Abstract
We report a case of monoclonal gammopathy of renal significance in a 63-year-old man who presented with nephrotic-range proteinuria and renal insufficiency. The kidney biopsy showed a membranoproliferative glomerulonephritis pattern with extensive crystalloid deposits in the glomerular capillary endothelial cells and very few in the tubular epithelial cells. The immunoperoxidase staining showed kappa light chain restriction. Subsequently, the bone marrow showed 6% plasma cells which confirmed the diagnosis of monoclonal gammopathy of renal significance. He responded well to bortezomib treatment with resolution of the nephrotic syndrome and normalization of renal function after 7 months.
Keywords: crystalloid glomerulopathy, monoclonal gammopathy of renal significance
Background
The most prevalent renal involvement in patients with plasma cell dyscrasia (PCD) is light chain cast nephropathy followed by amyloid light chain (AL) amyloidosis and monoclonal immunoglobulin deposit disease. Other diseases with such lesions include Fanconi syndrome, proliferative glomerulonephritis with monoclonal deposits and immunotactoid glomerulopathy [1, 2]. Monoclonal crystalloid inclusions are seen in proximal tubulopathy in patients with Fanconi syndrome. A crystalloid deposit of light chains in the capillary endothelial cells is a rare manifestation of multiple myeloma. Crystalloid inclusions are rarely identified in glomeruli [3–11]. Such inclusions of monoclonal light chains in capillary endothelial cells are a rare manifestation of PCD [3, 8].
We present the case of a 63-year-old man with new-onset nephrotic syndrome, renal insufficiency and no comorbidities. Kidney biopsy findings led to further PCD work-up, and the diagnosis of monoclonal gammopathy of renal significance (MGRS) was confirmed.
Case history
A 63-year-old man presented with progressively increasing pedal edema of 2 months duration and dyspnea for which he was hospitalized. At admission, he was found to have anasarca, massive ascites and anemia. His blood pressure was 120/80 mm/Hg, respiratory rate 22/min, heart rate 98/min. Laboratory investigations revealed a hemoglobin of 115 gm/L, white blood count (WBC) 5.4 × 109/L, platelet count 150 × 109/L, erythrocyte sedimentation rate (ESR) 95 mm/first hour, serum albumin 19 g/L, total protein 42 g/L, globulins 15 g/L [albumin/globulin (AG) ratio: 0.55], serum creatinine 274 μmol/L, blood urea nitrogen 21 mmol/L, serum potassium 6.6 mmol/L, serum bicarbonate 16.0 mmol/L, serum sodium 135 mmol/L, serum chloride 98 mmol/L, total serum calcium 1.82 mmol/L, serum uric acid 458 µmol/L, serum LDH 302 IU/L and blood sugar 12.89 mmol/L. Urine analysis revealed 3+ protein, 20–30 WBCs, 20–30 red blood cells, WBC cast and urine glucose 0.003 mmol/L. Urine protein/creatinine ratio was 13 418 mg/g. The 24-h urine protein excretion was 8.5 g. Urine culture was sterile. Serum C3 was 0.83 g/L. Viral tests (including human immunodeficiency virus, hepatitis B surface antigen and hepatitis C virus) and antineutrophil cytoplasmic antibodies (ANCA) serology profile were negative. He was started on high-dose diuretics and underwent a kidney biopsy.
The biopsy revealed a membranoproliferative glomerulonephritis ‘pattern’ (Figure 1) with numerous eosinophilic crystalloid structures of variable shapes within the cytoplasm of endothelial cells and a few in the podocytes (Figure 2). The tubules showed very few foci with crystalloid structures indicating proximal tubulopathy. Immunofluorescence with IgG, IgA, IgM, C3 and C1q were negative. Immonoperoxidase staining for kappa and lambda light chain showed a strong reaction with kappa light chains and negative reaction with lambda (Figure 3). Ultrastructure study of endothelial cells showed rhomboid to hexagonal shaped crystals with sharp edges within the cytoplasm. A diagnosis of crystalloid glomerulopathy with an membranoproliferative glomerulonephritis (MPGN) pattern and proximal tubulopathy consistent with Fanconi syndrome was made.
