Abstract
Objective. Sexual problems are common in people with diabetes. It is unknown whether early detection of diabetes and subsequent intensive multifactorial treatment (IT) are associated with sexual health. We report the prevalence of low sexual desire and low sexual satisfaction among people with screen-detected diabetes and compare the impact of intensive multifactorial treatment with the impact of routine care (RC) on these measures. Design. A cross-sectional analysis of the ADDITION-Denmark trial cohort six years post-diagnosis. Setting. 190 general practices around Denmark. Subjects. A total of 968 patients with screen-detected type 2 diabetes. Main outcome measures. Low sexual desire and low sexual satisfaction. Results. Mean (standard deviation, SD) age was 64.9 (6.9) years. The prevalence of low sexual desire was 53% (RC) and 54% (IT) among women, and 24% (RC) and 25% (IT) among men. The prevalence of low sexual satisfaction was 23% (RC) and 18% (IT) among women, and 27% (RC) and 37% (IT) among men. Among men, the prevalence of low sexual satisfaction was significantly higher in the IT group than in the RC group, p = 0.01. Conclusion. Low sexual desire and low satisfaction are frequent among men and women with screen-detected diabetes, and IT may negatively impact men's sexual satisfaction.
Key Words: Denmark, diabetes mellitus, general practice, personal satisfaction, primary health care, quality of life, sexual health, sexual desire
Sexual dysfunction is common among patients with type 2 diabetes and can negatively impact their quality of life. This study shows that:
Low sexual desire and low satisfaction are common among men and women with screen-detected type 2 diabetes.
Men receiving intensive treatment of type 2 diabetes report a lower level of sexual satisfaction than men receiving routine care.
Our results underline the need to distinguish between different aspects of sexual health, and to pay special attention among men when more intensive treatment strategies are used.
Introduction
Sexual problems are common among type 2 diabetes patients, and they can negatively impact the patients’ quality of life [1]. Previous studies evaluating the sexual consequences of diabetes tend to focus on sexual dysfunction, which represents only a limited part of sexual health. Calls have therefore been made for a broader evaluation of the sexual consequences of diabetes [2]. A parameter like sexual satisfaction correlates strongly with sexual quality of life, but is not always correlated with sexual function/ dysfunction. Sexual satisfaction covers an unlimited spectrum of sexual behaviours that are individually defined and influenced by many life and relational circumstances [2,3]. Lack of sexual desire is a commonly described sexual problem among men and women with type 2 diabetes [4], but the level of sexual desire also varies between sexes and with age in the normal population [5]. Hence, looking at sexual desire and satisfaction provides a description of the relationship between diabetes and two broader elements of sexual health.
Intensive multifactorial treatment (IT) of diabetes is advocated for many reasons [6]. Previous research of the effect of intensive treatment strategies in patients with type 2 diabetes finds neither benefits nor harms of such strategies to self-reported quality of life [7–9]. These results suggest that IT does not adversely affect patient-reported outcomes and patients’ quality of life. However, extant research offers no insight into the effect of IT on sexual health.
We here report the prevalence of low sexual desire and low sexual satisfaction among Danish men and women with screen-detected type 2 diabetes and examine the impact of intensive multifactorial treatment compared with routine care (RC) on these sexual issues.
