Abstract
Acute non-anion gap metabolic acidosis, also termed hyperchloremic acidosis, is frequently detected in seriously ill patients. The most common mechanisms leading to this acid–base disorder include loss of large quantities of base secondary to diarrhea and administration of large quantities of chloride-containing solutions in the treatment of hypovolemia and various shock states. The resultant acidic milieu can cause cellular dysfunction and contribute to poor clinical outcomes. The associated change in the chloride concentration in the distal tubule lumen might also play a role in reducing the glomerular filtration rate. Administration of base is often recommended for the treatment of acute non-anion gap acidosis. Importantly, the blood pH and/or serum bicarbonate concentration to guide the initiation of treatment has not been established for this type of metabolic acidosis; and most clinicians use guidelines derived from studies of high anion gap metabolic acidosis. Therapeutic complications resulting from base administration such as volume overload, exacerbation of hypertension and reduction in ionized calcium are likely to be as common as with high anion gap metabolic acidosis. On the other hand, exacerbation of intracellular acidosis due to the excessive generation of carbon dioxide might be less frequent than in high anion gap metabolic acidosis because of better tissue perfusion and the ability to eliminate carbon dioxide. Further basic and clinical research is needed to facilitate development of evidence-based guidelines for therapy of this important and increasingly common acid–base disorder.
Keywords: acidemia, base therapy, bicarbonate, hyperchloremic acidosis, non-anion gap acidosis
Introduction
Acute metabolic acidosis (defined temporally as lasting minutes to a few days) has traditionally been divided into two major categories based on the level of the serum anion gap: non-anion gap and high anion gap metabolic acidosis [1]. As implied, with the former acid–base disorder, the anion gap is within normal limits, whereas with the latter disorder it is increased. This categorization is primarily used to facilitate the differential diagnosis of metabolic acidosis. However, it also has relevance for predicting the clinical outcome and determining indications for treatment. Although many clinicians presume that acute metabolic acidosis in seriously ill patients will be due to a high anion gap acidosis, recent studies indicate that a non-anion gap metabolic acidosis or combination of non-anion gap and high anion gap metabolic acidosis might be more frequent [2, 3]. Based on these observations, it appears important to more clearly define the potential effects of non-anion gap metabolic acidoses on organ function as a basis for generating evidence-based guidelines for therapy.
In the present review, we summarize our current understanding of the pathophysiology of acute non-anion gap acidosis, its clinical characteristics, its adverse effects on cellular function, and also the benefits and complications of therapy.
Definition
In non-anion gap or hyperchloremic metabolic acidosis, a reduction in serum is matched by an approximately equivalent increase in the serum chloride concentration resulting in hypobicarbonatemia and hyperchloremia in the absence of an increase in the serum anion gap [4, 5]. In fact, since a decrease in blood pH alters the protonation of albumin (which normally makes up the majority of the anion gap), a slight decrease in the serum anion gap can actually be observed, particularly when the blood pH is very low [6].
The conclusion that a patient has a non-anion gap metabolic acidosis alone based on the finding that the anion gap calculated from examination of the serum electrolytes and serum albumin falls within the normal range can sometimes be incorrect [1, 4]. This might occur because the range of anion gap in the normal population is often large enough (often spanning 10 mEq/L from values at the low end of the anion gap to values at the high end of the gap) for an individual with a baseline anion gap at the lower range of normal to develop a high anion gap metabolic acidosis without causing the anion gap to exceed the normal range [7]. This difficulty can be minimized, but not necessarily eliminated, by utilizing a baseline value specific to the individual patient to calculate the change in the anion gap (ΔAG) [4].
Although it can sometimes be difficult for the aforementioned reasons, it is essential to try to identify whether a high anion gap acidosis is present: the type of acidosis has an important impact on clinical outcome, and therefore will have a bearing on when to initiate therapy and what therapy to utilize [8, 9].
Pathophysiologic mechanisms
The mechanisms commonly producing acute non-anion gap metabolic acidosis are shown in Table 1. They include loss of base from the body via either the urinary or gastrointestinal tract, administration of hydrochloric acid or substances that are metabolized to hydrochloric acid such as cationic amino acids and ammonium chloride, rapid infusion of sodium chloride-containing solutions such as 0.9% saline [3, 10–14] and loss of organic acid anions in the urine with replacement with filtered chloride (most commonly occurring with diabetes mellitus) [15].
Table 1.
