Abstract
Background
Some patients with thin basement membrane disease (TBMD) develop proteinuria, hypertension and different degrees of CKD, besides the persistent microhaematuria characteristic of the disease. Little is known about factors associated with this unfavourable outcome.
Methods
We reviewed clinical, pathological and radiological features of 32 patients with biopsy-proven TBMD. Patients were divided in two groups: those with persistent normal kidney function and negative or minimal proteinuria (n = 16) and those with persistent proteinuria >0.5 g/day (n = 16).
Results
Patients with proteinuria had a worse kidney function at baseline than those with negative proteinuria. Global or segmental glomerulosclerosis, together with interstitial fibrosis, was found in 37% of patients with proteinuria. All proteinuric patients were treated with renin–angiotensin system blockers. At the end of follow-up (198 months in proteinuric patients and 210 months in patients with negative proteinuria) the prevalence of hypertension was 68% in proteinuric patients (12% at baseline), compared with 12 and 6%, respectively, in non-proteinuric patients. A slow decline of renal function was observed in proteinuric patients, although no patient developed end-stage kidney disease. Ultrasound studies showed bilateral kidney cysts in nine patients (56%) with proteinuria. Cysts were bilateral and countless in six patients, and bilateral but with a limited number of cysts in the three remaining patients. No cysts were found in patients with negative proteinuria.
Conclusions
Some patients with TBMD develop hypertension, proteinuria and CKD. Multiple bilateral kidney cysts were found in a majority (56%) of these patients. Further studies are needed to investigate the pathogenesis and the influence on long-term outcome of this TBMD-associated multiple kidney cysts.
Keywords: cysts, proteinuria, renal function impairment, thin basement membrane disease
Introduction
Thin basement membrane disease (TBMD) is characterized by diffuse thinning of the glomerular basement membrane (GBM) [1, 2]. It accounts for a majority of familial persistent microhaematuria and some studies suggest that its prevalence can be remarkably high, affecting >1% of the general population [1–5].
Mutations in COL4A3 or COL4A4, the genes that codify for α-3 and α-4 chains of type IV collagen are the cause of the disease and such mutations are similar to those reported in autosomal recessive Alport syndrome [6–8]. It is thought that TBMD patients can represent heterozygote forms of these genetic abnormalities, since patients presenting mutations in both alleles of COL4A3 or COL4A4 develop a complete autosomal recessive Alport syndrome [6–10].
Clinically, TBMD is characterized by persistent microscopic haematuria and initial clinical studies depicted an excellent prognosis [1–5]. However, more recent publications have shown that hypertension, proteinuria and renal function impairment can appear in a significant number of cases [11–13]. In some series, proteinuria was found in 60% of the patients and it was >0.5 g/24 h in 5–10% of them [14]. Renal function impairment was clearly associated with the presence of proteinuria [11–14].
The risk to develop proteinuria and renal failure in TBMD has been shown to be associated with some specific mutations in COL4A3 or COL4A4 [15]. From a clinical point of view, however, no predictive factors associated with these unfavourable complications have been identified. On the other hand, many patients presenting with isolated microscopic haematuria whether or not accompanied by low-grade proteinuria are not submitted to a kidney biopsy. As a result, many patients with TBMD remain undiagnosed and the true incidence of proteinuria and renal function impairment in TBMD is unknown. In this context, a review of the clinical characteristics and outcomes of patients with biopsy-proven TBMD and long-term follow-up could provide important information about factors associated with the appearance of proteinuria and chronic kidney disease (CKD).
We reviewed all the patients who had received a biopsy-proven diagnosis of TBMD at our hospital. Those patients with a proteinuria persistently >0.5 g/day, either at presentation or during follow-up, were identified. Their clinical characteristics, radiological findings and long-term outcomes were compared with those of a group of patients with biopsy-proven TBMD who did not develop proteinuria throughout their clinical course.
Methods
Patients
All the patients with a biopsy-proven diagnosis of TBMD in our Hospital were identified. After the performance of renal biopsy, all the patients have been regularly visiting our outpatient clinic, at intervals of 6–12 months. TBMD patients were divided into two groups: those who presented with or had developed persistent proteinuria >0.5 g/day during follow-up (n = 16) and those without proteinuria (n = 16).
