Abstract
Objective
We aimed to examine associations of Lp(a) concentrations with coronary heart disease (CHD) and determine whether current Lp(a) clinical laboratory cut points identify risk of disease incidence in four races/ethnicities of the Multi-Ethnic Study of Atherosclerosis (MESA).
Approach and Results
A subcohort of 1,323 Black, 1,677 Caucasian, 548 Chinese-American, and 1,044 Hispanic MESA participants were followed over a mean 8.5 year period in which 235 incident CHD events were recorded. Lp(a) mass concentrations were measured using a turbidimetric immunoassay. Cox regression analysis determined associations of Lp(a) with CHD risk with adjustments for lipid and non-lipid variables. Lp(a) concentrations were continuously associated with risk of CHD incidence in Black [hazard ratio (HR)=1.49; 95% CI: 1.09 – 2.04] and Caucasian participants (HR=1.22; 95% CI: 1.02 – 1.45). Examining Lp(a) risk by the 50 mg/dL cut point revealed higher risks of incident CHD in all races except Chinese Americans: Blacks (HR=1.69; 95% CI: 1.03 – 2.76); Caucasians (HR=1.82; 95% CI: 1.15 – 2.88); Hispanics (HR=2.37; 95% CI: 1.17 – 4.78). The lower Lp(a) cut point of 30 mg/dL identified higher risk of CHD in Black participants alone (HR: 1.87; 95% CI: 1.08 – 3.21).
Conclusions
Our findings suggest that the 30 mg/dL cutoff for Lp(a) is not appropriate in Caucasian and Hispanic individuals, and the higher 50 mg/dL cutoff should be considered. In contrast, the 30 mg/dL cutoff remains suitable in Black individuals. Further research is necessary to develop the most clinically useful Lp(a) cutoff values in individual races/ethnicities.
Keywords: Lipoprotein (a), coronary heart disease, race, lipid, risk factor
INTRODUCTION
The American Heart Association/American College of Cardiology task force has recently issued guidelines for assessing 10-year atherosclerotic cardiovascular disease risk [1]. Within these guidelines, it is acknowledged that the risk algorithm for predicting disease remains imperfect, and further research is needed to improve risk evaluation—particularly in areas relating to genetic hyperlipidemias in Hispanic and Asian populations. To address this shortfall, the present study examines whether the lipid carrying particle, lipoprotein(a) [Lp(a)], imposes risk of incident coronary heart disease (CHD) across four race/ethnic groups in the Multi-Ethnic Study of Atherosclerosis (MESA).
Lp(a) is a well-studied subspecies of low density lipoprotein (LDL) that is recognized as a significant risk factor for CHD [2–9], and findings from a Mendelian randomization study suggest that elevated Lp(a) may directly contribute to CHD development [10]. Distinguishing it from other apolipoprotein B-containing lipoproteins, Lp(a) blood concentrations are primarily determined by the apo(a) gene, LPA [11, 12], and are negligibly affected by lifestyle modifications such as diet and exercise [9]. As a consequence of this strong genetic influence, race-based disparities in Lp(a) concentrations have been documented. Studies have consistently shown that Black individuals have 2–3 fold higher Lp(a) levels than Caucasians in numerous case-control and prospective studies [2, 4, 5, 13, 14]. Though fewer studies have been conducted in Chinese and Hispanic populations, it has been shown that Chinese have lower Lp(a) levels than Caucasians [15] while inconsistent results have been reported in Hispanics [5, 16, 17].
In addition to the race-based differences in Lp(a), it remains unclear whether elevated Lp(a) levels impose a significant risk of CHD across different races/ethnicities. Indeed, it has been observed that Black individuals have a substantially higher median level of Lp(a), but a correspondingly higher incidence of CHD is not observed [18] which suggests that Lp(a) does not impose the degree of CHD risk in Blacks as it does in Caucasians. This disparity between Blacks and Caucasians may necessitate race-specific Lp(a) cut points as well as re-evaluation of the existing 30 mg/dL cut point used by practitioners and clinical laboratories across the US. Supporting the latter, a recommendation by the 2010 European Atherosclerosis Society Consensus Panel [2] advocates a higher cut point of 50 mg/dL.