Common causes of acute non-anion gap metabolic acidosis
| Cause | Pathophysiological mechanisms | Comments |
|---|---|---|
| Diarrhea, enteric fistulae | Loss of bicarbonate from the gastrointestinal tract | Profound acidosis with profuse diarrhea, volume depletion and hypokalemia commonly accompany the metabolic acidosis |
| Administration of chloride-rich solutions, most commonly 0.9% saline | Dilution of bicarbonate stores? | Extremely common, in some studies accounts for as much as 50% of patients with metabolic acidosis |
| Conversion of high anion gap acidosis | Loss of circulating organic acid anions with replacement by chloride | 20% or more of patients with diabetic ketoacidosis will have a component of non-gap acidosis prior to or after treatment with saline containing solutions |
| Administration of cationic amino acids (present in common total parenteral nutrition solutions) intravenously; administration of NH4Cl either intravenously or orally. | Metabolism of substances to hydrochloric acid; consumption of in the liver during the metabolism of NH4Cl | Minimization of metabolic acidosis from TPN solutions has been accomplished by including a higher concentration of organic acid anions (potential base) |
The particular mechanism producing the non-anion gap acidosis will often have an impact on the nature of the adverse effects experienced by the patient and the eventual clinical outcome. For example, diarrhea or loss of fluid from enteric fistulae is frequent causes of non-anion metabolic gap acidosis accounting for a large percentage of cases with this acid–base disorder, particularly in areas where cholera is endemic [16–18]. The gastrointestinal losses of base are accompanied by ample quantities of sodium, potassium and water. Indeed, with some cases of secretory diarrhea, fluid loss can exceed 3 L/day [19]. As a consequence, volume depletion and hypokalemia often accompany the metabolic acidosis. The volume depletion reduces GFR which is adaptive in that it decreases bicarbonate excretion. Depending on the extent of renal tubular damage, there is also potentially an associated decrease in new renal bicarbonate generation. If volume depletion and hypokalemia are severe, hypotension and lactic acidosis can ensue [17]. The combined effects of these perturbations can have deleterious effects on organ function that are additive to those related to the acidemia.
The non-anion gap metabolic acidosis arising from aggressive administration of sodium chloride-containing solutions during operative procedures [12], treatment of volume depletion [3] and shock states [3] is becoming increasingly more prevalent. Indeed, in one study involving 70 patients in an intensive care unit, almost 50% had a non-anion gap pattern produced in this fashion [9, 20]. A non-anion gap metabolic acidosis can also be observed in patients with diabetic ketoacidosis or other high anion gap metabolic acidoses where the loss of organic anions (potential base) in the urine is replaced with administered sodium chloride [21]. The prevalence of a non-anion gap pattern is highest after treatment with saline, but can also be present prior to administration of fluids or insulin if patients are not volume depleted and are therefore able to excrete organic anions as they are produced [15]. The conversion of the acidosis to a non-anion gap pattern will have an impact on the use of base or other treatment modalities. Taken together, these three mechanisms account for the vast majority of acute non-anion gap metabolic acidoses encountered by the clinician.
Several disorders including various types of renal tubular acidosis and chronic kidney disease are associated with the development of a non-anion gap metabolic acidosis alone or a combination of non-anion gap and high anion gap acidosis [22]. Most commonly the metabolic acidosis is chronic in nature, the hypobicarbonatemia developing over many weeks or longer. Therefore, we have elected to not address their pathogenesis or treatment in the present manuscript. The pathogenesis, indications for treatment and complications of treatment are addressed by the authors and others in recent studies [23, 24].
Diagnosis
The approach to diagnosis of a non-anion gap metabolic acidosis has been considered in an in-depth review published previously [5]. Differentiating disorders associated with volume depletion from those associated with volume expansion is a critical step with important implications for treatment. This generally can be accomplished by obtaining a complete history and performing a focused physical examination. Measures of renal function including serum creatinine or cystatin C are valuable. They will not only alert the clinician as to the possible contribution of renal disease to the metabolic acidosis, but also govern his/her decision about initiation of dialysis. On the other hand, because in many cases the cause of the non-anion gap metabolic acidosis will be obvious, additional tests such as the assessment of the excretion rate of ammonium (by calculation of the urine anion, urine osmolal gap or direct measures of NH4 excretion) or documentation of renal bicarbonate wasting is rarely required.
Adverse effects
Acute metabolic acidosis is associated with several adverse effects including depression of cardiovascular function and a predisposition to cardiac arrhythmias, vasodilatation with hypotension, increased inflammation, suppression of immunity and an increase in mortality [25]. These effects are particularly prominent with lactic acidosis and their occurrence with non-anion gap metabolic acidosis has not been well detailed.
Animal studies in which lactic acidosis was produced by various methods including hypoxia and hypoperfusion followed by a lactic acid infusion [26] were associated with depression of cardiac contractility and cardiac output. When the link between the severity of the acidemia and changes in contractility was examined, it was found that contractility actually improved when blood pH was reduced from 7.4 to 7.2, but subsequently decreased when it fell below 7.2 [26]. The initial rise in cardiac contractility was attributed to increased systemic catecholamines.