Data collection
The following data both at baseline and during follow-up were recorded: blood pressure, serum creatinine, eGFR, proteinuria and urine sediment. All of the treatments received by every patient throughout follow-up were recorded.
Ultrasound studies performed during follow-up were reviewed: kidney size, the presence of renal cysts as well as their number and size and the presence of urolithiasis were recorded. Renal biopsies were reviewed in the present study and the following histological data were recorded: number of segmental or global sclerosed glomeruli, severity of mesangial proliferation and interstitial fibrosis, podocyte effacement and GBM width on electron microscopy studies. For the measurement of GBM thickness, a minimum of five capillary loops were studied. Ten transverse measurements between epithelial and membranes were performed on each loop in areas away from the mesangium. Those areas with no sharp outlined membranes were discarded. The severity of interstitial fibrosis was graduated between 0 and +++ (absent, mild, moderate and severe).
Definitions
The diagnosis of TBMD was established by the presence of diffuse thinning of GBM (mean GBM thickness <264 nm) and no images of GBM splitting or thickening were found on electron microscopy studies [16].
The presence of a blood pressure over 140/85 was considered hypertension. Nephrolithiasis was defined by the finding of kidney stones in radiological examinations or history of passing calculi. Follow-up was defined as the interval between renal biopsy performance (baseline) and the last visit, lost to follow-up or development of end-stage kidney disease (ESKD). ESKD was defined by an estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2 or the need of kidney transplantation. eGFR was calculated by the Modifications in Diet and Renal Disease four-variable equation.
Statistical analysis
Normally distributed variables are displayed as mean ± SD and compared using the t-test, one-way ANOVA or Pearson correlation coefficients. Proteinuria is expressed as median (range). Categorical variables are expressed as percentage and compared with the χ2 test. Loss of renal function (eGFR), expressed in mL/min/years, was calculated in every patient.
A P-value of <0.05 was considered significant. Statistical analysis was performed with the SPSS software (version 17.0 for Windows).
Results
Baseline characteristics
Baseline clinical characteristics of TBMD patients with and without proteinuria are shown in Table 1. Patients with negative proteinuria were younger, although this difference did not reach statistical significance. Kidney function was lower in proteinuric patients, with a serum creatinine significantly higher and a lower eGFR. Three patients with proteinuria presented an eGFR <60 mL/min/1.73 m2 at baseline (two with CKD Stage III, one with CKD Stage IV), whereas all patients with negative proteinuria showed an eGFR >60 mL/min/1.73 m2. Proteinuria was already significantly higher at baseline in proteinuric patients. Ocular abnormalities or sensorineural deafness were not detected at presentation in any patient. Familial history of microscopic haematuria was reported in 15/16 patients with proteinuria and in 14/16 patients with negative proteinuria (Table 1).
Table 1.
Clinical characteristics at baseline
| TBMD with proteinuria (n = 16) | TBMD without proteinuria (n = 16) | P | |
|---|---|---|---|
| Familial history of microhaematuria | 15 | 14 | NS |
| Age (years) | 35 ± 17 | 23 ± 13 | NS |
| Gender | 10 M, 6 F | 6 M, 10 F | NS |
| Hypertension | 2 (12%) | 1 (6%) | NS |
| Obesity (BMI > 30 kg/m) | 4 (25%) | 4 (25%) | NS |
| Smokers | 4 (25%) | 3 (18%) | NS |
| Proteinuria (g/day) (range) | 0.60 (0.05–1.45) | 0.00 (0.00–0.02) | 0.000 |
| Serum creatinine | 93.70 ± 32.70 µmol/L (1.06 ± 0.37 mg/dL) | 74.25 ± 13.26 µmol/L (0.84 ± 0.15 mg/dL) | 0.017 |
| eGFR (mL/min/1.73 m2) | 88 ± 38 | 121 ± 57 | NS |
| CKD stage | |||
| I | 7 | 10 | NS |
| II | 6 | 6 | NS |
| III | 2 | 0 | NS |
| IV | 1 | 0 | NS |
| V | 0 | 0 | NS |
NS, not significant.
Kidney biopsies
Table 2 summarizes the main histological findings in patients with and without proteinuria. The finding of glomerulosclerosis lesions (segmental or global) was significantly higher among patients with proteinuria (37 versus 0%). Interstitial fibrosis was exclusively found in proteinuric patients.
