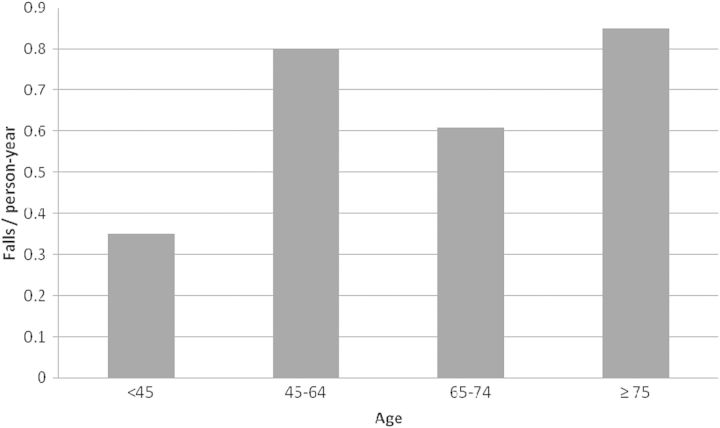Abstract
Background
Falls among patients undergoing maintenance hemodialysis (HD) have significant consequences for quality of life and functional independence, morbidity, healthcare utilization and even mortality, but studies on the etiology of falls within large HD cohorts are limited.
Methods
Falls during the past 12 months were ascertained for a prevalent multi-center HD cohort (n = 762) aged 20–92 years, and associations with demographic and treatment characteristics, comorbidities, cognitive function, prescribed medications, balance tests, frailty and depressive symptoms were assessed.
Results
Falls were sustained by 28.4% of participants. In multivariable-adjusted analyses, participants classified as frail were over two times more likely to report falls [odds ratio (OR): 2.39, 95% confidence interval (CI): 1.22–4.71, P = 0.01], and participants with a CES-D score 18+ and/or prescribed antidepressants were over 80% more likely to be fallers (OR: 1.83, 95% CI: 1.23–2.74, P = 0.003) than were participants with a CES-D score <18 and no prescribed antidepressants.
Conclusions
Frailty and depressed mood, factors that are potentially modifiable, are prominently associated with falls.
Keywords: depression, falls, frailty, hemodialysis, USRDS
Introduction
Falls may occur annually in 25% or more of end-stage renal disease (ESRD) patients undergoing maintenance hemodialysis (HD), with significant consequences for quality of life and functional independence, morbidity, healthcare utilization and even mortality [1–9]. Beaubrun et al. [9], using HD patient Medicare claim files in the United States Renal Data System (USRDS) for 2000–09, showed that a history of falls was a major contributor to fracture-related hospitalization. However, there have been few large studies on the etiology of falls occurring among HD patients [2].
Recently, an association between falls and frailty was demonstrated in a single-center study of 95 HD patients [8]. Frailty is well documented as a predictor of falls in the geriatric literature [10–16], but the recent work by McAdams-DeMarco et al. found a strong association between frailty and falls among HD patients regardless of their age [8]. However, the sample size limited their investigation of other variables such as depression that have previously been identified as posing significant risks for falling in the HD population [2, 7]. We used data from a large contemporary cohort of prevalent patients undergoing maintenance HD to examine the associations among falling, frailty and depressed mood/antidepressant use, within a multifactorial framework of risk of falling drawn from previous research (see Table 1).
Table 1.
Summary of findings from previous studies of falls among patients undergoing hemodialysis
| Location | # Participants # Dialysis units |
Participant age | Fall prevalence | Fall risk factor evidence |
|---|---|---|---|---|
| The UK [1] | 47 patients 1 unit |
 = 78.2 (5.3) = 78.2 (5.3) |
30% over 12 months |
|
| Belgium [2] | 308 patients 7 units |
Median = 71 | 12.7% over 8 weeks |
|
| Canada [3, 4] | 135 (studied retrospectively) 162 (studied prospectively) 1 center |
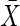 = 74.9 (6.2) = 74.9 (6.2)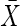 = 74.7 (6.1) = 74.7 (6.1) |
27% over 12 months 47% over a median of 468 days |
|
| The USA [6] | 76 patients 2 units |
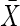 = 62.4(16.1) = 62.4(16.1) |
26.3% over 12 months |
|
| Switzerland [7] | 84 patients 1 center |
Median = 69.5 | a28.6% over a mean of 20.6 months |
|
| The USA [8] | 95 patients 1 unit |
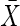 = 60.5 (12.6) = 60.5 (12.6) |
28.3% over a median of 6.7 months |
|
CCI, Charlson comorbidity index.