In pigs given an intravenous infusion of HCl to produce a blood pH of 7.1, a fall in stroke volume without a reduction in cardiac output was detected [27]. In studies in which metabolic acidosis (pH ≤ 7.1) was induced in rats by infusion of HCl, with or without preexisting sepsis, a severe reduction in blood pressure occurred similar to the situation with lactic acidosis [28, 29]. The hypotension was associated with a marked increase in nitric oxide production and plasma nitrate/nitrate levels. Pretreatment with a specific inhibitor of nitric oxide production, aminguanidine, reduced the levels of nitric oxide and prevented the fall in blood pressure. Non-anion gap metabolic acidosis produced in this fashion was also associated with an increase in circulating inflammatory cytokines [30]. Similar in vivo studies have not been done in humans. However, exposure of ventricular trabeculae obtained from the failing hearts of humans to an external pH < 7.2 (in HEPES-buffered solution) caused a decrease in contractility of the muscle and a blunted response to β-adrenergic stimulation [31]. These findings suggest the impact of an acidic milieu in humans will mimic that observed in animal studies.
The non-anion gap metabolic acidosis might even have a greater tendency to induce inflammation than lactic acidosis: addition of HCl to the media of macrophage-like RAW cells led to an increase in pro-inflammatory markers in response to liposacharide stimulation including NO, IL 10/IL6 ratios and NF-kappa B DNA binding [32]. A similar decrease in pH produced by lactic acid addition to the media had less of an effect. The relevance of these observations to clinical situations is unclear, but these in vitro data could provide potential clues as to what might occur in patients.
The clinical outcome of non-anion gap metabolic acidosis when compared with high anion gap metabolic acidosis has been examined in a few studies. A retrospective review of almost 9000 seriously ill patients hospitalized in an intensive care unit revealed that mortality of those with metabolic acidosis was significantly higher than those without metabolic acidosis (59 versus 25%) [8]. However, this increased mortality was primarily confined to those with lactic acidosis or other types of high anion gap acidosis, since the mortality of those with non-anion gap metabolic acidosis was not different than those without acidosis (29 versus 25%). The severity of the metabolic acidosis in both groups was not given, and therefore a difference between the groups in this important parameter could not be excluded as a factor. In this regard, an observational study of 75 consecutive patients admitted to a surgical ICU [9], almost half of whom had a non-anion gap metabolic acidosis and the remaining had a mixed high and non-anion gap metabolic acidosis, revealed that the mortality in the group with high and non-anion gap metabolic acidosis was three times that of the non-anion gap group (34 versus 10.8%). The hypobicarbontemia was slightly greater in the high anion gap group (−7.8 ± 5.4 versus −5.3 ± 2.5 mEq/L). However, in the group as a whole, there was no relationship between the severity of the acidosis and clinical outcome. These results seem to indicate that disorders associated with a high anion gap acidosis are associated with a higher mortality than those with a non-anion gap metabolic acidosis. On the other hand, in a longitudinal study of 60 patients with sepsis from a single intensive care unit, the presence of hyperchloremia early in the course of the disease was associated with a poor clinical outcome [33].
Up until a few years ago, the elevation of serum chloride in non-gap metabolic acidosis was not considered to have any impact on cellular function, per se. However, studies in animals and humans reported a deleterious effect of hyperchloremia resulting from the administration of large quantities of saline, which appeared to be separate from that produced by the metabolic acidosis [34]. In a study on 22 851 surgical patients with normal preoperative serum chloride concentration and renal function, those who developed acute postoperative hyperchloremia were at increased risk for 30-day postoperative mortality [3.0 versus 1.9%; odds ratio (95% CI): 1.58 (1.25–1.98)] and had a longer median hospital stay (7.0 days versus 6.3 days) [35]. They also had a greater prevalence of renal impairment. The mechanism underlying renal impairment was hypothesized to be hyperchloremic-induced vasoconstriction with reduced GFR [34]. However, the mechanism underlying the increased mortality is unclear, and future studies will be required to separate the potential independent effects of hyperchloremia, a decrease in systemic pH and hypobicarbonatemia.
Prevention
To the extent that non-anion gap metabolic acidosis is associated with administration of large quantities of chloride-containing solutions, this complication can be minimized or eliminated by limiting the quantity of chloride-containing solutions or interchanging it with balanced salt solutions [36]. Thus, administration of balanced salt solutions was shown to lessen the severity of the non-anion gap metabolic acidosis frequently developing during the treatment of diabetic ketoacidosis [36]. Also, administration of balanced salt solutions to more than 800 critically ill patients in an intensive care unit reduced the incidence of severe hyperchloremia and severe metabolic acidosis compared with a matched group receiving chloride-rich solutions [37].