Table 2.
Kidney biopsy findings
| TBMD with proteinuria (n = 16) | TBMD without proteinuria (n = 16) | P | |
|---|---|---|---|
| Patients showing segmental or global glomerulosclerosis | 6 (37%) | 0% | 0.007 |
| Glomeruli showing segmental or global glomerulosclerosis | 8% ± 14% | 0% | 0.024 |
| Patients showing interstitial fibrosis | 6 | 0 | 0.007 |
| Grade of interstitial fibrosis | |||
| I | 5 | 0 | 0.149 |
| II | 1 | 0 | NS |
| III | 0 | 0 | NS |
| IV | 0 | 0 | NS |
| Podocyte effacement | 4 (25%) | 2 (12.25%) | NS |
| GBM thickness (nm) | 215 ± 16 | 217 ± 21 | NS |
NS, not significant.
Final outcome
As shown in Table 3, the mean follow-up was 198 months in proteinuric patients and 210 in those patients with negative proteinuria. All of the patients in the proteinuric group were treated with angiotensin converting-enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), whereas only two patients with negative proteinuria received these drugs. The percentage of hypertensive patients significantly increased among proteinuric patients (from 12% at baseline to 68% at the end of follow-up) and kidney function showed a slow decline throughout follow-up [baseline serum creatinine and eGFR 93.70 ± 32.70 µmol/L (1.06 ± 0.37 mg/dL) and 88 ± 38 mL/min/1.73 m2, respectively, and 115.80 ± 62.76 µmol/L (1.31 ± 0.71 mg/dL) and 74 ± 39 mL/min/1.73 m2, respectively, at the end of follow-up]. CKD progressed from Stage I to II in one patient and from II to III in four patients. The mean slope of eGFR loss was −2.46 ± 6.78 mL/min/1.73 m2/year. Proteinuria at baseline and at the end of follow-up were similar, probably influenced by ACEI/ARB treatment.
Table 3.
Final outcomes in patients with and without proteinuria
| TBMD with proteinuria | TBMD without proteinuria | P | |
|---|---|---|---|
| Hypertensiona | 11 (68%) | 2 (12%) | 0.001 |
| Proteinuria (g/day) (range)a | 0.42 (0.27–1.7) | 0.00 (0.00–0.02) | 0.000 |
| Serum creatinine at baseline/at the end of follow-up (µmol/L, in parentheses mg/dL) | 93.70 ± 32.70 (1.06 ± 0.37)/115.80 ± 62.76 (1.31 ± 0.71) | 74.24 ± 13.36 (0.84 ± 0.15)/75.15 ± 13.36 (0.85 ± 0.15) | 0.017/0.015 |
| eGFR at baseline/at the end end of follow-up (mL/min/1.73 m2) | 88 ± 38/74 ± 39 | 121 ± 57/95 ± 22 | NS |
| 100% Increase in baseline serum creatininea | 1 | 0 | NS |
| CKD stages I/II/III/IV/Va | |||
| I | 6 | 7 | NS |
| II | 3 | 9 | 0.028 |
| III | 6 | 0 | 0.006 |
| IV | 1 | 0 | NS |
| V | 0 | 0 | NS |
| Follow-up (months) | 198 (96–238) | 210 (97–286) | NS |
| Treatment with ACEI/ARB | 16 | 2 | 0.000 |
| Kidney cysts | 9(56%) | 0 | 0.000 |
NS, not significant.
aAt the end of follow-up.
In patients with negative proteinuria, the percentage of hypertensive patients was 6% at baseline and 12% at the end of follow-up. As shown in Table 3, serum creatinine concentrations remained stable. CKD progressed from Stage I to Stage II in three patients, but no patient had progressed to Stage III at the end of follow-up.
No patient reached ESKD nor received a kidney transplantation. No ocular abnormalities, deafness or other extrarenal complications were detected throughout follow-up.