Given the discrepancies in the literature and the limited research conducted in Hispanic and Chinese populations, we aimed to examine racial/ethnic differences in: 1) Lp(a) mass levels and distribution patterns; 2) associations of Lp(a) with incident CHD; 3) the utility of existing 30 or 50 mg/dL cut points in identifying CHD risk in a prospective study of 1,323 Black, 1,677 Caucasian, 548 Chinese-American, and 1,044 Hispanic MESA participants over a median 8.5 year follow-up period.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
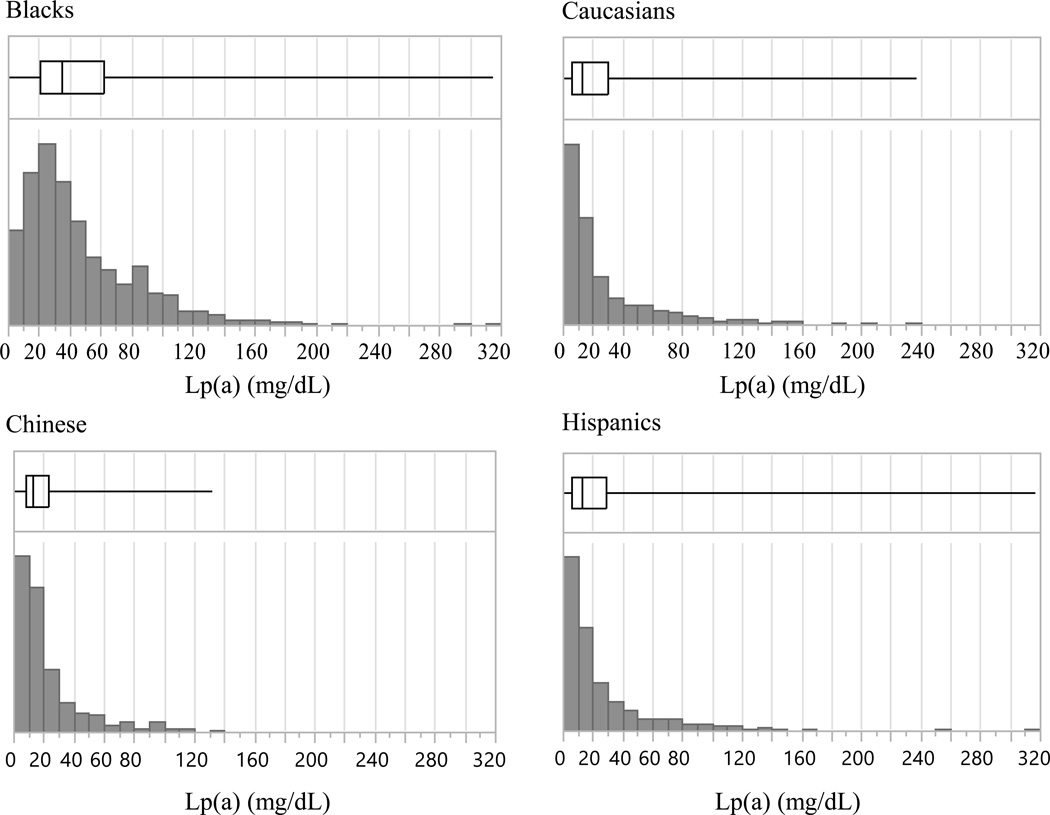
Characteristics of MESA participants across the four races/ethnic groups at baseline are shown in Table 1. The age and gender distributions at baseline are comparable. Blacks had the highest median level of Lp(a) compared to Hispanics, Chinese Americans, or Caucasians. Distributions of Lp(a) in Blacks, Caucasians, Chinese Americans and Hispanics are shown in Figure 1. Lp(a) levels in all ethnic groups were right-skewed, although the Black population showed less skewness. The median levels of Lp(a) for the four groups were: Blacks, 35.1 mg/dL; Caucasians, 13.0 mg/dL; Chinese, 12.9 mg/dL; Hispanics, 13.1 mg/dL.