The impact of using balanced salt solutions, thereby potentially ameliorating the effect of chloride-containing solutions on renal function and clinical outcome, was studied in a few large studies. The impact of isotonic saline versus a balance salt solution (in this case PlasmaLyte containing acetate as potential base, Baxter) was examined in a large retrospective study comparing over 30 000 patients receiving saline with more than 900 receiving PlasmaLyte who underwent abdominal surgery [38]. Mortality was 50% lower in the group receiving PlasmaLyte. In a separate prospective open-label study examining the impact of chloride-containing solutions on renal function, restricting chloride administration decreased the risk of acute kidney injury and also substantially reduced the need for renal replacement therapy [39]. Moreover, in controlled study of patients with sepsis, administration of balanced salt solutions was associated with a lower risk of death than in those receiving saline [40]. Also, a Cochrane Data Base analysis of 13 studies in which balance solutions were compared with saline for resuscitation during surgery revealed less hyperchloremia and a higher serum in the patients getting the balanced salt solutions [41].
As a result of these findings, some investigators have recommended giving balanced salt solutions as the primary resuscitation fluid to individuals with volume depletion or hypotension [42]. The utilization of a balanced salt solution (particularly one with a lowered chloride concentration) would likely have two benefits: first, the incidence and severity of hyperchloremia will be lessened. Secondly, the depression of serum will be avoided. At present, it is difficult to determine the individual contributions of the changes in acid–base balance and serum chloride to a poor clinical outcome and it is possible both effects are important.
The composition of commonly used crystalloids is shown in Table 2. Although saline has been compared with individual balanced salt solutions as a resuscitation fluid as described above, no comparison of individuals balanced buffer solution has been performed. Therefore, in making decisions about which buffered crystalloids to use, particular attention should be paid to the potential benefits and adverse effects of individual constituents in each solution. For example, although the chloride concentration of Ringer's lactate and Hartmann's solution is less than in normal saline, it is still >100 mmol/L. On the other hand, both contain adequate concentration of calcium. This is important, because it more likely to stabilize ionized calcium during their administration, particularly during the correction of acidemia whereas the rise in blood pH would tend to increase binding of calcium to protein and thereby reduce free Ca2+. In this regard, studies in animals and in patients have documented impairment of hemodynamic parameters associated with a fall in ionized calcium [43]. Also, infusion of calcium and hyperventilation of rats with hypotensive-induced lactic acidosis improved the response to infused catecholamines during sodium bicarbonate administration [44].
Table 2.
Commonly used crystalloid solutions
| Solution | 0.9% NaCl | 0.45% NaCl | Ringer's lactate | Ringer's acetate | Hartmann's | PlasmaLyte |
|---|---|---|---|---|---|---|
| Na+ (mmol/L) | 154 | 77 | 130 | 130 | 131 | 140 |
| Cl− (mmol/L) | 154 | 77 | 109 | 112 | 111 | 98 |
| K+ (mmol/L) | 0 | 0 | 4 | 5 | 5 | 5 |
| (mmol/L) | 0 | 0 | 0 | 0 | 0 | 0 |
| Ca2+ (mmol/L) | 0 | 0 | 1.4 | 1 | 2 | 0 |
| Lactate (mmol/L) | 28 | 0 | 29 | 0 | ||
| Mg2+ (mmol/L) | 0 | 0 | 0 | 1 | 0 | 1.5 |
| Acetate (mmol/L) | 0 | 27 | 0 | 27 | ||
| Glucose (mmol/L) | 0 | 0 | 0 | 0 | 0 | 0 |
| Osmolality (mOsm/L) | 308 | 278 | 273 | 276 | 278 | 294 |
| Comments | Most common solution used for resuscitation; non-anion gap acidosis and hypocalcemia common after administration volume overload potential risk | Solution used for treatment of hyperosmolal states; complications similar to that with 0.9% saline |
Effective resuscitation fluid; commonly used in surgical treatment; contains calcium to prevent hypocalcemia; lactate metabolized to base even in presence of lactic acidosis |
Effective resuscitation fluid; contains calcium to prevent hypocalcemia | Effective resuscitation fluid; contains calcium to prevent hypocalcemia | Effective resuscitation fluid; does not contain calcium |
On the other hand, although more laborious preparation of a customized solution by the clinician might be the most effective method of delivering base while stabilizing ionized calcium and preventing hyperchloremia. Addition of bicarbonate to 5% glucose solution can provide the base desired. However, calcium will have to be provided by a separate portal to prevent its precipitation in an alkaline medium.
In some situations oral base therapy will be sufficient for the treatment or prevention of non-anion gap metabolic acidosis. Indeed, rehydration with oral solutions has been found to be as successful as intravenous solutions in the treatment of severe diarrheal states associated with metabolic acidosis such as cholera [45].