Radiological findings
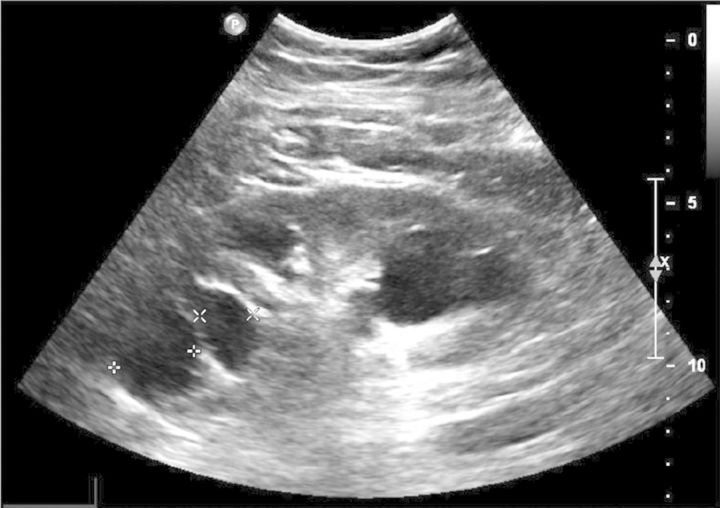
A majority of patients with proteinuria showed multiple kidney cysts on ultrasound abdominal examination (Figure 1). Bilateral kidney cysts were detected in nine patients (56%) with proteinuria, whereas no patient with negative proteinuria showed kidney cysts or other radiological abnormalities (P = 0.002). Individualized main radiological findings in patients with TBMD and proteinuria are shown in Table 4. Multiple and bilateral renal cysts were found in six patients, and the other three patients showed a limited number of cysts. Normal size kidneys were observed, with the exception of Patient 1 (Table 4), who showed multiple and bilateral kidney cysts with a kidney length pole to pole of 15 cm in both kidneys. Kidney size was slightly reduced in those patients with a more advanced CKD. When comparing proteinuric TBMD patients with or without kidney cysts, we found that patients with cysts were significantly older than those without cysts (Table 5). Kidney function tended to be worse in patients with cysts, both at baseline and at the end of follow-up, when compared with patients without cysts, but these differences were not statistically significant.
Fig. 1.
Kidney ultrasound in a patient with TBMD, proteinuria and CKD, showing bilateral kidney cysts.
Table 4.
Clinical, histological and radiological findings in TBMD patients with proteinuria at the end of follow-up
| Patient | Age | Gender | SCr | eGFR | Proteinuria (g/day) | GBM thickness (nm) | Kidney size RK/LK (cm) | Kidney cysts | Size of largest cyst (cm) | Lithiasis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | Male | 138.79 (1.57) | 67 | 1.85 | 240 | 15/15 | Multiple, bilateral | 7 | No |
| 2 | 54 | Female | 44.20 (0.50) | 136 | 0.43 | 225 | 11.6/11.2 | 2 in RK, 1 in LK | 4.5 | Yes |
| 3 | 61 | Male | 82.21 (0.93) | 87 | 1.34 | 200 | 12/12 | Multiple, bilateral | 2 | No |
| 4 | 46 | Male | 115.80 (1.31) | 59 | 1.15 | 230 | 11.8/11.3 | Multiple, bilateral | 2 | No |
| 5 | 71 | Female | 156.47 (1.77) | 30 | 0.29 | 190 | 9/10 | Multiple, bilateral | 2 | No |
| 6 | 68 | Male | 173.26 (1.96) | 36 | 0.26 | 210 | 9.4/10 | Multiple, bilateral | 5.1 | No |
| 7 | 37 | Male | 84.86 (0.96) | 93 | 3.76 | 205 | 12/13 | 1 in RK, 10 in LK | 3 | No |
| 8 | 66 | Female | 52.15 (0.59) | 108 | 0.34 | 230 | 12.2/12.8 | Multiple, bilateral | 1 | Yes |
| 9 | 64 | Male | 277.58 (3.14) | 21 | 0.32 | 210 | 10/10 | 4 in RK, 2 in LK | 4 | No |
| 10 | 53 | Male | 78.67 (0.89) | 95 | 0.42 | 220 | 9.5/11.5 | No | No | |
| 11 | 23 | Female | 45.96 (0.52) | 155 | 0.65 | 200 | 12.3/12 | No | No | |
| 12 | 39 | Male | 167.08 (1.86) | 43 | 2 | 250 | 11.5/12 | No | Yes | |
| 13 | 36 | Female | 87.51 (0.99) | 67 | 0.18 | 210 | 10.8/11.6 | No | No | |
| 14 | 56 | Female | 104.31 (1.18) | 50 | 0.16 | 190 | 9/8,8 | No | No | |
| 15 | 25 | Male | 188.29 (2.13) | 40 | 4.9 | 240 | 12.5/11 | No | No | |
| 16 | 43 | Male | 70.72 (0.80) | 112 | 0.27 | 210 | 11/9 | No | No |
SCr, serum creatinine (µmol/L, in parentheses mg/dL); eGFR, estimated glomerular filtrate rate (mL/min/1.73 m2); GBM, glomerular basement membrane; RK, right kidney; LK, left kidney
Table 5.