aThe study focus was severe falls, defined as ‘fall(s) requiring presentation to an emergency department and/or hospitalization’ [7].
Materials and methods
Data sources and collection
ACTIVE-ADIPOSE (A Cohort Study to Investigate the Value of Exercise in ESRD/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD) is a multi-center study of prevalent patients on HD coordinated by the USRDS [17]. At seven outpatient dialysis clinics in the Atlanta, Georgia metropolitan area and seven outpatient dialysis clinics in the San Francisco Bay Area, CA, 771 prevalent HD patients were enrolled and participated in baseline assessments during 2009–11. Participating clinics were affiliated with large dialysis organizations, medium dialysis organizations and academic medical centers. The number of study participants per dialysis clinic ranged from 33 to 99 (median = 50). Institutional review boards at Emory University and the University of California, San Francisco approved the study.
Eligible study participants were adults (≥18 years old), English or Spanish speaking, on HD for at least 3 months, and capable of giving informed consent. The exclusion criteria were current treatment by peritoneal dialysis or home HD and evidence of active malignancy, including brain tumor and expected geographic relocation. Vulnerable populations (pregnant women, prisoners, persons with significant mental illness) were also excluded. Single and double amputees and patients with prior or pending transplantation were considered eligible. Among eligible patients, 85% supplied informed consent and were enrolled. Reasons most frequently given by those who declined to participate were that they were ‘not interested,’ ‘too busy’ or ‘enrolled in another study’.
Falls incurred over the past 12 months were reported by 762 study participants. A fall was defined as an event that resulted in a person coming to rest inadvertently on the ground, floor or other lower level [18]. Study coordinators conducted a brief interview with study participants, measured physical performance and reviewed medical records. Each study site (Atlanta and San Francisco) had one primary study coordinator who conducted the majority of the assessments; the primary coordinator also trained and supervised an assistant coordinator. Standardized training for data collection was provided by the investigators and a physical activity specialist. Consistency of measurement procedures was monitored throughout the study, using repeated demonstration/review of physical performance techniques and office quality control of recorded interview and medical record data. All physical performance and body composition assessments were conducted pre-HD on the midweek treatment day.
Race, gender, age and ESRD treatment start date were ascertained from patient report and the USRDS Medical Evidence Standard Analysis Files. Race was patient reported; for the small number of participants who declined to specify their race, race information was taken from the USRDS Medical Evidence file. Patients reported whether they lived alone and completed the Kidney Disease Quality of Life-Cognitive Function scale (KDQOL-CF) [19] and the Center for Epidemiologic Studies-Depression (CES-D) scale [20] during the interview.
In addition to history of vision problems, comorbidities abstracted from dialysis clinic medical records included diabetes, chronic obstructive pulmonary disease (COPD) and cardiovascular conditions, i.e. congestive heart failure (CHF), coronary artery disease/myocardial infarction, cerebrovascular accident/transient ischemic attack, peripheral vascular disease and other cardiac diseases (cardiac dysrhythmia, atrial fibrillation, tachycardia, pericarditis, cardiac arrest). Study coordinators measured patients’ standing height and obtained the most recent pre- and post-dialysis weights from dialysis clinic medical records. Body mass index (BMI) was calculated as kg/m2. Receipt of vitamin D therapy, HD prescription and most recent pre- and post-dialysis blood pressures, Kt/V, serum albumin and serum creatinine were obtained from the dialysis clinic medical records. Currently prescribed home medications were identified in the medical chart.