Sodium bicarbonate or sodium citrate given orally can often be utilized to treat a non-anion gap metabolic acidosis, in a stable patient particularly in the absence of severe volume contraction. With severe volume contraction, the clinician might utilize one of the commercially available rehydration solutions [45].
Treatment
Given the possible evidence linking development of non-anion gap metabolic acidosis to a decreased GFR and an increase in mortality, it seems reasonable to consider administration of base to correct or lessen the acidemia. Indeed, based on an online survey administered to nephrologists and critical care physicians, one can infer that the majority of both groups (≥86%) will recommend base therapy for patients with acute non-anion gap metabolic acidosis when it is severe (pH 7.1–7.2) [46] regardless of the hemodynamic state of the patient.
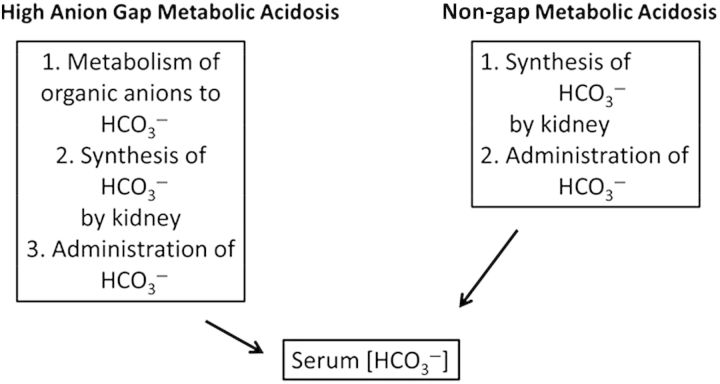
The mechanisms involved in correction of acute non-anion gap metabolic acidosis when compared with that of high anion gap acidosis are shown in Figure 1. In the former, the improvement in acid–base balance depends on synthesis of bicarbonate by the kidney and retention of administered base. This is in direct contrast to high anion gap metabolic acidosis such as ketoacidosis or lactic acidosis where the circulating organic acid anions can serve as a source of base. Given that renal impairment is very common in patients with either diarrhea or hypotension, generation of base by the kidney might be constrained. Should acute kidney injury with or without acute tubular necrosis develop, the need for base administration by the clinician might become even more necessary as renal generation of base is likely to be impaired.
Fig. 1.
Contrasting mechanisms for correction of metabolic acidosis with non-gap and high anion gap acidosis. With high anion gap acidosis, conversion of circulating organic acid anions to base can theoretically produce a large rise in serum Other sources of base include that given by the physician, and bicarbonate synthesized by the kidney. In contrast, there is no contribution from metabolism of circulating anions with non-gap acidosis. The only sources of base include that given by the physician and that synthesized by the kidney. Since many of these patients have renal impairment, there might be a constraint on base delivered from this source.
Limited studies have examined the use of base in the treatment of non-anion gap metabolic acidosis. In a single study, the effect of base treatment on clinical and acid–base parameters was examined in patients with non-anion gap acidosis produced by administration of saline prior to abdominal surgery [47]. This treatment resulted in a fall in serum to 18 mEq/L. Subsequent administration of bicarbonate or THAM led to a rise in serum to baseline. The bicarbonate administration led to an average increase in CO2 generation as reflected by need to increase ventilation by 40% to maintain a stable pCO2. In contrast, ventilation could be reduced by 60% with THAM.
The level of serum bicarbonate and blood pH which should trigger the initiation of therapy and the appropriate level of these parameters to attempt to achieve have remained elusive. As noted in previous publications, a blood pH of 7.1 to 7.2 has been considered the critical level at which to initiate therapy for common serious acid–base disorders such as lactic acidosis, based on in vivo animal studies [25] but supported by studies using trabeculae isolated from the myocardium of humans [31]. Extrapolating from these data, we have recommended a similar threshold for initiation of therapy of non-anion gap metabolic acidosis.
However, even if cardiac function is not impaired in the face of a blood pH of 7.2 to 7.4, cardiac contractility appears to be maintained by an influx of catecholamines, since it can be blocked by pretreatment with catecholamines [26]. This augmented catcholamine response could sensitize the myocardium to arrhythmias. Also, in light of evidence summarized above that non-anion metabolic gap acidoses might have a negative impact on clinical outcome even when the acidemia is not severe, initiation of base at a less severe degree of metabolic acidosis might be considered.
In addition to a reduction in intracellular and interstitial pH, recent evidence suggests that activation of the Na+–H+ exchanger, NHE1 contributes to cellular damage in hypoxic lactic acidosis [48]. As a result, treatment with a specific NHE1 inhibitor was shown to improve cellular function and reduce mortality [25]. The value of this modality of therapy in non-anion gap metabolic acidosis has not been studied and so its role in the treatment of this acid–base disorder remains undefined [49].