Table 1.
Characteristics of MESA participants in 4 ethnic groups at visit 1. Median values (inter-quartile rage, IQR) are specified for continuous variables and (%) for categorical variables. Median values are shown for triglycerides and Lp(a).
| Blacks | Caucasians | Chinese Americans | Hispanics | |
|---|---|---|---|---|
| Number | 1323 | 1677 | 548 | 1044 |
| Age (years) | 61 (52–70) | 62 (54–71) | 62 (53–71) | 61 (52–69) |
| Gender (male) | 621 (46.1%) | 813 (47.6%) | 217 (38.8%) | 517 (48.6%) |
| Smoker | 726 (53.9%) | 929 (54.4%) | 137 (24.5%) | 504 (47.4%) |
| Diabetes | 196 (14.6 %) | 86 (5.0 %) | 55 (9.8 %) | 171 (16.1%) |
| Hypertensive | 428 (31.8%) | 325 (19.0%) | 126 (22.5%) | 257 (24.2%) |
| On hypertension medicine | 613 (45.5%) | 493 (28.8%) | 138 (24.7%) | 305 (28.7%) |
| Non-Lp(a) LDL-C (mg/dL) | 113 (92–133) | 115 (97–136) | 114 (96–132) | 116 (97–137) |
| HDL-C (mg/dL) | 50 (41–61) | 50 (41–62) | 48 (40–58) * | 45 (38–54) * |
| Triglycerides (mg/dL) | 89 (66–122) * | 110 (75–160) * | 121 (85–169) * | 133 (94–189) * |
| Lp(a) (mg/dL) | 35.1 (20.4–61.6) * | 12.9 (5.8–29.6) | 12.9 (7.7–23.4) | 13.1 (6.3–28.8) |
indicates significantly different (P <0.05) from all 3 other ethnic groups.
Definitions: smoker = former or current; diabetic = treated or untreated; hypertensive = systolic blood pressure ≥ 140 mmHg; Lp(a) = lipoprotein(a); HDL-C = high density lipoprotein-cholesterol; LDL-C = low density lipoprotein-cholesterol.
Figure 1.
Histograms of Lp(a) distribution frequency and quartile box plots of Lp(a) levels in the four race/ethnic groups of MESA. The left and right box edges correspond to 25% and 75% Lp(a) percentiles, and the vertical line within the box indicates the median level.
Associations between baseline Lp(a) levels and CHD incidence over 8.5 years of follow-up are shown in Table 2. Lp(a) was log-transformed to account for non-normal distributions. For all 4,593 participants, 1 unit increase in log-Lp(a) led to a significantly higher risk of developing CHD [hazard ratio (HR)=1.26; 95% CI: 1.11 – 2.03] after adjusting for race/ethnicity and other CHD risk factors including age, sex, education, smoking, hypertension medication, systolic blood pressure, diabetes, race, high density lipoprotein-cholesterol (HDL-C), non-Lp(a) low density lipoprotein-cholesterol (LDL-C), and log-triglycerides. After stratifying the subcohort by race/ethnicity, Black individuals showed an association between Lp(a) and CHD incidence (HR=1.49; 95% CI: 1.09 – 2.04) as well as Caucasians (HR=1.22; 95% CI: 1.02 – 1.45). No significant associations were found in Chinese Americans (HR= 1.08; 95% CI: 0.65 – 1.80) or Hispanics (1.14; 95% CI: 0.86 – 1.50).
Table 2.