For patients who could stand unassisted without the use of a cane or walker, study coordinators assessed and recorded participants' performance on three balance tests from the Short Physical Performance Battery (SPPB) protocol [21], i.e. the side-by-side stand, semi-tandem stand and tandem stand. Participants received 1 point for maintaining balance for 10 s on each of the first two tests and 1 point for maintaining balance for 3–9.99 s and 2 points for maintaining balance for 10 s on the tandem stand. Thus, the total balance tests score could range from 0 to 4.
Participants were classified as non-frail, pre-frail or frail based on the Fried frailty index [10], which includes five indicators: (i) shrinking, i.e. 10 pounds or greater unintentional weight loss in the past 12 months; (ii) self-reported exhaustion ‘In the past week, everything I did was an effort most or all of the time’ or ‘In the past week, I could not get going most or all of the time’; (iii) weakness, i.e. dynamometer-measured grip strength of participants scoring in the lowest quintile, adjusted for sex and BMI; (iv) slowness, i.e. timed walk speed of participants in the slowest quintile on a 15-foot walk, adjusted for sex and height and (v) low physical activity, i.e. the lowest quintile for each sex of a weighted score of kilocalories expended per week in physical activities ‘you have done in the past 2 weeks’ as reported on the Minnesota Leisure Time Activity questionnaire [22]. Participants unable to walk were classified in the slowest quintile on the walk speed indicator, consistent with other frailty research [23]. The Fried methodology defines participants positive for three or more of the five indicators as ‘frail’ and defines participants positive for one or two indicators as ‘prefrail’ [10].
Depressive symptomatology was assessed using the CES-D scale [20]. Among dialysis patients, a CES-D score of 18 or higher is considered suggestive of clinical depression [24]. Prescribed antidepressant medications, identified in the medical chart, included selective serotonin reuptake inhibitors (SSRIs), atypical antidepressants and tricyclic antidepressants. Depressive symptoms and antidepressant prescription or use are closely related variables. Thus, for inclusion in logistic regression analyses to estimate factors associated with participants' likelihood of incurring falls, we created a four-level variable summarizing the possible combinations of non-elevated (<18) and elevated (18+) CES-D scores and prescription of antidepressants: CES-D score <18 and no prescribed antidepressant(s) (the reference category) and CES-D score 18+ in conjunction with antidepressant(s), CES-D score 18+ not in conjunction with antidepressant(s) and CES-D score <18 in conjunction with antidepressant(s). A binary variable was also constructed to summarize elevated CES-D score/antidepressant use versus non-elevated CES-D score/no antidepressant use.
Statistical analyses
The association of sociodemographic, clinical, physiological (balance, frailty) and depression score/medication variables with the occurrence of falls was estimated in univariable and multivariable logistic regression models. Multivariable models included participants with data for all covariates; no missing data were imputed. The interaction of frailty and age was examined. Multivariable analysis was stratified by dialysis units. Statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Table 2 shows basic demographic characteristics of the study population and corresponding characteristics of the overall US prevalent in-center HD population. Study participants ranged in age from 20 to 92 years, with a mean (SD) of 57.1 (14.1) and median of 57.5. Forty-one percent were women. The representation of African-American patients in the cohort was higher than in the overall US in-center HD population, consistent with the selected study sites, which contributed to the younger average age of the study cohort compared with the overall US in-center HD population. The primary cause of ESRD was diabetes or hypertension in 72% of the study cohort, similar to the overall US in-center HD population. Participants' median time since ESRD treatment start was 3 years. In terms of socioeconomic status, ∼76% had a high school education or above and 10% were currently employed.
Table 2.