Complications of therapy
Ultimately the benefits of therapy have to be weighed against the potential complications. Complications of base therapy have often been considered related to its use in the treatment of lactic acidosis and ketoacidosis. Theoretically they can occur during the treatment of non-anion gap metabolic acidosis just as with high anion gap metabolic acidosis. The major complications of bicarbonate therapy with high anion gap metabolic acidosis have been twofold: (i) generation of CO2 during the buffering process resulting in entry of CO2 into the cell and aggravation of intracellular acidosis and (ii) a reduction in ionized calcium as blood pH is increased.
The latter complication is likely to be frequent as base therapy with non-anion gap metabolic acidosis raises pH similar to that achieved with treatment of high anion gap metabolic acidosis [47]. However, the former complication might not be inevitable with base treatment of acute non-anion gap metabolic acidosis. Studies in animals and humans which examined changes in intracellular pH in response to bicarbonate administration as a surrogate for increased entry of CO2 have showed mixed results. Although in several studies a decrease in intracellular pH was observed, in some no change, or even an increase in intracellular pH was found [50, 51]. Additional factors that might affect its occurrence include whether tissue perfusion or respiratory elimination of CO2 is compromised. Both factors will predispose to the exacerbation of intracellular acidosis [25]. Thus, the mechanism leading to non-anion gap metabolic acidosis might affect the occurrence of this complication. For example, severe diarrhea with hypotension and/or administration of chloride-containing solutions for resuscitation in individuals in which tissue perfusion is not restored are two clinical scenarios more likely to be associated with this complication. In contrast, if tissue perfusion and pulmonary function are intact, the risk of this complication would be lessened.
The previous discussion applies to the intravenous administration of base. No comparative studies have examined the impact of oral base therapy on the aforementioned potential complications. Since the reduction in free Ca2+ concentration with base therapy is dependent solely on a rise in blood pH, the impact of oral therapy on the cardiovascular system will depend primarily on the speed and magnitude of the correction of acidemia.
On the other hand, since oral therapy will provide base at a slower rate than parenteral therapy, it is presumed the generation of CO2 and therefore the exacerbation of intracellular acidosis will be less. However, this possibility has not been subject to rigorous examination.
Conclusions and future directions
Acute non-anion gap metabolic acidosis is now recognized to be as a common cause of metabolic acidosis, particularly in the ICU. Further examination of its impact on cellular function and clinical outcome are needed. Most important, controlled studies in humans to determine at what level of serum should base therapy be initiated, and what level of serum should be targeted are needed. The potential of other modalities of treatment should also be explored. This research should facilitate the generation of evidence-based guidelines for the treatment of patients with this important and increasingly common form of acute metabolic acidosis.
Conflict of interest statement
The authors state there are no conflicts of interest. The manuscript has not been presented previously or submitted to another journal.
Acknowledgements
This work was done with support from the Veterans Administration (J Kraut) and UCLA Academic Senate (J Kraut). Dr. Kurtz is supported in part by funds from the NIH (R01-DK077162), the Allan Smidt Charitable Fund, the Factor Family Foundation, and the Arvey Foundation.
References
- 1.Kraut JA, Madias NE. Serum anion gap: its uses and limitation in clinical medicine. Clin J Am Soc Nephrol. 2006;3:208–225. doi: 10.2215/CJN.03020906. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghe C, Dadu R, Blot C, et al. Hyperchloremic metabolic acidosis following resuscitation of shock. Chest. 2010;138:1521–1522. doi: 10.1378/chest.10-1458. [DOI] [PubMed] [Google Scholar]
- 3.O'Dell E, Tibby SM, Durward A, et al. Hyperchloremia is the dominant cause of metabolic acidosis in the postresuscitation phase of pediatric meningococcal sepsis. Crit Care Med. 2007;35:2390–2394. doi: 10.1097/01.CCM.0000284588.17760.99. [DOI] [PubMed] [Google Scholar]