Material and methods
Study population
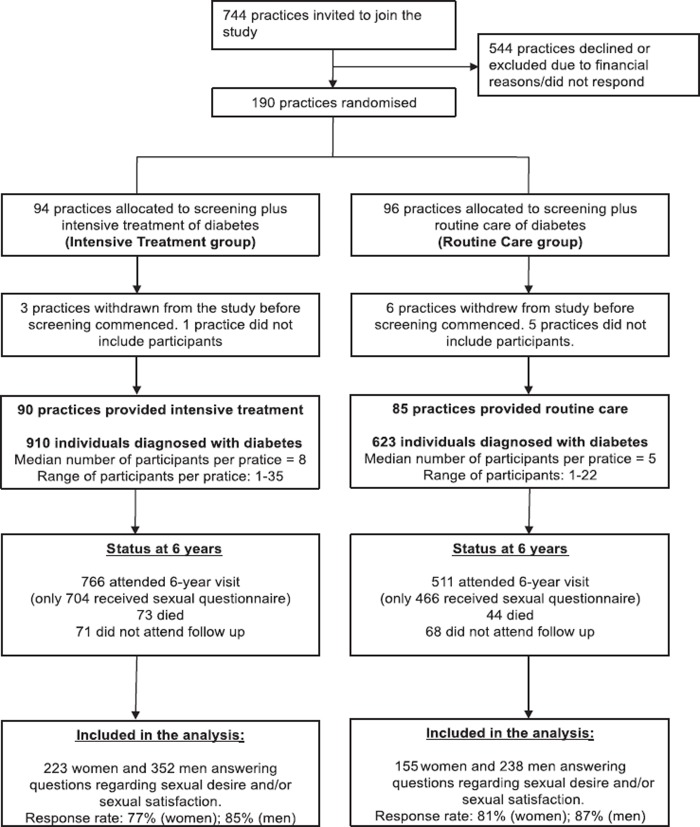
Data from the ADDITION-Denmark study was used. The design and the rationale of the ADDITION-Denmark study have already been reported [10]. Briefly, ADDITION-Denmark consists of two phases: a population-based stepwise screening programme for type 2 diabetes and a randomized controlled trial of early intensive multifactorial treatment among those found to have type 2 diabetes. The original aim of the ADDITION study was to evaluate the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with type 2 diabetes detected by screening. Overall, 1533 eligible participants with screen-detected diabetes in Denmark agreed to take part in the trial. After an average of six years, 1170 participants were re-examined and asked to complete self-report questions regarding their sexual health. Figure 1 displays the practice and participant flow.
Figure 1.
Practice and participant flow: ADDITION-Denmark.
Intervention
The specific characteristics of the intervention have been described previously [10,11]. The intervention was based on evidence from randomized controlled trials demonstrating the benefits of IT on cardiovascular risk factors in people with type 2 diabetes [12]. Hence, IT was promoted through the addition of several features to the existing diabetes care, i.e. routine care. The intervention included no extra initiatives addressing sexual health.
In the RC group, patients received the standard pattern of diabetes care according to Danish national recommendations [13].
Outcomes
ADDITION-Denmark participants were asked to complete a questionnaire regarding their sexual health, including their sexual desire and their sexual satisfaction. This asked: “How often do you have sexual desire?” (Often, occasionally, rarely, never) and, “I am satisfied with my sexual life” (agree a lot, agree, neither agree nor disagree, disagree, disagree a lot). The questions stem from the “National Health Interview Survey 2000 [14]. Two outcomes were defined: low sexual desire was defined as reporting sexual desire “rarely” or “never”; low sexual satisfaction was defined as reporting “disagree a lot” or “disagree”. Information on sexual health was only obtained at the six-year follow-up health assessment.
Study procedures
Information on health factors believed to potentially influence sexual health [15] was obtained by questionnaires and by a physical examination undertaken by centrally trained staff unaware of the study group allocation. The examination was performed according to standard operating procedures [12]. Detailed descriptions of the test methods have been reported elsewhere [11,16]. Data on health status and erectile dysfunction were collected using validated self-report questionnaires: the 36-item short Form Health Survey (SF36) and the abbreviated International Index of Erectile Function (IIEF-5), respectively [17].