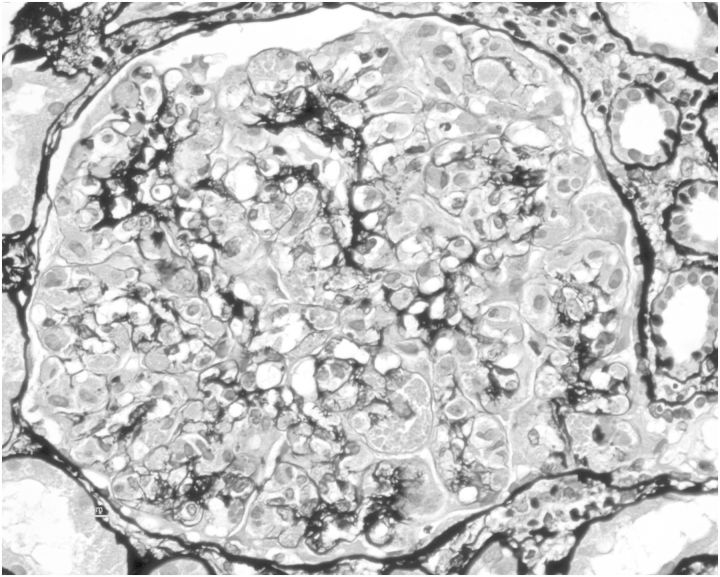
Fig. 1.
Proliferative glomerular tufts with global endothelial proliferation, obliteration of capillary lumen, double contours and eosinophilic crystals (×40, periodic acid-Schiff methenamine silver stain).
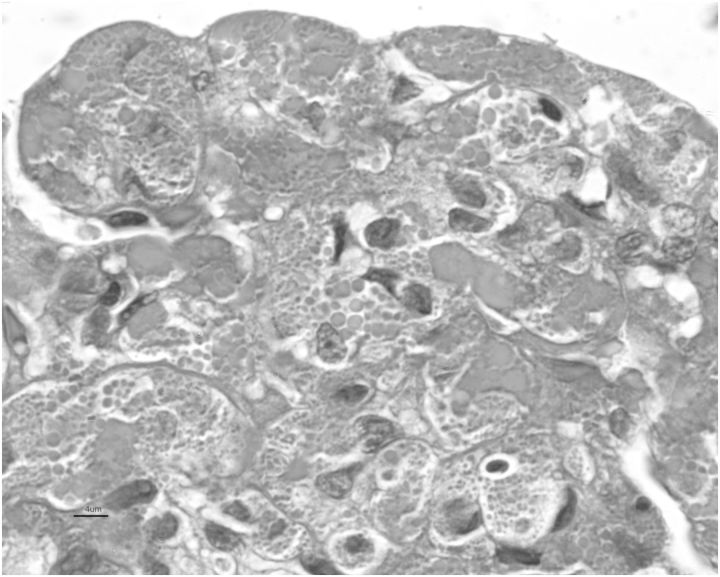
Fig. 2.
Bright fuchsinophilic round to ovoid crystals in endothelial cytoplasm bordered by bluish colored basement membranes (×100, Masson's trichrome stain). The crystals were Congo red stain negative (not shown).
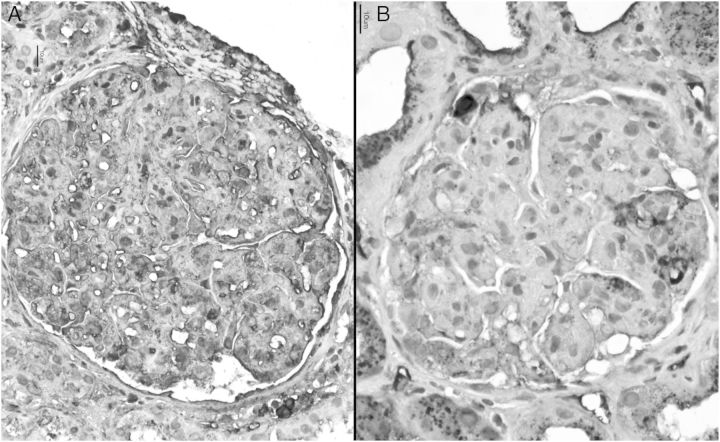
Fig. 3.
Crystals showing kappa light chain restriction (A) and negative lambda light chain (B) (×40, immunoperoxidase). Immunofluorescence revealed linear capillary wall deposits with IgG of 1+ intensity on a scale of 0–4+; non-descript pattern with kappa light chain (2+) in the crystals and negative lambda light chain reaction (not shown).