- 4.Kraut JA, Nagami GT. The serum anion gap in the evaluation of acid-base disorders: what are its limitations and can its effectiveness be improved? Clin J Am Soc Nephrol. 2013;8:2018–2024. doi: 10.2215/CJN.04040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraut JA, Madias NE. Differential diagnosis of nongap metabolic acidosis: value of a systematic approach. Clin J Am Soc Nephrol. 2012;7:671–679. doi: 10.2215/CJN.09450911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adrogue HJ, Brensilver J, Madias NE. Changes in plasma anion gap during chronic metabolic acid-base disturbances. Am J Physiol. 1978;235:F291–F297. doi: 10.1152/ajprenal.1978.235.4.F291. [DOI] [PubMed] [Google Scholar]
- 7.Iberti TJ, Leibowitz AB, Papadakos PJ, et al. Low sensitivity of the anion gap as a screen to detect hyperlactatemia in critically ill patients. Crit Care Med. 1990;18:275–277. doi: 10.1097/00003246-199003000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Gunnerson KJ, Saul M, He S, et al. Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Crit Care. 2006;10:R22–R31. doi: 10.1186/cc3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brill SA, Stewart TR, Brundage SI, et al. Base deficit does not predict mortality when secondary to hyperchloremic acidosis. Shock. 2002;17:459–462. doi: 10.1097/00024382-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Miller LR, Waters JH. Mechanism of hyperchloremic nonanion gap acidosis. Anesthesiology. 1997;87:1009–1010. doi: 10.1097/00000542-199710000-00050. [DOI] [PubMed] [Google Scholar]
- 11.Kellum JA, Bellomo R, Kramer DJ, et al. Etiology of metabolic acidosis during saline resuscitation in endotoxemia. Shock. 1998;9:364–368. doi: 10.1097/00024382-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Prough DS, Bidani A. Hyperchloremic metabolic acidosis is a predictable consequence of intraoperative infusion of 0.9% saline. Anesthesiology. 1999;90:1247–1249. doi: 10.1097/00000542-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kellum JA. Saline-induced hyperchloremic metabolic acidosis. Crit Care Med. 2002;30:259–261. doi: 10.1097/00003246-200201000-00046. [DOI] [PubMed] [Google Scholar]
- 15.Adrogue HJ, Wilson H, Boyd AE, et al. Plasma acid-base patterns in diabetic-ketoacidosis. N Engl J Med. 1982;307:1603–1610. doi: 10.1056/NEJM198212233072603. [DOI] [PubMed] [Google Scholar]
- 16.Zalunardo N, Lemaire M, Davids MR, et al. Acidosis in a patient with cholera: a need to redefine concepts. Q J Med. 2004;07:681–696. doi: 10.1093/qjmed/hch110. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Butler T, Rabbani GH, et al. The acidosis of cholera. Contributions of hyperproteinemia, lactic acidemia, and hyperphosphatemia to an increased serum anion gap. N Engl J Med. 1986;315:1591–1595. doi: 10.1056/NEJM198612183152506. [DOI] [PubMed] [Google Scholar]
- 18.Halperin ML, Kamel KS. Some observations on the clinical approach to metabolic acidosis. J Am Soc Nephrol. 2010;21:894–897. doi: 10.1681/ASN.2009080794. [DOI] [PubMed] [Google Scholar]
- 19.Gennari FJ, Weise WJ. Acid-base disturbances in gastrointestinal disease. Clin J Am Soc Nephrol. 2008;3:1861–1868. doi: 10.2215/CJN.02450508. [DOI] [PubMed] [Google Scholar]
- 20.Powner DJ, Kellum JA, Darby JM. Concepts of the strong ion difference applied to large volume resuscitation. J Intensive Care Med. 2001;16:169–176. [Google Scholar]
- 21.Adrogue HJ, Ecknoyan G, Suki WN. Diabetic ketoacidosis: role of the kidney in acid-base homeostasis reevaluated. Kidney Int. 1984;25:591–599. doi: 10.1038/ki.1984.62. [DOI] [PubMed] [Google Scholar]
- 22.Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45:978–993. doi: 10.1053/j.ajkd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Kraut JA, Madias NE. Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatr Nephrol. 2011;26:19–28. doi: 10.1007/s00467-010-1564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goraya N, Wesson DE. Acid-base status and progression of chronic kidney disease. Curr Opin Nephrol Hypertens. 2012;21:552–556. doi: 10.1097/MNH.0b013e328356233b. [DOI] [PubMed] [Google Scholar]
- 25.Kraut JA, Madias NE. Treatment of acute metabolic acidosis: a pathophysiologic approach. Nat Rev Nephrol. 2012;8:589–601. doi: 10.1038/nrneph.2012.186. [DOI] [PubMed] [Google Scholar]
- 26.Wildenthal K, Mierzwiak DS, Myers RW, et al. Effects of acute lactic acidosis on left ventricular performance. Am J Physiol. 1968;214:1352–1359. doi: 10.1152/ajplegacy.1968.214.6.1352. [DOI] [PubMed] [Google Scholar]
- 27.Stengl M, Ledvinova L, Chvojka J, et al. Effects of clinically relevant acute hypercapnic and metabolic acidosis on the cardiovascular system: an experimental porcine study. Crit Care. 2013;17:R303. doi: 10.1186/cc13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kellum JA, Song MC, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125:243–248. doi: 10.1378/chest.125.1.243. [DOI] [PubMed] [Google Scholar]
