Abstract
Our understanding of Gitelman syndrome (GS) and Bartter syndrome has continued to evolve with the use of genetic testing to more precisely define the tubular defects responsible. GS is caused by mutations in the SLC12A3 gene encoding the Na+–Cl− co-transporter of the distal convoluted tubule (NCCT) and tends to be associated with a milder salt-losing phenotype. We describe two female siblings presenting in infancy with a severe salt-losing tubulopathy and failure to thrive due to compound heterozygous mutations in the SLC12A3 gene encoding the NCCT. Both children were treated with indomethacin resulting in improved linear growth and polyuria. Some atypical biochemical findings in our cases are discussed including raised urinary prostaglandin (PGE2) excretion that normalized with intravenous fluid repletion.
Keywords: child, Gitelman syndrome, indomethacin, infant, prostaglandin
Background
Gitelman syndrome (GS) is an autosomal recessive salt-losing tubulopathy (OMIM 263800) characterized by hypokalemic metabolic alkalosis, hypomagnesemia and hypocalciuria [1]. GS is caused by mutations in the SLC12A3 gene encoding the Na+–Cl− co-transporter (NCCT) of the distal convoluted tubule (DCT) [2]. To date, >240 SLC12A3 mutations have been identified in GS [3, 4]. As DCT-mediated salt reabsorption accounts for only ∼5% of the filtered sodium load, GS has normally been described as having a mild salt-wasting phenotype that is often not detected until adolescence or early adulthood [3, 5, 6].
Bartter syndrome (BS) is a heterogeneous autosomal recessive salt-wasting condition that tends to present earlier in childhood with a more severe phenotype including significant salt wasting, polyuria and failure to thrive [7]. Mutations affecting the Na+2Cl−K+ co-transporter (NKCC2; OMIM 601678) or the renal outer medullary potassium channel (ROMK; OMIM 241200) in the thick ascending limb (TAL) often present antenatally with polyhydramnios, prematurity and severe salt wasting requiring significant electrolyte supplementation [8, 9]. This subtype of BS is sometimes referred to as ‘antenatal Bartter syndrome’ or ‘hyperprostaglandin E syndrome’ following the description of elevated prostaglandin E2 (PGE2) levels in such cases [10]. It was this discovery that led to the successful treatment of these patients with the cyclo-oxygenase inhibitor indomethacin, which remains an important part of therapy along with salt and water supplementation [11].
There are several other subtypes of BS with distinct phenotypes. Type III BS results from mutations that affect the basolateral chloride channel (ClC-Kb) in the DCT and TAL (OMIM 607364) [12]. Type IVa BS with sensorineural deafness is the result of mutations in the Barttin subunit of the ClC-Ka and ClC-Kb channels, which are expressed in the TAL and inner ear (OMIM 602522) [13]. Digenic mutations of both the ClC-Ka and ClC-Kb channels have also been described causing BS with sensorineural deafness (Type IVb BS; OMIM 613090) [14]. Type V BS results from mutations leading to upregulation of the calcium sensing receptor (CaSR) and therefore hypocalcemia and hypercalciuria in addition to the typical salt-losing phenotype (OMIM 601198) [15, 16]. Most recently, the combination of epilepsy, ataxia, sensorineural deafness and salt wasting tubulopathy similar to GS has been associated with mutations of the inward-rectifying potassium channel Kir 4.1 (EAST syndrome; OMIM 612780) [17].
We describe two female siblings presenting in infancy with hypokalemic metabolic alkalosis, severe failure to thrive, polyuria and increased PGE2 excretion. Both siblings revealed a dramatic clinical response to indomethacin including improvements in growth and polyuria. These features were consistent with a diagnosis of BS. However, genetic studies later confirmed a diagnosis of GS with the identification of compound heterozygous mutations in SLC12A3.
Case report
The first case is a Caucasian female born at term weighing 3.2 kg (appropriate for gestational age). No polyhydramnios was noted antenatally. This was the mother's first child with no history of spontaneous losses, consanguinity or family history of renal disease. She first came to medical attention at the age of 5 months for failure to thrive with a weight of 4.8 kg and length of 59 cm (height SDS −1.2, weight SDS −2.1; Figure 1). She was solely breast fed and thus supplementary formula feeds were introduced over the next month, but her weight fell further to 4.7 kg. At this time, she was reported to feed hourly with frequent vomiting, but no diarrhea. A history of polyuria was noted by the parents with frequent changing of wet diapers. Gross motor delay was also noted as she had only just begun to roll and had significant head lag. The treating pediatrician arranged for admission to the local hospital for further investigations and management.