Differences between TBMN patients with and without multiple kidney cysts
| Patients with kidney cysts (n = 9) | Patients without kidney cysts (n = 7) | P | |
|---|---|---|---|
| Age (years) | 57 ± 11.2 | 39 ± 12.65 | 0.008 |
| Gender | 6 M; 3 F | 4 M; 3 F | NS |
| Hypertensiona | 7 | 4 | NS |
| Proteinuria (g/day) (range)a | 0.43(0.30–1.59) | 0.42 (0.18–2) | NS |
| Serum creatinine at baseline/at the end of follow-up (µmol/L, in parentheses mg/dL) | 100.77 ± 41.54 (1.14 ± 0.47)/124.64 ± 72.49 (1.41 ± 0.82) | 83.98 ± 15.91 (0.95 ± 0.18)/105.19 ± 51.27 (1.19 ± 0.58) | NS |
| eGFR at baseline/at the end of follow-up (mL/min/1.73 m2) | 84 ± 50/69 ± 39 | 94 ± 14/80 ± 42 | NS |
| 100% Increase in baseline serum creatinine | 0 | 1 | NS |
| CKD stagesa | |||
| I | 3 | 3 | NS |
| II | 2 | 1 | NS |
| III | 3 | 3 | NS |
| IV | 1 | 0 | NS |
| V | 0 | 0 | NS |
| Follow-up (years) | 195 (85–231) | 201 (84–270) | NS |
| Treatment with ACEI/ARB | 9 | 7 | NS |
NS, not significant.
aAt the end of follow-up.
Nephrolithiasis (kidney lithiasis in radiological examinations and/or history of passing calculi) was found in six patients (18%), four of them with proteinuria (25%) and two without proteinuria (12%).
Discussion
Our study reports, for the first time, that a significant number (56%) of TBMD patients with proteinuria and CKD shows bilateral renal cysts, compared with TBMD patients without proteinuria.
TBMD accounts for a great majority of the so-called familial benign haematuria. Earlier studies reported an almost uniform long-term favourable prognosis in patients with biopsy-proven TBMD [3–5, 19]. Later studies [11–14] showed that some TBMD patients can develop proteinuria and CKD and, consequently, some authors have proposed to abandon the term familial benign haematuria [15, 20]. The results of our study are in agreement with such considerations, since 16 out of 32 patients (50%) with biopsy-proven TBMD developed proteinuria, hypertension and different stages of CKD. However, it should be considered that the true incidence of proteinuria and CKD in TBMD patients is unknown and probably overestimated, taking into account that kidney biopsies are not usually performed in patients with persistent microscopic haematuria unless proteinuria or kidney function impairment develops.
The reasons for some patients with biopsy-proven TBMD to maintain persistent microhaematuria with a normal kidney function and minimal or negative proteinuria, whereas others show a progressive proteinuria and kidney function decline, are not known. Some specific mutations in COL4A3/COL4A genes have been associated with the appearance of proteinuria and CKD in TBMD [15]. On the other hand, some studies [21, 22] have suggested a role of some genetic modifiers, like the R229Q mutation in NPHS2.
Most of the renal biopsies performed in TBMD patients with proteinuria and CKD have shown different types of focal segmental glomerulosclerosis lesions [11–15]. In agreement with these studies, we found global or segmental lesions of glomerulosclerosis in six (37%) of our TBMD proteinuric patients, whereas patients with negative proteinuria showed an almost normal histology, with the exception of the characteristic diffuse thinning of GBM on electron microscopy.