Self-reported low mental health and self-reported low physical health were defined as having a score in the 25% lowest quartile in the mental and the physical component of the SF-36 questionnaire, respectively. Erectile dysfunction was defined as having a cut-off score ≤ 21 [17]. Information on taking beta-blockers and having macro-vascular complications was obtained using a self-report questionnaire. Having a macro-vascular complication was defined as having had at least one of the following conditions: previous myocardial infarction, stroke, or operation/instrumentation on the heart. Peripheral neuropathy was detected using a vibration detection threshold and light touch sensory testing and defined as at least one positive test [16]; nephropathy was defined as micro-albuminuria or macro-albuminuria. Micro- albuminuria was defined as albumin/creatinine ratio on spot urine: men, 2.5–25mg/mmol; women, 3.5–25 mg/mmol; macro-albuminuria, > 25 mg/mmol. Retinopathy was defined as non-proliferative or proliferative retinopathy according to the ETDRS classification [18]. Body mass index (BMI) was measured estimating weight and height, and people were grouped into categories of normal weight, overweight, and obesity according to the WHO definition.
Data analyses and statistics
Prevalences were estimated separately within each treatment group and stratified into age groups. To compare treatment groups, Fisher's exact test was used. P < 0.05 was considered statistically significant.
For assessing the validity of the declared prevalences, non-attenders (participants who entered the ADDITION study, but who for different reasons did not answer sexual questions) were compared with attenders on baseline characteristics.
Logistic regression was used to examine whether a potential impact of IT on sexual desire or satisfaction could be linked to specific, sexual health risk factors (erectile dysfunction, self-reported health, BMI, macro-vascular complication, peripheral neuropathy, nephropathy, retinopathy, and taking beta-blockers). Risk factors were dichotomized into yes/no; and logistic regression was performed, stratified accordingly, adjusting for age, erectile dysfunction, and BMI, except where these variables were stratified themselves. All analyses were tested for interaction. Multiple testing was accounted for using the Bonferroni correction, and a 99.5% confidence interval (CI) was used instead of 95% CI. To clarify whether a potential impact of IT could be explained by contradictory characteristics of non-responders compared with responders, we compared non-responders (participants who received the sexual questionnaire, but did not answer it) with responders in the RC and IT group, respectively.
To compare non-attenders with attenders and non-responders with responders, we used the chi-squared test for categorical variables, the two tailed t-test for continuous data, and the Mann–Whitney U-test for data not normally distributed. P < 0.05 was considered statistically significant.
All analyses were conducted in men and women separately, and the analyses were performed using STATA version 12 (STATA Corp LP, College Station, Texas, USA.
Results
The study participants’ baseline characteristics are presented in Table I. At follow-up, 55% of women and 83% of men reported having been sexually active during the past 12 months (including masturbation), with no difference between treatment groups, p > 0.9. A total of 55% (IT) and 62% (RC) of the men had erectile dysfunction, p = 0.26. Twenty-nine (IT) and 23% (RC) of men were taking beta-blockers, p = 0.1.