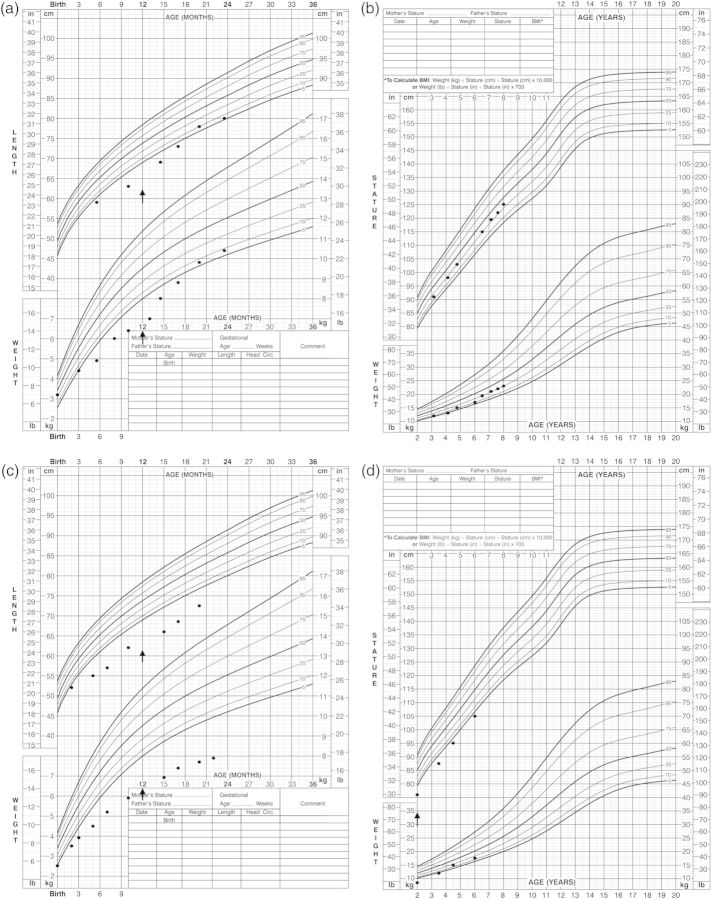
Fig. 1.
Growth profile of cases. (a) Case 1 (birth—2 years); arrow indicates start of indomethacin. (b) Case 1 (2 years—present). (c) Case 2 (birth—2 years); arrow indicates start of indomethacin. (d) Case 2 (2 years—present); arrow indicates increase in dose of indomethacin.
On admission, her chemistry profile demonstrated severe hyponatremia (Na+ 124 mmol/L) and a hypokalemic (K+ 2.1 mmol/L), hypochloremic (Cl− 72 mmol/L) metabolic alkalosis (pH 7.59, HCO3− >45 mmol/L). Serum magnesium was low (0.57 mmol/L). Blood urea nitrogen (BUN) and creatinine were within normal limits at 2.8 mmol/L and 29 μmol/L, respectively. Urinalysis was benign with no hematuria, proteinuria or pyuria. Urine specific gravity (SG) was <1.005 suggesting a urinary concentrating defect. Her urine sodium was 10 mmol/L (fractional excretion 0.63%) and urine potassium 23.2 mmol/L (fractional excretion 81%). Urine calcium:creatinine (UCa:Cr) ratio was elevated at 6 mmol:mmol with a normal serum calcium (2.53 mmol/L). She was normotensive despite elevated plasma renin and aldosterone levels at 44 ng/L/s (normal range 0.13–0.87 ng/L/s) and 5795 pmol/L (normal range 180–2380 pmol/L), respectively. Renal ultrasound was normal with no evidence of dysplasia, cysts, stones or nephrocalcinosis.
She was initially treated with intravenous normal saline and added potassium chloride to stabilize the electrolyte abnormalities. She was also commenced on oral supplementation of magnesium glucoheptonate (6 mg/kg/day of elemental Mg++) and potassium chloride (5.5 mmol/kg/day). With increasing doses of supplements, her hypokalemia, hypomagnesemia and metabolic alkalosis gradually improved over the next few months. However, at 1 year of age, her growth remained poor (height SDS −2.5, weight SDS −2.9; Figure 1). Consequently, the decision was made to start oral indomethacin (2 mg/kg/day divided bid). Over the next 6–12 months, she showed an excellent response to indomethacin with less polyuria, improved motor development and marked catch-up growth (current height SDS −0.37, weight SDS −1.05; Figure 1). Her UCa:Cr ratio, which was initially elevated, has been in the hypocalciuric range (<0.05 mmol:mmol) since the age of 2 years.