Associations of Lp(a) (per 1 log unit increase) and risk of CHD are presented as hazard ratios and adjusted for age, sex, smoking, hypertension medication, systolic blood pressure, diabetes, non-Lp(a) LDL-C, HDL-C, and log-triglycerides. An adjustment for race was made in the column denoted ‘All Groups.’
| Blacks | Caucasians | Chinese Americans |
Hispanics | All Groups | |
|---|---|---|---|---|---|
| N | 1323 | 1677 | 548 | 1044 | 4593 |
| CHD events (%) | 66 (4.9) | 102 (6.0) | 18 (3.3) | 49 (4.6) | 235 (5.0) |
| Estimated HR | 1.49 | 1.22 | 1.08 | 1.14 | 1.26 |
| 95% CI | (1.09, 2.04) | (1.02, 1.45) | (0.65, 1.80) | (0.86, 1.50) | (1.11, 2.03) |
| P value | 0.014* | 0.028* | 0.77 | 0.36 | <0.001* |
P value of <0.05 indicates significance.
Further analyses were carried out using two cut points for Lp(a) (Table 3). Lp(a) levels ≥50 mg/dL revealed higher CHD risk in all races except Chinese Americans: Hispanics (HR=2.37; 95% CI: 1.17 – 4.78); Blacks (HR=1.69; 95% CI: 1.03 – 2.76); Caucasians (HR=1.82; 95% CI: 1.15 – 2.88). In contrast, only Black participants showed significant risk of CHD in those with Lp(a) levels ≥30 mg/dL (HR: 1.87; 95% CI: 1.08 – 3.21).
Table 3.
Associations of Lp(a) and risk of CHD are presented as hazard ratios and adjusted for age, sex, smoking, hypertension medication, systolic blood pressure, diabetes, non-Lp(a) LDL-C, HDL-C, and log-triglycerides. Lp(a) was examined as a categorical variable (30 and 50 mg/dL cut points).
| Blacks | Caucasians | Chinese Americans |
Hispanics | All Groups | |
|---|---|---|---|---|---|
| N | 1323 | 1677 | 548 | 1044 | 4593 |
| CHD events (%) | 66 (4.9) | 102 (6.0) | 18 (3.3) | 49 (4.6) | 5.03% |
| ≥ 30 mg/dL | |||||
| N (%) † | 774 (57.5) | 423 (24.8) | 108 (19.3) | 258 (24.2) | 1563 (33.4) |
| Estimated HR | 1.87 | 1.44 | 1.39 | 1.46 | 1.6 |
| 95% CI | (1.08, 3.21) | (0.95, 2.18) | (0.47, 4.08) | (0.78, 2.75) | (1.21, 2.10) |
| P value | 0.024* | 0.088 | 0.55 | 0.23 | <0.001* |
| ≥ 50 mg/dL | |||||
| N (%) ** | 445 (33.0) | 255 (14.9) | 54 (9.7) | 140 (13.2) | 894 (19.1) |
| Estimated HR | 1.69 | 1.82 | 1.04 | 2.37 | 1.83 |
| 95% CI | (1.03, 2.76) | (1.15, 2.88) | (0.22, 4.98) | (1.17, 4.78) | (1.36, 2.46) |
| P value | 0.037* | 0.010* | 0.96 | 0.017* | <0.001* |
P value of <0.05 indicates significance.
number of individuals with Lp(a) ≥ 30 mg/dL;
number of individuals with Lp(a) ≥ 50 mg/dL.
Net reclassification index (NRI) scores and c-statistics were assessed to determine whether adding Lp(a) to a baseline risk model would more accurately predict CHD cases and non-cases in Black, Caucasian, Chinese, and Hispanic participants as well as in the population as a whole. Models treating Lp(a) as a continuous (per 1 log unit increase) or categorical variable (>30 mg/dL or >50 mg/dL) are presented in Supplemental Table I. Significant NRI results were observed where Lp(a) > 30 mg/dL in Black individuals and the entire subcohort. The significant improvement in reclassification was driven largely by correctly predicting a higher risk in those that suffered events: 73% or 48 cases in Black participants and 42% or 100 cases in the subcohort. Using this cutoff also incorrectly predicted a lower risk in those that did not suffer events: 55% or 692 non-cases in Black individuals and 32% or 1,394 non-cases for the subcohort.