Table I.
Baseline characteristics of the ADDITION-Denmark trial cohort (n = 968).
| Women | Men | |||
| RC (n = 155) | IT (n = 223) | RC (n = 238) | IT (n = 352) | |
| HbA1c (% of haemoglobin)* | 6.3 (6.0;6.8) | 6.4 (6.0;7.0) | 6.4 (6.0;7.2) | 6.3 (6.0;7.0) |
| HbA1c (mmol/mol)* | 45 (42;51) | 46 (42;53) | 46 (42;55) | 45 (42;53) |
| Systolic blood pressure (mmHg) | 149.0 (20.2) | 144.4 (19.2) | 149.5 (18.4) | 149.1 (18.2) |
| Diastolic blood pressure (mmHg) | 87.2 (11.0) | 84.1 (10.2) | 89.3 (11.1) | 89.2 (10.2) |
| Total cholesterol (mmol/L) | 5.9 (1,2) | 5.7 (1,1) | 5.7 (1,2) | 5.5 (1,1) |
| Age (years) | 59.7 (6.57) | 59.4 (7.3) | 59.9 (6.7) | 59.7 (6.5) |
| Body mass index (kg/m2) | 31.3 (5.8) | 31.7 (6.5) | 30.2 (4.3) | 30.5 (4.4) |
| Smoking, current smoker, n (%) | 42 (27%) | 55 (25%) | 80 (34%) | 103 (30%) |
| Alcohol (units/week)* | 2 (1;7) | 2 (1;7) | 10 (3;19) | 10 (3;18) |
| White ethnic origin, n (%) | 149 (98%) | 206 (98%) | 230 (98%) | 331 (99%) |
| Marital status | ||||
| Married, n (%) | 102 (67%) | 149 (68%) | 173 (73%) | 258 (74%) |
| Unmarried, n (%) | 14 (9%) | 16 (7%) | 35 (15%) | 35 (10%) |
| Separated, n (%) | 18 (12%) | 33 (15%) | 20 (6%) | 39 (11%) |
| Widow/widower, n (%) | 19 (12%) | 21 (10%) | 8 (3%) | 16 (4%) |
| Nephropathy | ||||
| Micro-albuminuria | 18 (12%) | 18 (9%) | 44 (21%) | 57 (18%) |
| Macro-albuminuria | 3 (2%) | 4 (2%) | 2 (1%) | 7 (2%) |
Notes: Data are means (SD), unless otherwise indicated. *Median (25th; 75th percentile). IT = intensive treatment; RC = routine care.
Prevalence of sexual desire and sexual satisfaction (Table II)
Table II.
Prevalence in percentages of low sexual desire and low sexual satisfaction among men and women in the ADDITION population answering sexual questions.
| Men | Women | |||||||
| RC | IT | p-value | RC | IT | p-value | |||
| Low sexual desire, % (95% CI): | n | n | ||||||
| In total | 145 | 24.5 (19.0–30.0) | 24.7 (20.2–29.2) | 0.95 | 202 | 53.2 (45.3–61.2) | 53.8 (47.2–60.4) | 0.91 |
| 44–59 years | 15 | 7.4 (0.2–14.6) | 14.3 (6.3–22.3) | 0.22 | 31 | 43.3 (24.5–62.2) | 40.0 (25.1–54.9) | 0.77 |
| 60–69 years | 72 | 22.9 (15.2–30.6) | 25.2 (18.9–31.7) | 0.64 | 101 | 50.6 (39.4–61.9) | 53.0 (43.8–62.3) | 0.74 |
| 70–78 years | 58 | 41.5 (29.2–53.8) | 32.0 (22.5–41.4) | 0.21 | 70 | 64.4 (49.9–79.0) | 65.1 (53.0–77.2) | 0.95 |
| Low sexual satisfaction, % (95% CI): | n | n | ||||||
| In total | 185 | 27.4 (21.5–33.3) | 37.0 (31.8–42.2) | 0.01 | 64 | 22.7 (15.3–30.0) | 17.7 (12.3–23.0) | 0.27 |
| 44–59 years | 40 | 20.4 (9.3–31.5) | 37.7 (26.6–48.7) | 0.03 | 19 | 31.0 (13.1–48.9) | 24.4 (10.7–38.1) | 0.59 |
| 60–69 years | 104 | 31.5 (22.8–40.3) | 40.4 (32.9–47.8) | 0.13 | 32 | 24.2 (13.6–34.9) | 15.2 (8.2–22.2) | 0.16 |
| 70–78 years | 41 | 25.9 (14.2–37.5) | 29.9 (20.1–39.7) | 0.60 | 13 | 12.1 (3.7–23.9) | 17.3 (6.7–27.9) | 0.76 |
Notes: Data are prevalence % (95% CI). People were categorized as having low sexual desire if reporting sexual desire rarely or never when asked “How often do you have sexual desire?”. People were categorised as having low sexual satisfaction if reporting disagree or disagree a lot with the statement “I am satisfied with my sexual life”.
Overall, the prevalence of low sexual desire rose significantly with increasing age among men and women, p < 0.01. In all age groups, more women than men reported low sexual desire, p < 0.01.