Serum electrophoresis showed an intense monoclonal M band in the gamma region, with elevated gammaglobulins of 37.05% (normal 12–22%) and serum IgM 7.33 g/L (normal 0.45–2.45 g/L). Bone marrow biopsy revealed 6% plasma cells. Immunofixation of serum and urine showed IgG-kappa monoclonality. Following the diagnosis of PCD, the patient was treated with six cycles of intravenous bortezomib and oral dexamethasone in standard dose recommendations. Lenalidomide was added after three cycles. At 7 months after initiation of therapy, he was symptom free with edema and ascites resolved; normalization of serum creatinine to 79 µmol/L, albumin/globulin ratio 1.31:1 and normal serum electrophoretic pattern. Hemoglobin improved to 126 gm/L. The 24-h urine protein excretion declined to 1.2 g. However, he was lost to follow-up for 2 months and has recently returned with an increased serum creatinine 230 µmol/L, elevated IgM 3020 g/L and faint monoclonal band in serum electrophoresis.
Discussion
Renal involvement occurs often in patients with multiple myeloma. The incidence of renal failure is 19–56% and proteinuria 70–80% [1, 2]. Organ damage is predominantly attributed to two broad mechanisms, i.e. the immunoglobulin-mediated and non-immunoglobulin-mediated type in PCD [1]. Renal impairment in monoclonal gammopathy, without fulfilling other criteria of multiple myeloma, is categorized in the recent literature as monoclonal gammopathy of renal significance [12].
Clinical bedside teaching and the foremost differential diagnoses considered in the case of an elderly patient presenting with nephrotic syndrome with or without renal failure include membranous nephropathy, primary amyloidosis focal-segmental glomerulosclerosis and minimal-change disease. The severity of renal failure at presentation is often mild to moderate in these conditions. Hypotension, if present, is in favor of a diagnosis of amyloidosis. A histological approach to the etiologic classification of MPGN was recently reviewedby Sethi et al. [13]. Work-up to exclude dysproteinemia is recommended in cases with MPGN with monoclonality on immunofluorescence. Histologic findings of eosinophilic inclusions in the glomerular tufts with an MPGN pattern raises other possibilities of systemic disease (in order of prevalence) such as class IV lupus nephritis, cryoglobulinemic MPGN and, rarely, intracapillary monoclonal immunoglobulin deposit disease.
A physicochemical property inherent in filtered light chains is the main reason for crystal formation. Leboulleux et al. [14] have shown that crystals in proximal tubulopathy are resistant to proteolytic cleavage in vitro. Light chain crystalline inclusions in PCD are more commonly seen within the proximal tubular epithelial cells appearing as bright eosinophilic crystals that are needle-, ovoid- or rhomboid-shaped. However, crystal deposition within the glomerular cells is uncommon; only nine case reports have been published in the literature [3–11]. Of the nine cases, five were males and four were females. IgG-kappa was seen in all of them; the diagnoses were multiple myeloma in seven cases and MGRS in two cases. Six of these nine patients presented with non-nephrotic proteinuria and full nephrotic syndrome was seen in three cases. Renal insufficiency was seen in eight cases. Rossmann et al. [9] and Matsuyama et al. [6] have described one case each of crystalloid inclusions in patients with MGRS in women aged 44 and 40 years, respectively. Both had IgG-kappa monoclonality and presented with nephrotic-range proteinuria and normal renal function. Histologically, glomerular crystalloid inclusions were detected in podocytes in both cases. Our case has similarities with the two previously described cases; however, in addition, our patient had renal insufficiency and crystalloid deposits located predominantly in the glomerular endothelial cells. Reviewing all previous cases, the common factor is monoclonal light chain with different physicochemical characteristics. Immunohistochemistry performed better compared with the immunofluorescence technique in demonstrating monoclonality due to the denaturing process and the exposure of the antigenic sites sequestered in the crystalline lattice.