In contrast, improvements in c-statistics were observed across all models for the vast majority of races/ethnicities (Supplemental Table I). The largest c-statistic improvement was observed in Caucasians where Lp(a) > 50 mg/ml (0.011). The lowest c-statistic improvements were observed in Chinese participants using either cutoff value or Lp(a) (0.001 for 50mg/dL; 0.002 for 30 mg/dL) and in Hispanic individuals where Lp(a) > 30 mg/dL cutoff (0.001).
To further test which cutoff value more accurately reflects the relationship between Lp(a) and CHD events within each race, log-likelihoods of both Cox regression models were compared. In the entire population (n=4593), increasing the Lp(a) cutoff from 30 to 50 mg/dL resulted in a 2 unit increase in log-likelihood. Similarly, changing the cutoff from 30 to 50 mg/dL in Caucasians and Hispanics resulted in an increase of 1.6 and 1.9, respectively. In Black participants, the higher 50 mg/dL cutoff resulted in a decrease of 0.6 compared to the 30 mg/dL cutoff, though the difference may not be significant.
DISCUSSION
In this prospective study of 4,593 MESA participants, we aimed to determine whether Lp(a) levels associate with CHD incidence in Hispanic, Black, Caucasian and/or Chinese participants over a median study follow up period of 8.5 years. We found that Lp(a) was associated with higher risk of CHD in all participants; however, after stratifying the population by race/ethnicity, significant association only remained in Black and Caucasian subgroups. Further analyses using Lp(a) cut points revealed that 50 mg/dL identified higher CHD risk in all races except Chinese Americans, while the 30 mg/dL cut point detected a significant risk of CHD in Black study participants alone.
Lp(a) levels across race/ethnicity
It is well-documented that Lp(a) levels are strongly influenced by race/ethnicity. Black individuals have been shown to have 2–3 fold higher median Lp(a) levels relative to Caucasians in prospective studies and clinical trials [2, 4, 5, 13, 14]—a finding confirmed in this MESA subcohort (median of 35.1 mg/dL in Blacks versus 13.0 mg/dL in Caucasians, Table 1). By comparison, only a few studies have been conducted in Hispanic and Asian populations. Haffner et al. [17] reported significantly lower levels of Lp(a) in 316 Mexican Americans compared to 242 Caucasians. In contrast, Kamboh et al. [16] observed significantly higher Lp(a) levels in 215 Hispanics compared to 309 Caucasians. Similar to the MESA population, the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial was composed of four races and reported that Lp(a) levels were highest in Blacks (n=853, median=60 nmol/L), followed by Asians (n=138, median=38 nmol/L), then Hispanics (n=784, median=24 nmol/L) and finally Caucasians (n=7746, median=23 nmol/L) [5]. The present analysis supports the JUPITER finding that there are no significant differences in Lp(a) levels between Caucasians and Hispanics. In contrast with JUPITER, we showed no significant differences in Lp(a) levels in Chinese Americans compared to Caucasian participants; however, it should be recognized that the Asian population in JUPITER was smaller (n=138) than the Chinese American population in this MESA subcohort (n=548), and confirmation is therefore warranted.
Apart from differences in median Lp(a) concentrations, Lp(a) distribution patterns also varied by race (Figure 1). Caucasians, Hispanics, and Chinese populations showed right-skewed distribution patterns, while the Black population showed a relatively more symmetric distribution pattern with a right-handed tail. These race-based differences in median Lp(a) concentrations and distribution patterns likely have implications for clinical reference ranges and are discussed further below.
Lp(a) cutoffs and CHD risk
Lp(a) has largely been found to be a modest, independent risk factor for CHD and atherothrombotic forms of stroke [2–10]. Despite such evidence, guidelines that define ‘normal’ and ‘elevated/at risk’ levels have yet to be established. Clinical laboratories in the United States generally designate elevated levels at ≥30 mg/dL; however, a number of investigators and the 2010 European Atherosclerosis Society Consensus Panel recommend that 50 mg/dL serve as the clinical Lp(a) cut off to signify higher risk of disease [2]. Given this controversy, we tested these two cut points. Our findings agreed with the latter investigators that 50 mg/dL is an appropriate cut point with an important caveat—30 mg/dL may be a suitable for Black individuals given that it detected significant risk of CHD. Though such a recommendation may invite controversy, Lp(a) levels ≥30 mg/dL corresponded to an 87% greater risk of CHD in Black study participants (p=0.02), but a significantly higher risk was not observed in the 24.8% of Caucasians or 24.2% of Hispanics with Lp(a) levels (≥30 mg/dL), underscoring the importance of having a clinically meaningful Lp(a) cut point specific for Black populations. By comparison, Caucasians and Hispanics only showed a greater risk of CHD at the 50 mg/dL threshold.
