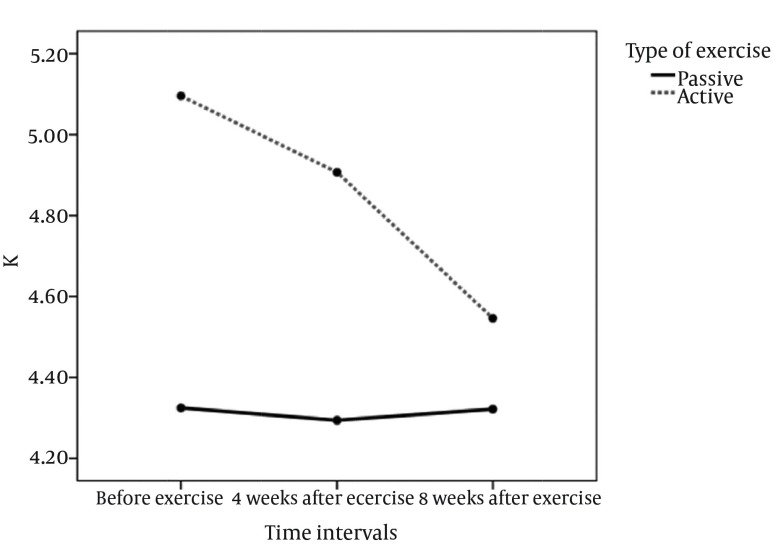
The repeated measure analysis showed that the type of the exercise as a factor had significant relation with the phosphorus and hematocrit levels. The phosphorus level decreased significantly in active exercise (Table 4, Figure 2). There was a noticeable decrease in Potassium level after the active exercise; although, this change was not statistically significant (Figure 3). However, the hematocrit level was significantly increased in the passive exercise. Compared to the baseline, the mean diastolic blood pressure was significantly decreased at the end of the fourth and eighth weeks of the passive intradialytic exercise. However, no significant changes were observed in the mean systolic and diastolic blood pressures in the group that performed active intradialytic exercise (Table 5). A significant relationship was found between the postexercise overall QOL and marital status and level of education (P < 0.0001). The mean overall QOL was 63.78 ± 21.15 at the beginning of the study, which was increased to 77.07 ± 21.14 at the end of the eight weeks of intradialytic exercise (P = 0.007). Moreover, the mean scores were significantly increased in the domains of general health, changes in health, physical functioning, and limitations due to physical problems. However, no significant differences were observed in the other dimensions (Table 6).
Table 4. Repeated-Measure ANOVA of the Variables Between the Passive and Active Exercises a.
| Variables | Degree of Freedom | Mean of Square | F statistics | P Value |
|---|---|---|---|---|
| Dialysis efficacy | 1 | 0.02 | 0.431 | 0.517 |
| Dialysis efficacy × Type of exercise | 1 | 0.133 | 2.89 | 0.1 |
| URR | 1 | 0.033 | 0.001 | 0.973 |
| URR × Type of exercise | 1 | 38.25 | 1.347 | 0.256 |
| Hgb | 1 | 1.63 | 1.32 | 0.259 |
| Hgb × Type of exercise | 1 | 0.799 | 0.647 | 0.428 |
| Hct | 1 | 2.11 | 0.326 | 0.573 |
| Hct× Type of exercise | 1 | 45.99 | 7.081 | 0.013 |
| Potassium | 1 | 1.14 | 3.01 | 0.094 |
| Potassium × Type of exercise | 1 | 1.11 | 2.94 | 0.097 |
| Calcium | 1 | 1.01 | 3.29 | 0.08 |
| Calcium × Type of exercise | 1 | 0.121 | 0.198 | 0.66 |
| phosphorus | 1 | 1.127 | 3.56 | 0.069 |
| phosphorus × Type of exercise | 1 | 1.76 | 4.91 | 0.035 |
aAbbreviations: URR, urea reduction ratio; Hgb, Hemoglobin; Hct, Hematocrit
Figure 2. Comparing the Level of Phosphorus in the Passive and Active Exercises.
Figure 3. Comparing the Level of Potassium in Passive and Active Exercises.