A significant number of PCD cases have indolent clinical manifestations, falling short of hematologic criteria but demonstrating monoclonality in serum/urine. Renal impairment has been proven to be an independent prognostic feature for survival [15]. These cases have high rates of post-transplant recurrences. An index case showed only 6% plasma cells and hence could not be called a multiple myeloma. In light of recent advances in management approach, he was managed with bortezomib and steroid therapy. The response observed in this case according to criteria [15] was a very good partial response for 7 months. However, there was progression-free survival for 9 months with serum immunofixation demonstrating IgG-kappa light chain restriction and elevated IgM levels.
In summary, this is a case of nephrotic syndrome with acute kidney injury as the presenting symptom of monoclonal gammopathy of renal significance. Crystalloid deposits in the capillary endothelial cells, podocytes and tubular epithelial cells are also a rare presentation in multiple myeloma. The kidney biopsy findings were paramount to a speedy diagnosis, treatment and remission in this patient. This case highlights how monoclonal gammopathy may present and progress with few clinical symptoms, an isolated involvement of the kidney, a very good partial response and a short progression-free survival necessitating re-induction.
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgements
We sincerely thank Mrs Tulasi Kumari, Mrs Hema Nagaraj and Mr Nagaraj for their outstanding technical support in the histopathology section.
References
- 1.Heher EC, Rennke HG, Laubach JP, et al. Kidney disease and multiple myeloma. Clin J Am Soc Nephrol. 2013;8:2007–2017. doi: 10.2215/CJN.12231212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Carstens PH, Woo D. Crystalline glomerular inclusions in multiple myeloma. Am J Kidney Dis. 1989;14:56–60. doi: 10.1016/s0272-6386(89)80095-2. [DOI] [PubMed] [Google Scholar]
- 4.Keller LS, Faull RJ, Smith P, et al. Crystalloid deposits in the kidney. Nephrology (Carlton) 2005;10:81–83. doi: 10.1111/j.1440-1797.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 5.Kowalewska J, Tomford RC, Alpers CE. Crystals in podocytes: an unusual manifestation of systemic disease. Am J Kidney Dis. 2003;42:605–611. doi: 10.1016/s0272-6386(03)00794-7. [DOI] [PubMed] [Google Scholar]
- 6.Matsuyama N, Joh K, Yamaguchi Y, et al. Crystalline inclusions in the glomerular podocytes in a patient with benign monoclonal gammopathy and focal segmental glomerulosclerosis. Am J Kidney Dis. 1994;23:859–865. doi: 10.1016/s0272-6386(12)80141-7. [DOI] [PubMed] [Google Scholar]
- 7.Nasr SH, Preddie DC, Markowitz GS, et al. Multiple myeloma, nephrotic syndrome and crystalloid inclusions in podocytes. Kidney Int. 2006;69:616–620. doi: 10.1038/sj.ki.5000144. [DOI] [PubMed] [Google Scholar]
- 8.Papla B, Spolnik P, Rzenno E, et al. Generalized crystal-storing histiocytosis as a presentation of multiple myeloma: a case with a possible pro-aggregation defect in the immunoglobulin heavy chain. Virchows Arch. 2004;445:83–89. doi: 10.1007/s00428-004-1031-3. [DOI] [PubMed] [Google Scholar]
- 9.Rossmann P, Hornych A, Englis M. Histologie und Ultrastruktur kristalloider Einlargerungen in den Podocyten bei Paraproteinamie. Virchows Arch A Pathol Anat Histopathol. 1968;344:151–158. [PubMed] [Google Scholar]
- 10.Tomioka M, Ueki K, Nakahashi H, et al. Widespread crystalline inclusions affecting podocytes, tubular cells and interstitial histiocytes in the myeloma kidney. Clin Nephrol. 2004;62:229–233. doi: 10.5414/cnp62229. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Hishida A, Honda N, et al. Crystal-storing histiocytosis and crystalline tissue deposition in multiple myeloma. Arch Pathol Lab Med. 1991;115:351–354. [PubMed] [Google Scholar]
- 12.Leung N, Bridoux F, Hutchison CA, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120:4292–4295. doi: 10.1182/blood-2012-07-445304. [DOI] [PubMed] [Google Scholar]
- 13.Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 14.Leboulleux M, Lelongt B, Mougenot B, et al. Protease resistance and binding of Ig light chains in myeloma-associated tubulopathies. Kidney Int. 1995;48:72–79. doi: 10.1038/ki.1995.269. [DOI] [PubMed] [Google Scholar]
- 15.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.