It is important to stress that, in the absence of electron microscopy studies, many patients with TBMD can receive an erroneous diagnosis of focal segmental glomerulosclerosis. Suspicion of TBMD should be raised by the presence of persistent microscopic haematuria, and the finding of haematuria in other first-degree relatives should reinforce such suspicion. Some authors have pointed out that TBMD can be an important cause of CKD and even ESKD, given its remarkable prevalence among the general population [23]. A better knowledge of its different phenotypes and presentations would help with the differential diagnosis of TBMD with IgA nephropathy, focal segmental glomerulosclerosis and other chronic glomerulonephritis that can also present persistent microhaematuria, non-nephrotic proteinuria and progressive CKD.
Our finding of a remarkable prevalence of renal cysts in TBMD patients with proteinuria could help when making such a differential diagnosis. More than a half of our proteinuric TBMD patients (9/16, 56%) showed bilateral kidney cysts that were multiple and countless in six of them. In fact, one patient (Patient 1, Table 4), who had a kidney volume slightly above normal values, had received a tentative diagnosis of polycystic kidney disease superimposed to TBMD. The absence of progressive kidney enlargement on subsequent echographies and of a familial history of polycystic kidney disease made this diagnosis unlikely. Normal size kidneys were observed in the remaining patients, although those with more advanced degrees of CKD had slightly reduced kidneys.
Ultrasound morphology of kidney cysts was similar to that of simple cysts, with thin, regular walls and absence of masses or haemorrhages in their inside (Figure 1). A wide range of cyst sizes was observed, the largest ones achieving 4.5–7 cm (Table 4). Unfortunately, ultrasound studies in our patients were performed at very different intervals after kidney biopsy, and repeated examinations at regular intervals were performed in only some cases. Hence, we cannot provide a precise report of cyst appearance and growth.
Pathogenesis of these TBMD-associated kidneys cysts is unknown. The prevalence of isolated kidney cysts in general population increases with age and can be observed in >30% of people >70 years of age [24, 25]. However, bilateral cysts are much less common and are particularly rare in subjects <50 years of age [24, 25]. Therefore, the striking prevalence of bilateral cysts (56%) among our proteinuric TBMD patients, whose age at the end of follow-up was 49 ± 15 years, is clearly higher than that in the normal population. However, we found that proteinuric TBMD patients with cysts were significantly older than those without cysts (Table 5), thus suggesting an influence of ageing on the appearance or enlargement of kidney cysts in our patients.
Remarkably, no TBMD patient with negative proteinuria or CKD showed cysts, suggesting a possible link between kidney cysts and such complications. Simple kidney cysts have been related to the appearance of hypertension and prehypertension [26]. The prevalence of hypertension among our proteinuric patients (more than a half of them having kidney cysts) increased from 12% at baseline to 68% at the end of follow-up, whereas these percentages were 6 and 12%, respectively, in patients with negative proteinuria. However, there were no significant differences in the prevalence of hypertension between proteinuric patients with or without cysts (Table 5).
Our study is the first formal report of multi-cystic kidneys in TBMD. Pierides et al. in a genetic and clinico-pathological study of 11 large Cypriot pedigrees [20] found multiple kidney cysts in many older patients in four of the pedigrees, but a precise description of the prevalence of cysts or their possible association with proteinuria or CKD was not given.
Further studies in larger cohorts of TBMD patients are needed to confirm our findings and to investigate the possible existence of other genetic mutations associated with cyst formation. Interestingly, kidney cysts, renal function impairment and haematuria have been reported in patients with mutations in the COL4A1 gene, encoding for the α1-chain of type IV collagen. Mutations in this gene cause a systemic phenotype characterized by hereditary angiopathy, nephropathy, aneurysms and muscle cramps (HANAC syndrome) [27–29].
We found a remarkable prevalence of nephrolithiasis among our patients: renal stones in abdominal ecographies or history of passing calculi were detected in six patients (18%). A previous study of our group [16] reported an association of TBMD with hypercalciuria, hyperuricosuria and nephrolithiasis. Interestingly, some studies have reported an association between renal cysts and nephrolithiasis, perhaps as a consequence of intratubular obstruction with crystals [17, 18]. However, as shown in Table 4, we found no relationship between the presence of cysts and nephrolithiasis in the present study.