Lp(a) in risk reclassification
The addition of Lp(a) to CHD risk models has been shown to improve NRI and c-statistics (i.e. area under the curve) in previous studies of Caucasians [20, 21], yet no corresponding research has been conducted in other races. NRI scores are presented for all four race groups across all models (Supplemental Table I), but results are most relevant where HRs of Lp(a) and CHD were found to be significant. Significant NRI scores were only observed in Black individuals (p=0.05) and for the entire population (p=0.04) where Lp(a) >30 mg/dL. In contrast, modest increases in c-statistics were observed across all races and models except in Hispanic and Chinese participants where Lp(a) was treated as a continuous variable. These contrasting NRI and c-statistic results as well as the inability to replicate previous findings in Caucasians [20, 21] are likely due to limited statistical power following race-stratification coupled with the modest to moderate influence of Lp(a) on CHD risk. Further investigation is needed in larger, racially diverse populations to determine which cutoff optimally improves risk classification within each race.
Comparing Lp(a) cutoff values using log-likelihood
Log-likelihood functions have previously been used to determine optimal cutoff values [19] and to compare model fit among datasets. Although p-values cannot be directly computed in this type of comparison, a 1 unit increase in log-likelihood was used as an a priori threshold that is suggestive of the superior cutoff value. Selecting the higher 50 mg/dL in Caucasians and Hispanics resulted in an increase of 1.6 and 1.9 in log-likelihoods, respectively—signifying that the 50 mg/dL cutoff is a better fit than 30 mg/dL. In Black participants, however, the higher cutoff of 50 mg/dL resulted in a decrease of 0.6 compared to the 30 mg/dL cutoff, suggesting that 30 mg/dL may serve as the superior cutoff in Black individuals, though the difference may not be significant because it did not breach the 1 unit threshold.
Lp(a) in Chinese Americans
Unlike findings in other racial/ethnic groups, no relation between Lp(a) and CHD was observed in Chinese Americans—in contrast to results from previous studies, albeit in native East Asian populations [22–24]. Our null results are likely due to multiple factors, but the relatively fewer number of Chinese participants (n=548) and lower incidence of CHD events (n=18; 3.28%) limited our statistical power for this subgroup. The possibility that a higher Lp(a) cut point would identify CHD risk in Chinese individuals should not be discounted, and follow-up studies in larger Chinese American populations are warranted.
Strengths and Limitations
This study provided the first large-scale prospective evaluation of Lp(a) and risk of CHD incidence across four major race/ethnic groups in the United States. Despite the limited number of CHD events in this MESA subcohort, particularly following race-stratification, significant relationships between Lp(a) and CHD risk were observed nonetheless. With respect to Lp(a), its immunochemical measurement has historically been challenging due to the variable size of its apo(a) component—a product of the heterogeneity of kringle IV type 2 repeats within apo(a) among individuals. The current study employed an Lp(a) assay with antibodies targeted to the uniform region of apo(a) and included five apo(a) calibrators with mixed molecular weights to minimize apo(a) size-dependent biases associated with Lp(a) measurement [25].
In terms of limitations, it should be acknowledged that analyses that included the entire subcohort may have led to an imprecise estimate of the Lp(a)-associated risk of CHD due to racial disparities in triglycerides and blood pressure that may not have been overcome with statistical adjustments. In addition, conflicting results for risk reclassification analyses suggest that further investigation is needed in larger populations to determine which Lp(a) value optimally improves event prediction in individual races. Finally, adjustments were made for multiple confounding variables, but the presence of residual confounders remains possible.