- 29.Pedoto A, Caruso JE, Nandi J, et al. Acidosis stimulates nitric oxide production and lung damage in rats. Am J Respir Crit Care Med. 1999;159:397–402. doi: 10.1164/ajrccm.159.2.9802093. [DOI] [PubMed] [Google Scholar]
- 30.Kellum JA, Song MC, Schmigel J, et al. Effects of hyperchloremic acidosis on hemodynamics and circulating inflammatory molecules in experimental sepsis. Crit Care Med. 2001;29:A46. [Google Scholar]
- 31.Schotola H, Toischer K, Popov AF, et al. Mild metabolic acidosis impairs the beta-adrenergic response in isolated human failing myocardium. Crit Care. 2012;16:R153. doi: 10.1186/cc11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song MC, Kellum JA. Effect of lactic acidosis on LPS-induced production of inflammatory cytokines and nitric oxide in raw 264.7 macrophages. Crit Care Med. 2002;30:A54. [Google Scholar]
- 33.Noritomi DT, Soriano FG, Kellum JA, et al. Metabolic acidosis in patients with severe sepsis and septic shock: a longitudinal quantitative study. Crit Care Med. 2009;37:2733–2739. doi: 10.1097/ccm.0b013e3181a59165. [DOI] [PubMed] [Google Scholar]
- 34.Lobo DN, Awad S. Should chloride rich crystallods remain the mainstay of fluid resuscitation to prevent pre-renal acute kidney injury. Kidney Int. 2014;86:1096–1105. doi: 10.1038/ki.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCluskey SA, Karkouti K, Wijeysundera D, et al. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;117:412–421. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- 36.Mahler SA, Conrad SA, Wang H, et al. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29:670–674. doi: 10.1016/j.ajem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Yunos NM, Kim IB, Bellomo R, et al. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39:2419–2424. doi: 10.1097/CCM.0b013e31822571e5. [DOI] [PubMed] [Google Scholar]
- 38.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 39.Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 40.Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med. 2014;42:1585–1591. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 41.Burdett E, Dushianthan A, Bennett-Guerrero E, et al. Perioperative buffered versus non-buffered fluid administration for surgery in adults. Cochrane Database Syst Rev. 2012;12:CD004089. doi: 10.1002/14651858.CD004089.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Severs D, Hoom EJ, Rookmaaker MB. A critical appraisal of intravenous fluids from the physiologic basis to clinical evidence. Nephrol Dial Transpl. 2014 doi: 10.1093/ndt/gfu005. doi:10.1093/ndt/gfu005. [DOI] [PubMed] [Google Scholar]
- 43.Cooper DJ, Walley KR, Wiggs BR, et al. Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. Ann Intern Med. 1990;112:492–498. doi: 10.7326/0003-4819-112-7-492. [DOI] [PubMed] [Google Scholar]
- 44.Kimmoun A, Ducrocq N, Sennoun N, et al. Efficient extra- and intracellular alkalinization improves cardiovascular functions in severe lactic acidosis induced by hemorrhagic shock. Anesthesiology. 2014;120:926–934. doi: 10.1097/ALN.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 45.Atia AN, Buchman AL. Oral rehydration solutions in non-cholera diarrhea: a review. Am J Gastroenterol. 2009;104:2596–2604. doi: 10.1038/ajg.2009.329. [DOI] [PubMed] [Google Scholar]
- 46.Kraut JA, Kurtz I. Use of base in the treatment of severe acidemic states. Am J Kidney Dis. 2001;38:703–727. doi: 10.1053/ajkd.2001.27688. [DOI] [PubMed] [Google Scholar]
- 47.Rehm M, Finsterer U. Treating intraoperative hyperchloremic acidosis with sodium bicarbonate or tris-hydroxymethyl aminomethane: a randomized prospective study. Anesth Analg. 2003;96:1201–1208. doi: 10.1213/01.ANE.0000048824.85279.41. [DOI] [PubMed] [Google Scholar]
- 48.Wu DM, Bassuk J, Arias J, et al. Cardiovascular effects of Na+/H+ exchanger inhibition with BIIB513 following hypovolemic circulatory shock. Shock. 2005;23:269–274. [PubMed] [Google Scholar]
- 49.Wu D, Kraut JA. Role of NHE1 in the cellular dysfunction of acute metabolic acidosis. Am J Nephrol. 2014;40:36–42. doi: 10.1159/000364783. [DOI] [PubMed] [Google Scholar]
- 50.Forsythe S, Schmidt GA. Sodium bicarbonate for the treatment of lactic acidosis. Chest. 2000;117:260–267. doi: 10.1378/chest.117.1.260. [DOI] [PubMed] [Google Scholar]
- 51.Sessler D, Mills P, Gregory G, et al. Effects of bicarbonate on arterial and brain intracellular pH in neonatal rabbits recovering from hypoxic lactic acidosis. J Pediatr. 1987;111:817–823. doi: 10.1016/s0022-3476(87)80194-4. [DOI] [PubMed] [Google Scholar]