The prevalence of low sexual satisfaction did not change significantly with age. Among patients aged above 59 years, statistically significantly more men than women expressed low sexual satisfaction, p < 0.01. Non-attending women were more likely to be current smokers and older; and non-attending men were less likely to be married and more likely to be current smokers. Differences between attenders and non-attenders were the same across treatment groups.
Impact of intensive multifactorial treatment on sexual desire and sexual satisfaction
Among men, the prevalence of low sexual satisfaction was significantly higher in the IT group (37%) than in the RC group (27%), whereas no statistically significant difference was observed among women. Furthermore, IT had no impact on self-reported sexual desire among either men or women.
As IT was found to impact on sexual satisfaction in men, logistic regression was used to study whether this impact was linked to specific sexual health risk factors. This analysis showed a clear tendency that IT was associated with lower self-reported sexual satisfaction than RC (data not shown) among men with better health (normal weight; no micro-vascular or macro-vascular complications, better self-reported mental and physical health, and no beta-blocker consumption); among men with better self-perceived mental health, the association was found to be statistically significant (p < 0.005). There was no difference in the impact of IT compared with RC among men with and without erectile dysfunction.
Non-responders’ follow-up characteristics differed from the responders’ follow-up characteristics. Among women, there were no differences in the IT group. In the RC group, non-responding women were significantly older and had a higher self-rated mental health score than responders. Among men, no differences were seen in the RC group, whereas non-responding men in the IT group had a higher total cholesterol, a higher HbA1c, drank less alcohol, were less likely to take beta-blockers and had a higher diastolic blood pressure. Other characteristics were comparable between the groups.
Discussion
Principal findings
In this randomized study we found that low sexual desire and low sexual satisfaction were common among middle-aged and older Danish Caucasian women and men with screen-detected type 2 diabetes. The prevalence patterns differed by age and sex. Compared with routine care, intensive multifactorial treatment was associated with lower sexual satisfaction in men, but not in women. There was a trend for this association to be particularly pertinent among men with better health.
Strengths and weaknesses
A main strength of the present study compared with other studies of sexual health among middle-aged and older patients with type 2 diabetes is that our patient population was large, homogeneous and well described and that the questionnaire response rate was relatively high. Our data relied on self-administered questionnaires, which in general motivate more honest answers to questions regarding intimate matters [19]. However, it is possible that some groups of people are more likely than others to answer questions on sexual health. Thus, people who are not sexually active may find answering such questions less relevant than people who are sexually active. However, this would probably apply to the same extent in the IT and RC group.
Non-attenders differed from attenders, but they differed in similar ways across the treatment groups, therefore under- or overestimation of prevalences is most likely similar. Non-responders differed from responders in the RC and the IT group, respectively. However, the differences were small and not in conflicting directions. We are therefore confident that the calculated odds ratios are reliable.
We examined a post-hoc question in the ADDITION-Denmark trial cohort. The number of statistical tests performed in the logistic regression analysis to compare the IT with the RC group among men was high, which increased the risk of a type 1 error. This was taken into account by using 99.5% CI instead of 95% CI. However, our between-treatment group results should be interpreted with caution and they should be confirmed in future studies. Finally, information on sexual health was not obtained at baseline, which prevented us from observing longitudinal changes.
Findings in relation to other studies
In previous population-based studies of healthy middle-aged and older Danish citizens, the prevalence of low sexual satisfaction was found to be 10% among women and 14% among men (n = 1494) [20]. The prevalence of low sexual desire was found to be 20% among women and 8% among men, with a decrease in sexual desire with increasing age (n = 10 458) [5].
Unfortunately, most studies of sexual desire and satisfaction among men and women with type 2 diabetes are relatively small in size (n = 26–665), and methodological differences hamper comparison of results across studies. However, among middle-aged and older men and women with type 2 diabetes, the previously reported prevalence of low sexual desire lies in the range of 25–31% among men and in the range of 58–78% among women. Previously reported prevalences of low sexual satisfaction are reported to vary from 40% to 54% among men and from 26% to 45% among women [4,21–27]. These results are largely similar to our findings. This indicates that in a randomly selected population of Danish men and women with screen-detected type 2 diabetes, the levels of low sexual desire and low sexual satisfaction are comparable to those reported for other populations of men and women with type 2 diabetes.