Her current regimen at 8 years of age includes indomethacin (3.5 mg/kg/day divided bid), potassium chloride (3.5 mmol/kg/day divided tid), amiloride (0.8 mg/kg once daily) and magnesium glucoheptonate (13 mg of elemental Mg++/kg/day). She consumes large quantities of high salt foods including feta cheese and olives on a daily basis. Her most recent bloodwork at 8 years of age shows stable electrolytes and blood gas values (Na+ 138 mmol/L, K+ 3.6 mmol/L, Cl− 99 mmol/L, Mg++ 0.75 mmol/L, pH 7.40, HCO3− 29 mmol/L). Her serum creatinine is slightly elevated at 55 μmol/L [estimated glomerular filtration rate (eGFR) 84 mL/min/1.73 m2].
Case 2 is the second child (female) born to the same parents at 36 weeks gestation weighing 2.5 kg (appropriate for gestational age). There was no polyhydramnios reported. Given her older sister’s history (Case 1), blood work was drawn at 1 week of age which demonstrated mild hyponatremia (132 mmol/L) and metabolic alkalosis (pH 7.64, HCO3− 30 mmol/L). Serum potassium (4.1 mmol/L) and magnesium (0.83 mmol/L) were normal. The BUN was mildly elevated at 5.3 mmol/L, consistent with volume depletion, and creatinine normal at 20 μmol/L. Her first UCa:Cr ratio at 4 months of age was in the normal range (0.74 mmol:mmol) with a normal serum calcium (2.72 mmol/L).
Sodium chloride supplementation (1 mmol/kg/day) was initiated at 1 week. She did not require potassium chloride supplementation (1 mmol/kg/day) until 5 months of age when she became persistently hypokalemic (K+ 2.9 mmol/L). Indomethacin (2 mg/kg/day) was commenced at 1 year of age given for failure to thrive (height SDS −3.7, weight SDS −3.9). Her growth response to indomethacin was appreciable, but less marked than that of her sister. However, her growth has improved with a gradual increase in the dose to 3 mg/kg/day from 2 years of age (current height SDS −1.96, weight SDS −1.11; Figure 1). Magnesium glucoheptonate supplementation was also started at 2 years of age (3 mg of elemental Mg++/kg/day) with the evolution of hypomagnesemia (serum Mg++ 0.66 mmol/L). She has required multiple admissions to hospital for exaggerated electrolyte imbalances including severe hypokalemia (K+ <2.5 mmol/L) in association with routine childhood infections. Her UCa:Cr is now persistently in the hypocalciuric range (<0.05 mmol:mmol).
Her current treatment regime at 5 years of age includes potassium chloride (3.8 mmol/kg/day divided bid), magnesium glucoheptonate (4.7 mg/kg/day of elemental Mg++ divided tid), indomethacin (3.8 mg/kg/day divided bid) and amiloride (0.8 mg/kg once daily). She maintains a high salt diet similar to her sister. Electrolytes and blood gas have been stable on this regimen (current Na+ 137 mmol/L, K+ 3.6 mmol/L, Cl− 100 mmol/L, Mg++ 0.81 mmol/L, pH 7.4, HCO3− 30 mmol/L). Of note her serum potassium has improved since the recent addition of amiloride. The most recent serum creatinine was normal at 41 μmol/L (eGFR 94 mL/min/1.73 m2).
Genetic testing
Genetic testing identified biallelic SLC12A3 mutations in both siblings: c.473G > A encoding p.R158Q and c.631_642del (p.R211_E214del) (Figure 2). The c.473G > A mutation has been previously described in a child with GS [18] while the c.631_642del mutation has not been previously reported. The SLC12A1, KCNJ1, CLCNKA, CLCNKB, BSND genes were also screened with no mutations identified. The c.473G > A mutation was found to be maternal in origin and c.631_642del paternal. Both parents have normal serum electrolytes. The father is of normal height (180 cm, SDS 0.44); however, the mother is short (150 cm, SDS −2.05).
Fig. 2.

SLC12A3 gene displaying known exons with mutations identified in the cases marked.
Following the molecular analysis, both sisters were electively admitted to hospital to determine prostaglandin (PGE2) excretion while off indomethacin therapy. We felt that this admission would clarify the need for long-term indomethacin therapy in both patients, given this therapy is normally prescribed for cases of BS and carries significant long-term risks of renal and gastrointestinal toxicity.