Conclusion
The current study provides evidence that race is an important factor when considering the Lp(a) level that imposes a significant risk of CHD development. Our findings suggest that the 30 mg/dL cutoff for Lp(a) is not appropriate in Caucasian and Hispanic individuals, and the higher 50 mg/dL cutoff should be considered. In contrast, the 30 mg/dL cutoff remains suitable in Black individuals. Further research is necessary to develop the most clinically useful Lp(a) cutoff values in individual races/ethnicities.
Supplementary Material
Significance.
Lipoprotein(a) [Lp(a)] is a well-known lipid risk factor for coronary heart disease (CHD). Previous studies have shown that Lp(a) levels and distribution patterns vary by race, however, it remains unclear whether Lp(a) imposes risk across all races. We therefore aimed to examine the linear associations of Lp(a) with CHD and whether existing thresholds of 30 or 50 mg/dL identify CHD risk in Black, Caucasian, Chinese-American, and Hispanic MESA participants over a median 8.5 year follow-up period. Continuous associations of Lp(a) and CHD were only significant in Black and Caucasian study participants. It was found that the 50 mg/dL cut point identifies CHD risk in three of the four races/ethnicities, but the lower 30 mg/dL cut point may be appropriate for detecting CHD risk for Black individuals. Further study and development of clinical ranges is necessary to identify desirable and ‘at risk’ Lp(a) levels across individual races/ethnicities.
Acknowledgments
The authors thank the other investigators, staff, and participants of the MESA study for their contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding: research was supported by the following contracts, N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Abbreviations
- CHD
coronary heart disease
- CI
confidence interval
- HR
hazard ratio
- HDL-C
high density lipoprotein cholesterol
- JUPITER
Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin
- Lp(a)
lipoprotein(a)
- LDL
low density lipoprotein
- LDL-C
low density lipoprotein cholesterol
- MESA
Multi-ethnic Study of Atherosclerosis
- NRI
Net Reclassification Improvement
Footnotes
Conflict of interest: Russell Warnick, Daniel Hoefner, and Joseph McConnell are associated with Health Diagnostic Laboratory, Inc., the clinical laboratory responsible for obtaining Lp(a) data in the present study. Joseph McConnell serves as the Chief Executive Officer, Russell Warnick serves as the Chief Scientific Officer, and Daniel Hoefner serves as the Laboratory Director. There are no other conflicts of interest to disclose.
References
- 1.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kronenberg F, Kronenberg MF, Kiechl S, Trenkwalder E, Santer P, Oberhollenzer F, Egger G, Utermann G, Willeit J. Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis: prospective results from the Bruneck study. Circulation. 1999;100:1154–1160. doi: 10.1161/01.cir.100.11.1154. [DOI] [PubMed] [Google Scholar]
- 4.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, Folsom AR, Boerwinkle E, Ballantyne CM. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and Caucasian subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–249. doi: 10.1161/CIRCULATIONAHA.111.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) Circulation. 2014;129:635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emerging Risk Factors Collaboration. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiner PJ. Lipoprotein[a] as a risk factor for preclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 1993;13:826–833. doi: 10.1161/01.atv.13.6.826. [DOI] [PubMed] [Google Scholar]
- 8.Schreiner PJ, Heiss G, Tyroler HA, Morrisett JD, Davis CE, Smith R. Race and gender differences in the association of Lp(a) with carotid artery wall thickness. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 1996;16(3):471–478. doi: 10.1161/01.atv.16.3.471. [DOI] [PubMed] [Google Scholar]