Demographic characteristics of the ACTIVE-ADIPOSE study cohort and of the overall US prevalent hemodialysis population
| ACTIVE-ADIPOSE study cohort (n = 762) | 31 December 2010 US Point Prevalent Center Hemodialysis Population (n = 378 293)a |
|
|---|---|---|
| Age, % | ||
| <45 | 20.2 | 13.4 |
| 45–64 | 51.3 | 41.8 |
| 65–74 | 17.3 | 22.9 |
| 75+ | 11.2 | 21.8 |
| Male, % | 59.2 | 55.7 |
| Race, % | ||
| White | 23.8 | 55.1 |
| Black/African-American | 61.6 | 37.8 |
| Other | ||
| Native American | 0.4 | 1.5 |
| Asian | 11.3 | 5.0 |
| Other (Native Hawaiian, Other Pacific Islander, other) | 2.9 | 0.6 |
| Hispanic, % | 12.7 | 17.1 |
| Primary ESRD diagnosis, % | ||
| Diabetes | 36.9 | 45.2 |
| Hypertension | 34.9 | 28.8 |
aSource: Table D.11, 2012 USRDS Annual Data Report, p. 390.
Overall, 28.4% of the cohort (216 participants) sustained one or more falls over 12 months, and 124 of these participants (57%) reported multiple falls. With a total of 671 falls reported, the fall incidence rate was 0.88 falls/person-year and was not significantly different for amputees/non-amputees. Fractures were sustained by 11.2% of fallers and primarily involved the upper or lower limbs, in addition to two hip fractures and a forehead injury. Among patients who sustained these injuries, e.g. broken hip and broken vertebrae, 71% were hospitalized.
In unadjusted analyses, older age, female gender, CHF, PVD, COPD, higher number of prescribed medications, prefrail or frail status versus non-frail, and higher CES-D score and/or prescribed antidepressants were associated with increased odds of falling. A higher cognitive function (KDQOL-CF) score, a higher total balance tests score and higher level of serum albumin were associated with decreased odds of falling (Table 3).
Table 3.
OR for association of patient characteristics with fall(s)
| Characteristics | 1+ Falls in the past 12 months |
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI)a | P-value | |
| Age, years | 1.02 (1.01, 1.04) | <0.001 | 1.02 (1.01, 1.04) | 0.01 |
| Gender | ||||
| Male (reference) | 1.00 | 1.00 | ||
| Female | 1.40 (1.01, 1.93) | 0.04 | 1.35 (0.92, 1.98) | 0.12 |
| Race, % | ||||
| White (reference) | 1.00 | 1.00 | ||
| Black | 0.71 (0.49, 1.04) | 0.08 | 0.85 (0.46, 1.56) | 0.60 |
| Other | 0.77 (0.46, 1.31) | 0.34 | 0.77 (0.42, 1.41) | 0.39 |
| Vintage | 0.97 (0.94, 1.00) | 0.09 | 1.00 (0.96, 1.04) | 0.90 |
| Diabetes | 1.22 (0.89, 1.68) | 0.23 | 0.82 (0.54, 1.25) | 0.34 |
| CHF | 1.63 (1.16, 2.30) | 0.005 | 1.44 (0.96, 2.16) | 0.08 |
| CAD/MI | 1.18 (0.83, 1.68) | 0.36 | 0.80 (0.50, 1.27) | 0.34 |
| CVA/TIA | 1.51 (0.92, 2.48) | 0.10 | 1.19 (0.68, 2.10) | 0.55 |
| PVD | 1.70 (1.03, 2.82) | 0.04 | 1.39 (0.75, 2.57) | 0.30 |
| Other cardiac diseases | 1.34 (0.94, 1.91) | 0.11 | 0.77 (0.48, 1.24) | 0.29 |