Finally, another interesting finding of our study was the very slow progression of those patients with proteinuria or CKD. All of them were treated with renin–angiotensin system blockers and regularly controlled along a long-term follow-up (a mean follow-up of 16.5 years). None of them achieved ESKD and the mean slope of eGFR loss was −2.46 ± 6.78 mL/min/1.73 m2/year. This relatively encouraging outcome would agree with recently published studies showing a clear beneficial effect of ACEI or ARB on patients with Alport syndrome [30].
In conclusion, we found a remarkable prevalence (56%) of multi-cystic kidneys in patients with TBMD, showing proteinuria and CKD. In contrast, no cysts were detected in TBMD with normal kidney function and negative or minimal proteinuria. Further studies are needed to characterize the pathogenesis and the possible influence of these cysts on TBMD outcomes.
Conflict of interest statement
The results presented in this paper have not been published previously in whole or part, except in abstract format.
Acknowledgements
This study was supported by grants from FIS (10/02668) and REDINREN (RD012/0021).
References
- 1.Savige J, Rana K, Tonna S, et al. Thin basement membrane nephropathy. Kidney Int. 2003;64:1169–1178. doi: 10.1046/j.1523-1755.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 2.Tryggvason K, Patrakka J. Thin basement membrane nephropathy. J Am Soc Nephrol. 2006;17:813–822. doi: 10.1681/ASN.2005070737. [DOI] [PubMed] [Google Scholar]
- 3.Tiebosch AT, Wolters J, Frederik PF, et al. Epidemiology of idiopathic glomerular disease: a prospective study. Kidney Int. 1987;32:112–116. doi: 10.1038/ki.1987.179. [DOI] [PubMed] [Google Scholar]
- 4.Aarons I, Smith PS, Davies RA, et al. Thin membrane nephropathy: a clinico-pathological study. Clin Nephrol. 1989;32:151–158. [PubMed] [Google Scholar]
- 5.Goel S, Davenport A, Goode NP, et al. Clinical features and outcome of patients with thin and ultrathin glomerular membranes. Q J Med. 1995;88:785–793. [PubMed] [Google Scholar]
- 6.Badenas C, Praga M, Tazón B, et al. Mutations in the COL4A4 and COL4A3 genes cause familial benign hematuria. J Am Soc Nephrol. 2002;13:1248–1254. doi: 10.1681/ASN.V1351248. [DOI] [PubMed] [Google Scholar]
- 7.Lemmink HH, Nillesen WN, Mochizuki T, et al. Benign familial hematuria due to mutation of the type IV collagen alpha 4 gene. J Clin Invest. 1996;98:1114–1118. doi: 10.1172/JCI118893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mochizuki T, Lemmink HH, Mariyama M, et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet. 1994;8:77–81. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- 9.Heidet L, Arrondel C, Forestier L, et al. Structure of the human type IV collagen gene COL4A3 and mutations in autosomal Alport syndrome. J Am Soc Nephrol. 2001;12:97–106. doi: 10.1681/ASN.V12197. [DOI] [PubMed] [Google Scholar]
- 10.Torra R, Tazón-Vega B, Ars E, et al. Collagen type IV (alpha3-alpha4) nephropathy: from isolated haematuria to renal failure. Nephrol Dial Transplant. 2004;19:2429–2432. doi: 10.1093/ndt/gfh435. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwhof CM, de Heer F, de Leeuw P, et al. Thin GBM nephropathy: premature glomerular obsolescence is associated with hypertension and late onset renal failure. Kidney Int. 1997;51:1596–1601. doi: 10.1038/ki.1997.219. [DOI] [PubMed] [Google Scholar]
- 12.Dische FE, Weston MJ, Parsons V. Abnormally thin glomerular basement membranes associated with hematuria, proteinuria or renal failure in adults. Am J Nephrol. 1985;5:103–109. doi: 10.1159/000166914. [DOI] [PubMed] [Google Scholar]