We report diverse prevalences of low sexual desire and low sexual satisfaction, which illustrate that low sexual desire might not involve equivalently low sexual satisfaction. Concordantly, people engage in sexual behaviour for many other reasons than sexual desire and how to obtain sexual satisfaction is individual. Sexual satisfaction mainly derives from positive sexual experiences and cannot automatically be equated with absence of sexual performance or the presence of sexual desire problems [3]. Our results underline the importance of distinguishing between different aspects of sexual health, i.e. the need to include sexual satisfaction in the evaluation of sexual health.
To our knowledge, no previous studies have examined the impact of intensive treatment on the level of sexual desire and the level of sexual satisfaction among people with type 2 diabetes. Intensive treatment has been found not to negatively influence self-reported health status, well-being, quality of life, and treatment satisfaction among patients with type 2 diabetes [7–9].
However, our study suggests that IT in primary care could have a negative impact on self-reported sexual well-being in men with type 2 diabetes, especially in men with better health. This finding is unexpected. It is well documented that erectile dysfunction is strongly related to physical health problems and poor self-reported health [15,28,29]. Why, then, do men with better self-reported health and few physical health problems experience less sexual satisfaction when receiving IT compared with RC independently of erectile dysfunction? IT was provided with additional health information and health checks. More attention was therefore given to the disease among patients in the IT group than in the RC group. This may have raised the level of self-perceived illness, which may, again, have translated into a lower reported level of subjective sexual health. According to studies of men and women's health behaviour, men tend to avoid visiting their general practitioners because illness tends to be considered a weakness and visiting the general practitioner a sign of dependency, which may both cause men to experience self-doubts about their masculinity [30]. It is therefore possible that the advanced treatment programme may have caused self-doubts about masculinity and sexual performance in men, resulting in less subjective sexual satisfaction. It can also be speculated that, especially among men with better health, IT could cause men to have overly high expectations to sexual life, which increases the potential for distress and lower sexual satisfaction if such expectations cannot be met.
Implications for practice
Practitioners dealing with diabetes have previously focused almost exclusively on sexual dysfunction, but sexual desire and sexual satisfaction are also affected early in the trajectory of diabetes. This underlines the need to distinguish between different aspects of sexuality when addressing the sexual burden of diabetes. Furthermore, intensive multifactorial treatment could potentially negatively impact men's sexual satisfaction, and practitioners should therefore pay special attention to these issues to protect sexual health among men when more intensive treatment strategies are used in the treatment of type 2 diabetes.
Acknowledgements
The authors gratefully acknowledge the contribution of all participants, practice nurses, and GPs in the ADDITION-Denmark study.
Ethical approval
Ethical approval in accordance with the principles of the 1996 Helsinki Declaration was obtained from each of the relevant local ethics committees. All participants provided informed consent.
Funding
ADDITION-Denmark was supported by the National Health Services in the counties of Copenhagen, Aarhus, Ringkøbing, Ribe, and South Jutland in Denmark; the Danish Council for Strategic Research; the Danish Research Foundation for General Practice; Novo Nordisk Foundation; the Danish Centre for Evaluation and Health Technology Assessment; the Diabetes Fund of the National Board of Health; the Danish Medical Research Council; and the Aarhus University Research Foundation. The trial has been given unrestricted grants from Novo Nordisk AS, Novo Nordisk Scandinavia AB, Novo Nordisk UK, ASTRA Denmark, Pfizer Denmark, Glaxo-SmithKline Pharma Denmark, Servier Denmark A/S, and HemoCue Denmark A/S. Parts of the grants from Novo Nordisk Foundation, Danish Council for Strategic Research, and Novo Nordisk were transferred to the other centres. This study was granted from the Research Council for Medical Sciences.