Table 5. Mean and Standard Deviation of Systolic and Diastolic Pressures in Patients Performing Passive and Active Intradialytic Exercise.
| TimeBlood Pressure | Beginning of Study | End of 4th Week | End of 8th Week | P Value a | df | Chi-Square |
|---|---|---|---|---|---|---|
| Passive exercise | ||||||
| Systolic pressure | 13.38 ± 2.12 | 12.56 ± 1.50 | 13.31 ± 2.21 | 0.058 | 2 | 5.698 |
| Diastolic pressure | 8.06 ± 0.77 | 7.56 ± 0.62 | 7.69 ± 0.70 | 0.039 | 2 | 6.488 |
| Active exercise | ||||||
| Systolic pressure | 12.94 ± 1.91 | 13.31 ± 2.33 | 14.00 ± 2.03 | 0.255 | 2 | 2.735 |
| Diastolic pressure | 7.81 ± 0.91 | 8.00 ± 1.03 | 8.29 ± 0.72 | 0.296 | 2 | 2.438 |
aFriedman test.
Table 6. Comparing the Mean and Standard Deviation of Quality of Life Scores Before and After Performing Intradialytic Exercise a.
| General Health | Number of Questions | Before | After | P Value b |
|---|---|---|---|---|
| Changes in health | 5 | 42.50 ± 19.32 | 55.31 ± 20.69 | 0.028 |
| Physical functioning | 1 | 74.18 ± 26.64 | 70.31 ± 16.37 | 0.016 |
| Limitations due to physical problems | 10 | 58.43 ± 25.34 | 65.00 ± 20.08 | 0.057 |
| Limitations due to emotional problems | 4 | 57.81 ± 36.19 | 78.12 ± 36.37 | 0.027 |
| Social functioning | 3 | 53.75 ± 39.13 | 60.00 ± 44.72 | 0.538 |
| Bodily pain | 2 | 68.12 ± 28.33 | 76.25 ± 19.95 | 0.165 |
| Vitality | 2 | 55.62 ± 33.05 | 68.12 ± 25.87 | 0.076 |
| Mental health | 4 | 60.93 ± 22.22 | 65.93 ± 21.30 | 0.198 |
| Overall quality of life | 5 | 65.93 ± 25.50 | 77.50 ± 18.25 | 0.088 |
| General health | 36 | 510.31 ± 169.27 | 616.56 ± 169.16 | 0.007 |
aData are presented as Mean ± SD.
bFriedman test.
5. Discussion
This study aimed to compare the effects of the active and passive intradialytic pedaling exercises in hemodialysis patients. Results showed that intradialytic exercise for half an hour leads to positive changes. Although changes in serum electrolytes were not statistically significant in both exercises; however, these changes might be clinically important. In the present study, performing the eight weeks of passive exercise resulted in an increase in URR and dialysis efficacy up to 1.36% and 11%, respectively. Although these changes were not significant, they can be important from clinical viewpoint. According to the Disease Outcomes Quality Initiative (DOQI) clinical guideline recommendations, the minimum acceptable dialysis efficacy should be 1.2 for patients undergoing hemodialysis (2). The exercise programs in this study increased the dialysis efficacy to 1.43 and this is obviously higher than the minimum acceptable efficacy. Consistent with our results, Parsons et al. have reported that a 5-month intradialytic exercise program could increase the dialysis efficacy up to 11% at the end of the first month, which then increased to 19% at the end of the fifth month (1). In another study, Mohseni et al. have reported that an eight-week intradialytic exercise program could increase the URR and dialysis efficacy about 11% and 38%, respectively (7). Fallahi et al. have also conducted an eight-week intradialytic exercise program on 14 patients and reported that although the URR and dialysis efficacy were increased, changes were not statistically significant (2). It appears that intradialytic exercise increases the muscle-blood flow and opens the capillary surface area that consequently would increase the leak of urea from the tissue to the vascular compartment and finally enhances the serum urea clearance and improves the dialysis efficacy (1). The present study showed that the passive exercise had better effects on dialysis efficacy than active exercise, especially at the end of the study. This finding may be attributed to the weakness of musculoskeletal system in these patients. Such weakness may resulted in intolerance of active exercise while passive exercise can be tolerated and resulted in better consequences in patients with ESRD undergoing chronic hemodialysis. The current study showed that passive intradialytic exercise had better effects on systolic and diastolic blood pressures than active exercise. This finding is consistent with results reported