- 13.Van Paassen P, van Breda Vriesman PJ, van Rie H, et al. Signs and symptoms of thin basement membrane nephropathy: a prospective regional study on primary glomerular disease—the Limburg Renal Registry. Kidney Int. 2004;66:909–913. doi: 10.1111/j.1523-1755.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira M, Cartwright J, Horn K, et al. Thin basement membrane disease with heavy proteinuria or nephrotic syndrome at presentation. Am J Kidney Dis. 2000;35:E 15.1–8. doi: 10.1016/s0272-6386(00)70033-3. [DOI] [PubMed] [Google Scholar]
- 15.Voskarides K, Damianou L, Neocleous V, et al. COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J Am Soc Nephrol. 2007;18:3004–3016. doi: 10.1681/ASN.2007040444. [DOI] [PubMed] [Google Scholar]
- 16.Praga M, Martínez MA, Andrés A, et al. Association of thin basement membrane nephropathy with hypercalciuria, hyperuricosuria and nephrolithiasis. Kidney Int. 1998;54:915–920. doi: 10.1046/j.1523-1755.1998.00065.x. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Nieto V, Negrete-Pedraza F, Lopez-Garcia M, et al. Are simple renal cysts in childhood associated with kidney stones? Nephro-Urol Mon. 2012;4:596–598. doi: 10.5812/numonthly.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia Nieto V, Dublan Garcia K, Luis Yanes MI. Are simple renal cysts another manifestation of prelithiasis in infancy? Nefrologia. 2010;30:337–341. doi: 10.3265/Nefrologia.pre2010.Apr.10409. [DOI] [PubMed] [Google Scholar]
- 19.Rogers PW, Kurtzman NA, Bunn SM, Jr, et al. Familial benign essential hematuria. Arch Intern Med. 1973;131:257–262. [PubMed] [Google Scholar]
- 20.Pierides A, Voskarides K, Athanasiou Y, et al. Clinico-pathological correlations in 127 patients in 11 large pedigrees, segregating one of three heterozygous mutations in the COL4A3/COL4A4 genes associated with familial haematuria and significant late progression to proteinuria and chronic kidney disease from focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2009;24:2721–2729. doi: 10.1093/ndt/gfp158. [DOI] [PubMed] [Google Scholar]
- 21.Tonna S, Wang YY, Wilson D, et al. The R229Q mutation in NPHS2 may predispose to proteinuria in thin-basement-membrane nephropathy. Pediatr Nephrol. 2008;23:2201–2207. doi: 10.1007/s00467-008-0934-7. [DOI] [PubMed] [Google Scholar]
- 22.Voskarides K, Arsali M, Athanasiou Y, et al. Evidence that NPHS2-R229Q predisposes to proteinuria and renal failure in familial hematuria. Pediatr Nephrol. 2012;27:675–679. doi: 10.1007/s00467-011-2084-6. [DOI] [PubMed] [Google Scholar]
- 23.Deltas C, Pierides A, Voskarides K. Molecular genetics of familial hematuric diseases. Nephrol Dial Transplant. 2013;28:2946–2960. doi: 10.1093/ndt/gft253. [DOI] [PubMed] [Google Scholar]
- 24.Ravine D, Gibson RN, Donlan J, et al. An ultrasound renal cyst prevalence survey: specificity data for inherited renal cystic diseases. Am J Kidney Dis. 1993;22:803–807. doi: 10.1016/s0272-6386(12)70338-4. [DOI] [PubMed] [Google Scholar]
- 25.Rule A, Sasiwimonphan K, Lieske J, et al. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am J Kidney Dis. 2012;59:611–618. doi: 10.1053/j.ajkd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CT, Yang YC, Wu JS, et al. Multiple and large simple renal cysts are associated with prehypertension and hypertension. Kidney Int. 2013;83:924–930. doi: 10.1038/ki.2012.481. [DOI] [PubMed] [Google Scholar]
- 27.Plaisier E, Gribouval O, Alamowitch S, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 28.Plaisier E, Chen Z, Gekeler F, et al. Novel COL4A1 mutations associated with HANAC syndrome: a role for the triple helical CB3[IV] domain. Am J Med Genet. 2010;152A:2550–2555. doi: 10.1002/ajmg.a.33659. [DOI] [PubMed] [Google Scholar]
- 29.Kashtan CE, Segal Y. Genetic disorders of glomerular basement membranes. Nephron Clin Pract. 2011;118:c9–c18. doi: 10.1159/000320876. [DOI] [PubMed] [Google Scholar]
- 30.Gross O, Licht C, Anders HJ, et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 2012;81:494–501. doi: 10.1038/ki.2011.407. [DOI] [PubMed] [Google Scholar]