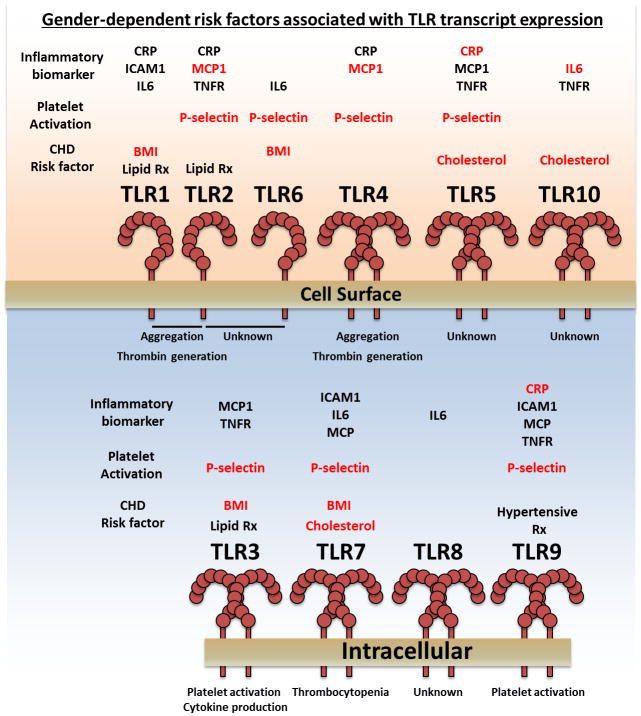
In this issue, Koupenova and colleagues demonstrate for that the transcripts of all 10 Toll-like receptors (TLRs) are expressed in platelets. Utilizing the Framingham Heart Offspring cohort the authors were able to identify a novel positive correlation between TLR transcript expression in platelets and coronary heart disease (CHD) risk factors including obesity, gender and inflammation (see figure).
Figure.
Gender-dependent risk factors associated with TLR transcript expression in platelets. Platelets express the transcripts of all 10 TLRs arranged above based on receptor subcellular location. Generally, the function of TLRs in platelets is pro-thrombotic and pro-inflammatory. Platelet TLR transcript expression correlates with inflammation, platelet activation, CHD risk factors, in a gender-dependent manner. Positive correlations between risk factor and TLR transcript expression for males (Blue) and females (Red) are depicted above each receptor.
TLRs are invariant, innate immune receptors that recognize evolutionarily conserved molecular patterns expressed by pathogens or endogenous molecules associated with cellular damage. TLRs can be characterized into two groups based on their subcellular location and substrate specificity. Surface expressed TLRs (TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10) recognize structural components (lipids and proteins), whereas intracellular TLRs (TLR3, TLR7, TLR8 and TLR9) recognize nucleic acids from pathogens.1
Recent studies have begun to characterize the function of TLRs in the platelet and how they contribute to inflammatory and thrombotic processes associated with CHD risk.2, 3 Interestingly, while the canonical TLR signaling pathway components are expressed in platelets,4 TLRs have also been shown to signal through non-canonical pathways including integrin and ITAM activation pathways to name a few.5 Thus it is not surprising that certain TLRs in platelets have been shown to have a pro-inflammatory and a pro-thrombotic effect. Ligation of TLRs for example may have the potential to signal in a pro-thrombotic manner by either directly causing (TLR9 and TLR2/1 heterodimer)6, 7 or potentiating aggregation (TLR4).8 Additionally, TLRs can modulate platelet reactivity through enhancement of thrombin generation which has been shown to be mediated through TLR2 and TLR4. Finally, TLR7 has been shown to mediate thrombocytopenia following exposure to the encephalomyocarditis virus, a TLR7-specific ligand.9
In the current issue, Koupenova et al. have expanded our understanding of TLRs by demonstrating that platelets have variable expression of all ten TLR transcripts. The transcripts for TLR5, TLR9 and TLR10 are present in platelets from greater than 80% of individuals, while transcripts for TLRs 1-4 were present in the platelets of 50–70% of individuals. Interestingly, less than 30% of individuals expressed TLR6, TLR7 and TLR8 transcripts. Unexpectedly, there was an increase in the transcript levels for all 10 TLRs in isolated platelets from women compared to men. The observed difference in TLR expression by gender may be highly informative as this may give some insight into functional differences in platelet reactivity by gender not previously considered. Further, the authors were able to show that this expression difference was not attributable solely to X-linked genes as the majority of TLRs are autosomal, except for TLR7 and TLR8.
While gender differences in the expression pattern and level of TLRs in human platelets is itself novel, the authors were additionally able to correlate inflammatory biomarkers with an increase in the number and level of platelet TLR transcripts in a gender-dependent manner. In addition, the authors were able to demonstrate that TLR transcript expression in women, but not men, correlates with platelet activation.
By virtue of its population and genetic underpinnings, this study has the potential to greatly expand exploration of the connections between TLRs and CHD risk factors. Future studies are required to determine how CHD risk factors impact TLR transcript expression and if expression is affected by age or ethnicity. This study opens up the exciting possibility that the TLR transcript profile of the readily accessible platelet could be used as a diagnostic tool to monitor CHD progression. However, further work is required to determine if TLR transcripts in platelets are predictive of CHD outcomes. Finally, while the functions of some members of the TLR family have been elucidated in platelets, this study supports a potential role for a diverse compliment of TLRs in inflammation and thrombosis.
Acknowledgments
Source of Funding
The authors are supported in part by the National Institutes of Health Grants (HL114405, GM105671, and MD007880).
Footnotes
Disclosures None
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nature immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circulation research. 2013;112:1506–1519. doi: 10.1161/CIRCRESAHA.113.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 4.Berthet J, Damien P, Hamzeh-Cognasse H, Pozzetto B, Garraud O, Cognasse F. Toll-like receptor 4 signal transduction in platelets: Novel pathways. British journal of haematology. 2010;151:89–92. doi: 10.1111/j.1365-2141.2010.08292.x. [DOI] [PubMed] [Google Scholar]
- 5.Falker K, Klarstrom-Engstrom K, Bengtsson T, Lindahl TL, Grenegard M. The toll-like receptor 2/1 (tlr2/1) complex initiates human platelet activation via the src/syk/lat/plcgamma2 signalling cascade. Cellular signalling. 2014;26:279–286. doi: 10.1016/j.cellsig.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circulation research. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circulation research. 2013;112:103–112. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet tlr2 and tlr4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K, Freedman JE. Platelet-tlr7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]