by Henrique et al. (11). McMurray et al. (9) and Fallahi et al. (2) who studied the effects of intradialytic exercise in patients under chronic hemodialysis. However, several studies could not confirm the positive effects of intradialytic exercise on blood pressure (9, 13). In the present study, phosphorus levels were significantly lower than the baseline both at the end of the fourth and eighth weeks of active exercise. However, passive exercise didn’t show the same effects. The effect of active exercise on phosphorus level was in line with findings of the several previous studies (16, 17). Borzou et al. also reported that increasing the blood flow to 250 mL/min and then to 300 mL/min can be effective in clearance of phosphorus from the blood (5). It is suggested that more time or the severity of exercise is required to see a noticeable change in phosphorus levels (2). The present study showed that passive exercise did not affect serum potassium level. However, serum potassium levels were decreased at the end of the fourth and eighth weeks of active exercise. Few studies have confirmed the effectiveness of exercise in the reduction of potassium level (16). However, some studies have shown that serum potassium levels did not significantly change after 12 weeks of intradialytic exercise (1). Although the dose of Eprex (erythropoietin) did not change during the study, the level of hemoglobin was increased after active and passive intradialytic exercise programs. This increase was up to 0.6 of µmol/L. In passive exercise. Although this increase was not statistically significant, it is clinically valuable. Similar results were reported by Fallahi et al. (2). However, Parsons et al. have reported that hemoglobin level was decreased after exercise during dialysis (1). It seems that intradialytic exercise can strengthen the effects of erythropoietin in patients with lower hemoglobin levels. Results of the present study indicated significant changes in patients' QOL after intradialytic exercise. Although another studies have reported no significant difference between the QOL before and after intradialytic exercise (1, 15) a recent study reported that an intradialytic exercise program could significantly improve the QOL of dialysis patients in the areas of physical functioning, physical limitations, bodily pain and mental health (18). The present study showed that the active intradialytic exercise had positive effects on blood pressure. The exercise program could also decrease the serum phosphorus and potassium levels and increase the hemoglobin, especially in active exercise. Also, the passive exercise had positive effects on blood pressure, dialysis efficacy and hemoglobin. Moreover, both exercise programs could significantly improve the QOL in hemodialysis patients. Therefore, it is suggested that some type of intradialytic exercise programs be used during a hemodialysis session based on the patients’ tolerance.
This study was conducted in one center and in a small sample size and the small sample size may affect not only on the statistical power of the results but also on the generalizability of the findings. Moreover, the exercise programs used in this study conducted in short duration and only in the first two hours of the dialysis. Then, replication of the same studies with larger sample size and longer periods are suggested.
Acknowledgments
The authors wish to acknowledge the support of Research Deputy of Kashan University of Medical Sciences and the head and staff of Hemodialysis Ward for their support and cooperation and also to thank all patients that participated in this study.
Footnotes
Authors' Contributions:Conception and design: Azra Sadat Musavian, Alireza Soleimani, Negin Masoudi Alavi, Alimohammad Baseri, Fatemeh Savari, data collection: Azra Sadat Musavian, data analysis: Azra Sadat Musavian, Negin Masoudi Alavi, manuscript Writing: Azra Sadat Musavian, Negin Masoudi Alavi, manuscript editing and revision Azra Sadat Musavian, Negin Masoudi Alavi, Alireza Soleimani.
Funding/Support:This study was approved and supported by the Deputy of Research, Kashan University of Medical Sciences.