Declaration of interests
TL received unrestricted grants for the ADDITION study (screening and intensive treatment of type 2 diabetes in primary care) from public foundations and the medical industry: Novo Nordisk AS, Novo Nordisk Scandinavia AB, ASTRA Denmark, Pfizer Denmark, GlaxoSmithKline Pharma Denmark, SERVIER Denmark A/S and HemoCue Denmark A/S. TL holds shares in Novo Nordisk.
The remaining authors have declared no conflict of interests. The authors alone are responsible for the content and writing of the paper.
References
- 1.De Berardis G, Pellegrini F, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, et al. Longitudinal assessment of quality of life in patients with type 2 diabetes and self-reported erectile dysfunction. Diabetes Care. 2005;28:2637–43. doi: 10.2337/diacare.28.11.2637. [DOI] [PubMed] [Google Scholar]
- 2.Chao JK, Lin YC, Ma MC, Lai CJ, Ku YC, Kuo WH, et al. Relationship among sexual desire, sexual satisfaction, and quality of life in middle-aged and older adults. J Sex Marital Ther. 2011;37:386–403. doi: 10.1080/0092623X.2011.607051. [DOI] [PubMed] [Google Scholar]
- 3.Pascoal PM, Narciso Ide S, Pereira NM. What is sexual satisfaction? Thematic analysis of lay people's definitions. J Sex Res. 2014;51:22–30. doi: 10.1080/00224499.2013.815149. [DOI] [PubMed] [Google Scholar]
- 4.Lindau ST, Tang H, Gomero A, Vable A, Huang ES, Drum ML, et al. Sexuality among middle-aged and older adults with diagnosed and undiagnosed diabetes: A national, population-based study. Diabetes Care. 2010;33:2202–10. doi: 10.2337/dc10-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eplov L, Giraldi A, Davidsen M, Garde K, Kamper- Jorgensen F. Sexual desire in a nationally representative Danish population. J Sex Med. 2007;4:47–56. doi: 10.1111/j.1743-6109.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- 6.Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: An update. Nat Rev Cardiol. 2010;7:369–75. doi: 10.1038/nrcardio.2010.35. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RT, Narayan KM, Feeney P, Goff D, Jr, Ali MK, Simmons DL, et al. Effect of intensive glycemic lowering on health-related quality of life in type 2 diabetes: ACCORD trial. Diabetes Care. 2011;34:807–12. doi: 10.2337/dc10-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study Group (UKPDS) Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control. Diabetes Care. 1999;22:1125–36. doi: 10.2337/diacare.22.7.1125. [DOI] [PubMed] [Google Scholar]
- 9.Van den Donk M, Griffin SJ, Stellato RK, Simmons RK, Sandbaek A, Lauritzen T, et al. Effect of early intensive multifactorial therapy compared with routine care on self-reported health status, general well-being, diabetes-specific quality of life and treatment satisfaction in screen-detected type 2 diabetes mellitus patients (ADDITION-Europe): A cluster-randomised trial. Diabetologia. 2013;56:2367–77. doi: 10.1007/s00125-013-3011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G. The ADDITION study: Proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. Int J Obes Relat Metab Disord. 2000;24(Suppl 3):S6–11. doi: 10.1038/sj.ijo.0801420. [DOI] [PubMed] [Google Scholar]
- 11.The ADDITION Study Screening and treatment study of type-2 diabetes. Available at: http://www.addition.au.dk/(accessed 21 February 2014)
- 12.Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbaek A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): A cluster-randomised trial. Lancet. 2011;378:156–67. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dansk selskab for almen medicin Type 2-diabetes i almen praksis – En evidensbaseret vejledning 2004 [Type 2 diabetes in general practice – An evidence-based guideline] Available at http://www.dsam.dk/files/9/type_2_diabetes_2004_rettet.pdf (accessed 28 October 2014)