- 9.Marcovina S, Koschinsky M. Lipoprotein (a): Structure, Measurement, and Clinical Significance. In: Rifai N, Warnick KF, Dominiczak M, editors. Handbook of Lipoprotein Testing. 2000. pp. 345–385. [Google Scholar]
- 10.Kamstrup PR1, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 11.Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest. 1992;901:52–60. doi: 10.1172/JCI115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mooser V, Scheer D, Marcovina SM, Wang J, Guerra R, Cohen J, Hobbs HH. The Apo(a) gene is the major determinant of variation in plasma Lp(a) levels in African Americans. Am J Hum Genet. 1997;61:402–417. doi: 10.1086/514851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard BV, Le NA, Belcher JD, Flack JM, Jacobs DR, Jr, Lewis CE, Marcovina SM, Perkins LL. Concentrations of Lp(a) in black and white young adults: relations to risk factors for cardiovascular disease. Ann Epidemiol. 1994;4:341–350. doi: 10.1016/1047-2797(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 14.Tsimikas S, Clopton P, Brilakis ES, Marcovina SM, Khera A, Miller ER, de Lemos JA, Witztum JL. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation. 2009;119:1711–1719. doi: 10.1161/CIRCULATIONAHA.108.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee D, Wong EC, Shin J, Fortmann SP, Palaniappan L. Racial and Ethnic Variation in Lipoprotein (a) Levels among Asian Indian and Chinese Patients. J Lipids. 2011;2011:291954. doi: 10.1155/2011/291954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamboh MI, Rewers M, Aston CE, Hamman RF. Plasma apolipoprotein A-I, apolipoprotein B, and lipoprotein(a) concentrations in normoglycemic Hispanics and non-Hispanic Caucasians from the San Luis Valley, Colorado. Am J Epidemiol. 1997;146:1011–1018. doi: 10.1093/oxfordjournals.aje.a009229. [DOI] [PubMed] [Google Scholar]
- 17.Haffner SM, Gruber KK, Morales PA, Hazuda HP, Valdez RA, Mitchell BD, Stern MP. Lipoprotein(a) concentrations in Mexican Americans and non-Hispanic Caucasians: the San Antonio Heart Study. Am J Epidemiol. 1992;136:1060–1068. doi: 10.1093/oxfordjournals.aje.a116571. [DOI] [PubMed] [Google Scholar]
- 18.Guyton JR, Dahlen GH, Patsch W, Kautz JA, Gotto AM., Jr Relationship of plasma lipoprotein Lp(a) levels to race and to apolipoprotein B. Arteriosclerosis. 1985;5:265–272. doi: 10.1161/01.atv.5.3.265. [DOI] [PubMed] [Google Scholar]
- 19.Faraggi D, Simon R. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Stat Med. 1996;15:2203–2213. doi: 10.1002/(SICI)1097-0258(19961030)15:20<2203::AID-SIM357>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64:851–860. doi: 10.1016/j.jacc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 21.Hobbs GA1, Kaplan IV, Levinson SS. Mechanized lipoprotein(a) assay as a marker for coronary artery disease illustrates the usefulness of high lipoprotein(a) levels. Clin Chim Acta. 1998;274:1–13. doi: 10.1016/s0009-8981(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 22.Yang WX, Yang Z, Wu YJ, Qiao SB, Yang YJ, Chen JL. Factors associated with coronary artery disease in young population (age ≤ 40): analysis with 217 cases. Chin Med Sci J. 2014;29:38–42. doi: 10.1016/s1001-9294(14)60022-5. [DOI] [PubMed] [Google Scholar]
- 23.Liang XH, Huang CZ. Detection of serum Lp(a) level of coronary heart disease and its clinical significance. Hunan Yi Ke Da Xue Xue Bao. 2001;26:227–228. [PubMed] [Google Scholar]
- 24.Qin SY, Liu J, Jiang HX, Hu BL, Zhou Y, Olkkonen VM. Association between baseline lipoprotein (a) levels and restenosis after coronary stenting: meta-analysis of 9 cohort studies. Atherosclerosis. 2013;227:360–366. doi: 10.1016/j.atherosclerosis.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Marcovina SM, Albers JJ, Scanu AM, Kennedy H, Giaculli F, Berg K, et al. Use of a reference material proposed by the international federation of clinical chemistry and laboratory medicine to evaluate analytical methods for the determination of plasma lipoprotein(a) Clin Chem. 2000;46:1956–1967. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.