References
- 1.Parsons TL, Toffelmire EB, King-VanVlack CE. Exercise training during hemodialysis improves dialysis efficacy and physical performance. Arch Phys Med Rehabil. 2006;87(5):680–7. doi: 10.1016/j.apmr.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 2.Fallahi M, Shahidi S, Farajzadegan Z. [The Effect of Intradialytic Exercise on Dialysis Efficacy, Serum Phosphate, Hemoglobin and Blood Pressure Control and Comparison between Two Exercise Programs in Hemodialysis Patients]. J Isfahan Med School. 2008;26(89):152–61. [Google Scholar]
- 3.Magnard J, Deschamps T, Cornu C, Paris A, Hristea D. Effects of a six-month intradialytic physical ACTIvity program and adequate NUTritional support on protein-energy wasting, physical functioning and quality of life in chronic hemodialysis patients: ACTINUT study protocol for a randomised controlled trial. BMC Nephrol. 2013;14:259. doi: 10.1186/1471-2369-14-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung TD, Park SH. Intradialytic exercise programs for hemodialysis patients. Chonnam Med J. 2011;47(2):61–5. doi: 10.4068/cmj.2011.47.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borzou SR, Gholyaf M, Zandiha M, Amini R, Goodarzi MT, Torkaman B. The effect of increasing blood flow rate on dialysis adequacy in hemodialysis patients. Saudi J Kidney Dis Transpl. 2009;20(4):639–42. [PubMed] [Google Scholar]
- 6.Smart N, McFarlane J, Cornelissen V. The Effect of Exercise Therapy on Physical Function, Biochemistry and Dialysis Adequacy in Haemodialysis Patients: A Systematic Review and Meta-Analysis. Open J Nephrol. 2013;3:25. doi: 10.4236/ojneph.2013.31005. [DOI] [Google Scholar]
- 7.Mohseni R, Emami Zeydi A, Ilali E, Adib-Hajbaghery M, Makhlough A. The effect of intradialytic aerobic exercise on dialysis efficacy in hemodialysis patients: a randomized controlled trial. Oman Med J. 2013;28(5):345–9. doi: 10.5001/omj.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen KL. Exercise in the end-stage renal disease population. J Am Soc Nephrol. 2007;18(6):1845–54. doi: 10.1681/ASN.2007010009. [DOI] [PubMed] [Google Scholar]
- 9.McMurray A, Blazey L, Fetherston C. The effect of intradialytic foot pedal exercise on blood pressure phosphate removal effi ciency and health related quality of life in haemodialysis patients. Ren Soc Aust J. 2008;4(2):38–44. [Google Scholar]
- 10.Montazeri A, Gashtasbi A, Vahdaninya M. [Translation and determining the validity and reliability of Persian version of SF-36] Payesh Health Monit. 2005;5(1) [Google Scholar]
- 11.Henrique DM, Reboredo Mde M, Chaoubah A, Paula RB. [Aerobic exercise improves physical capacity in patients under chronic hemodialysis]. Arq Bras Cardiol. 2010;94(6):823–8. doi: 10.1590/s0066-782x2010005000043. [DOI] [PubMed] [Google Scholar]
- 12.Girija K, Radha R. Beneficial Effect of Physical Activity in Hemodialysis Patients. Univers J Eng Sci. 2013;1(2):40–4. [Google Scholar]
- 13.Leung R. Physiological effects of exercise during dialysis on chronic renal failure Journal of Exercise Science and Fitness. J Exerc Sci Fit. 2004;2(1):30–5. [Google Scholar]
- 14.Aliasgharpour M, Hadiyan Z. Assessment of a Designed Exercise Program on Physical Capacity using Six-Minute Walking Test (6MWT) in hemodialysis patients. J Tehran Univ Med Sci sch Nurs Midwifery. 2011 [Google Scholar]
- 15.Parsons TL, Toffelmire EB, King-VanVlack CE. The effect of an exercise program during hemodialysis on dialysis efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. Clin Nephrol. 2004;61(4):261–74. doi: 10.5414/cnp61261. [DOI] [PubMed] [Google Scholar]
- 16.Makhlough A, Ilali E, Mohseni R, Shahmohammadi S. Effect of intradialytic aerobic exercise on serum electrolytes levels in hemodialysis patients. Iran J Kidney Dis. 2012;6(2):119–23. [PubMed] [Google Scholar]
- 17.Vaithilingam I, Polkinghorne KR, Atkins RC, Kerr PG. Time and exercise improve phosphate removal in hemodialysis patients. Am J Kidney Dis. 2004;43(1):85–9. doi: 10.1053/j.ajkd.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Heidarzadeh M, Zamanzadeh V, Maghvan AP, Oshvandi K. The effect of physical exercise on physical and psychological problems. Iran J Nurs Midwifery Res. 2010;15(1):20–6. [PMC free article] [PubMed] [Google Scholar]