- 14.Kjøller M, Rasmussen N. Danish Health and Morbidity Survey 2000 & trends since 1987. Copenhagen: National Institute of Public Health; 2002. [Google Scholar]
- 15.Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369:597–611. doi: 10.1016/S0140-6736(07)60280-3. [DOI] [PubMed] [Google Scholar]
- 16.Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: The ADDITION-Denmark study. Diabetes Care. 2011;34:2244–9. doi: 10.2337/dc11-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 18.Grading diabetic retinopathy from stereoscopic color fundus photographs An extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 19.McEwan RT, Harrington BE, Bhopal RS, Madhok R, McCallum A. Social surveys in HIV/AIDS: Telling or writing? A comparison of interview and postal methods. Health Educ Res. 1992;7:195–202. doi: 10.1093/her/7.2.195. [DOI] [PubMed] [Google Scholar]
- 20.Ventegodt S. Sex and the quality of life in Denmark. Arch Sex Behav. 1998;27:295–307. doi: 10.1023/a:1018655219133. [DOI] [PubMed] [Google Scholar]
- 21.Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Fernando DJ, Levy JC. Erectile dysfunction among men with diabetes is strongly associated with premature ejaculation and reduced libido. J Sex Med. 2008;5:2125–34. doi: 10.1111/j.1743-6109.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- 22.Mulligan T, Retchin SM, Chinchilli VM, Bettinger CB. The role of aging and chronic disease in sexual dysfunction. J Am Geriatr Soc. 1988;36:520–4. doi: 10.1111/j.1532-5415.1988.tb04022.x. [DOI] [PubMed] [Google Scholar]
- 23.Copeland KL, Brown JS, Creasman JM, Van Den Eeden SK, Subak LL, Thom DH, et al. Diabetes mellitus and sexual function in middle-aged and older women. Obstet Gynecol. 2012;120:331–40. doi: 10.1097/AOG.0b013e31825ec5fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erol B, Tefekli A, Ozbey I, Salman F, Dincag N, Kadioglu A, et al. Sexual dysfunction in type II diabetic females: A comparative study. J Sex Marital Ther. 2002;28(Suppl 1):55–62. doi: 10.1080/00926230252851195. [DOI] [PubMed] [Google Scholar]
- 25.Mezones-Holguin E, Blumel JE, Huezo M, Vargas R, Castro J, Cordova W, et al. Impact of diabetes mellitus on the sexuality of Peruvian postmenopausal. Gynecol Endocrinol. 2008;24:470–4. doi: 10.1080/09513590802273749. [DOI] [PubMed] [Google Scholar]
- 26.Burke JP, Jacobson DJ, McGree ME, Nehra A, Roberts RO, Girman CJ, et al. Diabetes and sexual dysfunction: Results from the Olmsted County study of urinary symptoms and health status among men. J Urol. 2007;177:1438–42. doi: 10.1016/j.juro.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 27.Taloyan M, Wajngot A, Johansson SE, Tovi J, Sundquist J. Ethnic differences in dissatisfaction with sexual life in patients with type 2 diabetes in a Swedish town. BMC Public Health. 2010;10:536. doi: 10.1186/1471-2458-10-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein R, Klein BE, Moss SE. Ten-year incidence of self-reported erectile dysfunction in people with long- term type 1 diabetes. J Diabetes Complications. 2005;19:35–41. doi: 10.1016/j.jdiacomp.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Christensen BS, Gronbaek M, Osler M, Pedersen BV, Graugaard C, Frisch M. Associations between physical and mental health problems and sexual dysfunctions in sexually active Danes. J Sex Med. 2011;8:1890–902. doi: 10.1111/j.1743-6109.2010.02145.x. [DOI] [PubMed] [Google Scholar]
- 30.Courtenay WH. Constructions of masculinity and their influence on men's well-being: A theory of gender and health. Soc Sci Med. 2000;50:1385–401. doi: 10.1016/s0277-9536(99)00390-1. [DOI] [PubMed] [Google Scholar]