Further investigations
Indomethacin was held for 48 h prior to admission (washout period), but regular supplements were continued in both siblings. Upon admission, initial bloodwork revealed severe hypokalemia (2.5 mmol/L in both) with metabolic alkalosis (Case 1: HCO3− 32 mmol/L, Case 2: HCO3− 33 mmol/L). Both siblings were polyuric upon presentation (Case 1: 5.5 mL/kg/h, Case 2: 8.8 mL/kg/h). Twenty-four-hour urine prostaglandin E2 (PGE2) excretion was significantly elevated at 2059 ng/day/1.73 m2 in Case 1 and 3609 ng/day/1.73 m2 in Case 2 (normal range 48–394 ng/24 h/1.73 m2) [10]. After a bolus of normal saline (20 mL/kg) and following 48 h of intravenous fluids (maintenance rate), urine PGE2 excretion decreased to normal ranges in both siblings (Case 1: 374 ng/24 h/1.73 m2, Case 2: 312 ng/24 h/1.73 m2 in Case 2). Based on these results, a decision was made to reintroduce indomethacin at their previous doses. It was thought unlikely that the siblings would be able to consume sufficient oral fluids and salt supplementation to replicate the effects of the intravenous volume expansion achieved in hospital. The polyuria and electrolyte/acid–base derangements again improved within 1 week of restarting therapy.
Discussion
The classic description of GS is of a benign salt-wasting condition most often diagnosed in adulthood [1, 3]. However, in more recent literature, GS appears to be responsible for a wider spectrum of disease than initially appreciated [4, 19, 20]. A limited number of reports have emerged describing children affected as early as the neonatal period [21, 22]. Also mentioned within larger cohort descriptions of GS are cases presenting at <2 years of age with symptoms including growth delay, hypotonia and muscular spasms from severe hypomagnesemia [4, 19, 20]. Previous case series have suggested that males may be more severely affected than females [23].
Aside from the severity of our described GS cases, there are some interesting biochemical findings worthy of discussion, in particular the demonstration of raised urinary PGE2 excretion in both siblings. Increased PGE2 is typically associated with NKCC2 or ROMK channel mutations resulting in antenatal BS where patients present in the neonatal period with a history of polyhydramnios, prematurity and severe salt wasting. In contrast, patients with GS tend to have normal PGE2 levels as described by Lüthy et al. [6]. It has been thought that this discrepancy in PGE2 levels explains why children with BS normally require non-steroidal anti-inflammatory drug therapy in addition to salt and fluid supplementation whereas children with GS do not [6, 20]. Nonetheless, improved growth in response to indomethacin therapy in GS has been described in the literature [21, 24]. Our demonstration of raised urine PGE2 level confirms that indomethacin is a rational therapy in patients with more severe salt-wasting phenotypes associated with SLC12A3 mutations.
Another feature in both patients was the initial absence of hypocalciuria, which has previously been considered a discriminating feature of GS [6, 20, 25]. More recent case series of patients with SLC12A3 mutations have demonstrated variability in urine calcium excretion [4, 23]. Interestingly, Case 1 demonstrated hypercalciuria upon presentation and Case 2 had normal urinary calcium excretion. To our knowledge, hypercalciuria has never been reported in GS. Both cases developed hypocalciuria with time. Thus, we confirm that urinary calcium excretion cannot be relied upon as a diagnostic feature in GS and that some patients with initially high or normal urinary calcium excretion will eventually develop hypocalciuria.
We would like to acknowledge some limitations in the genetic analysis of the described cases. We were unable to perform quantitative or semi-quantitative techniques such as multiplex ligation probe amplification screening. As a result, it is possible that a large deletion, duplication or rearrangement could have been missed [4]. Also, we did not screen for mutations in the CaSR given the absence of hypocalcemia and development of hypocalcuria.
In conclusion, given the range of phenotypic expression in the salt-losing tubulopathies, genetic testing is the only method capable of confirming the precise nature of the underlying tubular defect. Previously proposed discriminating features such as urine calcium excretion, hypomagnesemia and age of presentation have all been shown to be variable in GS and may evolve with time as illustrated by our cases. Despite this, we would suggest that the identification of SLC12A3 mutations should not preclude pediatric patients with a more severe salt-wasting phenotype from a trial of indomethacin.
Acknowledgements
The authors wish to thank Dr Mato Nagel and his laboratory for their work in the genetic analysis of our patients and their parents.
Conflict of interest statement. None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Gitelman HJ. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians. 1966;79:221–235. [PubMed] [Google Scholar]
- 2.Simon DB, Nelson-Williams C, Bia MJ, et al. Gitelman'ss variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na–Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 3.Knoers NV, Levtchenko EN. Gitelman syndrome. Orphanet J Rare Dis. 2008;3:22. doi: 10.1186/1750-1172-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargas-Poussou R, Dahan K, Kahila D, et al. Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol. 2011;22:693–703. doi: 10.1681/ASN.2010090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettinelli A, Bianchetti MG, Girardin E, et al. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr. 1992;120:38–43. doi: 10.1016/s0022-3476(05)80594-3. [DOI] [PubMed] [Google Scholar]
- 6.Lüthy C, Bettinelli A, Iselin S, et al. Normal prostaglandinuria E 2 in Gitelman's syndrome, the hypocalciuric variant of Bartter's syndrome. Am J Kidney Dis. 1995;25:824–828. doi: 10.1016/0272-6386(95)90563-4. [DOI] [PubMed] [Google Scholar]
- 7.Bartter FC, Pronove P, Gill JR, et al. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med. 1962;33:811–828. doi: 10.1016/0002-9343(62)90214-0. [DOI] [PubMed] [Google Scholar]
- 8.Simon DB, Karet FE, Hamdan JM, et al. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na–K–2Cl cotransporter NKCC2. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 9.Simon DB, Karet FE, Rodriguez-Soriano J, et al. Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 10.Seyberth HW, Rascher W, Schweer H, et al. Congenital hypokalemia with hypercalciuria in preterm infants: a hyperprostaglandinuric tubular syndrome different from Bartter syndrome. J Pediatr. 1985;107:694–701. doi: 10.1016/s0022-3476(85)80395-4. [DOI] [PubMed] [Google Scholar]
- 11.Seyberth HW, Schlingmann KP. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol. 2011;26:1789–1802. doi: 10.1007/s00467-011-1871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konrad M, Vollmer M, Lemmink HH, et al. Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol. 2000;11:1449–1459. doi: 10.1681/ASN.V1181449. [DOI] [PubMed] [Google Scholar]
- 13.Estévez R, Boettger T, Stein V, et al. Barttin is a Cl− channel β-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature. 2001;414:558–561. doi: 10.1038/35107099. [DOI] [PubMed] [Google Scholar]
- 14.Schlingmann KP, Konrad M, Jeck N, et al. Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med. 2004;350:1314–1319. doi: 10.1056/NEJMoa032843. [DOI] [PubMed] [Google Scholar]
- 15.Vargas-Poussou R, Huang C, Hulin P, et al. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol. 2002;13:2259–2266. doi: 10.1097/01.asn.0000025781.16723.68. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe S, Fukumoto S, Chang H, et al. Association between activating mutations of calcium-sensing receptor and Bartter’s syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 17.Bockenhauer D, Feather S, Stanescu HC. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;260:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syrén M-L, Tedeschi S, Cesareo L, et al. Identification of fifteen novel mutations in the SLC12A3 gene encoding the Na–Cl Co-transporter in Italian patients with Gitelman syndrome. Hum Mutat. 2002;20:78. doi: 10.1002/humu.9045. [DOI] [PubMed] [Google Scholar]
- 19.Riveira-Munoz E, Chang Q, Godefroid N, et al. Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol. 2007;18:1271–1283. doi: 10.1681/ASN.2006101095. [DOI] [PubMed] [Google Scholar]
- 20.Peters M, Jeck N, Reinalter S, et al. Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med. 2002;112:183–190. doi: 10.1016/s0002-9343(01)01086-5. [DOI] [PubMed] [Google Scholar]
- 21.Tammaro F, Bettinelli A, Cattarelli D, et al. Early appearance of hypokalemia in Gitelman syndrome. Pediatr Nephrol. 2010;25:2179–2182. doi: 10.1007/s00467-010-1575-1. [DOI] [PubMed] [Google Scholar]
- 22.Raza F, Sultan M, Qamar K, et al. Gitelman syndrome manifesting in early childhood and leading to delayed puberty: a case report. J Med Case Rep. 2012;6:331. doi: 10.1186/1752-1947-6-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin SH, Shiang JC, Huang CC, et al. Phenotype and genotype analysis in Chinese patients with Gitelman's syndrome. J Clin Endocrinol Metab. 2005;90:2500–2507. doi: 10.1210/jc.2004-1905. [DOI] [PubMed] [Google Scholar]
- 24.Liaw LC, Banerjee K, Coulthard MG. Dose related growth response to indometacin in Gitelman syndrome. Arch Dis Child. 1999;81:508–510. doi: 10.1136/adc.81.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naesens M, Steels P, Verberckmoes R, et al. Bartter's and Gitelman's syndromes: from gene to clinic. Nephron Physiol. 2004;96:65–78. doi: 10.1159/000076752. [DOI] [PubMed] [Google Scholar]