| COPD | 2.23 (1.30, 3.81) | 0.003 | 1.20 (0.63, 2.28) | 0.59 |
| KDQOL-CF score | 0.98 (0.97, 0.99) | <0.001 | 0.99 (0.97, 0.997) | 0.01 |
| # Of prescribed medications | 1.08 (1.05, 1.12) | <0.001 | 1.03 (0.98, 1.08) | 0.22 |
| Total balance tests score | 0.84 (0.76, 0.93) | <0.001 | 1.06 (0.91, 1.23) | 0.45 |
| Frailty classification | ||||
| Non-frail (reference) | 1.00 | 1.00 | ||
| Prefrail | 2.01 (1.33, 3.04) | 0.001 | 1.57 (0.99, 2.50) | 0.06 |
| Frail | 4.82 (2.85, 8.17) | <0.001 | 2.39 (1.22, 4.71) | 0.01 |
| CES-D score/antidepressant(s) | ||||
| CES-D <18/no antidepressant(s) (reference) | ||||
| CES-D 18+/antidepressant(s) | 4.03 (2.12, 7.66) | <0.001 | 2.19 (0.99, 4.84) | 0.05 |
| CES-D 18+/no antidepressant(s) | 2.56 (1.71, 3.84) | <0.001 | 2.02 (1.26, 3.26) | 0.004 |
| CES-D <18/antidepressant(s) | 2.25 (1.33, 3.80) | 0.003 | 1.42 (0.77, 2.61) | 0.27 |
| BMI | ||||
| <18.5 | 2.24 (0.80, 6.25) | 0.12 | 1.34 (0.39, 4.57) | 0.64 |
| [18.5, 25) | 1.00 | 1.00 | ||
| [25, 30) | 1.07 (0.71, 1.62) | 0.73 | 1.05 (0.66, 1.67) | 0.81 |
| ≥30 | 1.31 (0.89, 1.92) | 0.17 | 1.32 (0.84, 2.08) | 0.23 |
| Serum albumin | 0.42 (0.28, 0.65) | <0.0001 | 0.83 (0.49, 1.40) | 0.48 |
BMI, body mass index; CAD, coronary artery disease; CES-D, Center for Epidemiologic Studies-Depression; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; HD, hemodialysis; KDQOL-CF, Kidney Disease Quality of Life-Cognitive Function; MI, myocardial infarction; other antidepressant class, atypical antidepressant or tricyclic antidepressant; other cardiac disease, cardiac dysrhythmia, atrial fibrillation, tachycardia, pericarditis, cardiac arrest; PVD, peripheral vascular disease; SSRI, selective serotonin reuptake inhibitor; TIA, transient ischemic attack; Total balance tests score, sum (range 0–4) over three tests from the Short Physical Performance Battery.
an = 720 participants with information for all covariates; analysis stratified by dialysis units.
In the multivariable-adjusted analyses, the odds of incurring falls were higher in association with frail, compared with non-frail, status (OR: 2.39, 95% CI 1.22–4.71, P = 0.01), and the interaction of age and frailty status (P = 0.46) was not significant. Compared with a CES-D score <18 and no prescribed antidepressants, the odds of incurring falls increased in association with a CES-D score 18+ and no prescribed antidepressants (OR: 2.02, 95% CI 1.26–3.26, P = 0.004). The odds of incurring falls were also greater as age increased (OR: 1.02, 95% CI 1.01–1.04, P = 0.01) and were lower in association with a higher cognitive function score (OR: 0.99, 95% CI 0.97–0.997, P = 0.01). Predictors of incurring an injurious fall, compared with incurring a non-injurious fall or no fall, were similar (multivariable proportional odds models; data not shown).
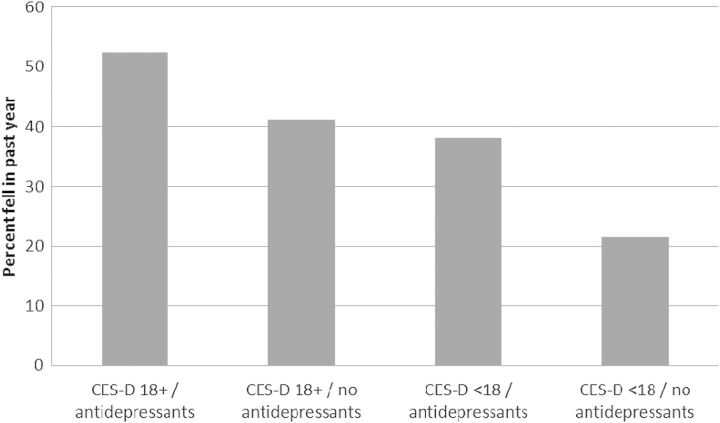
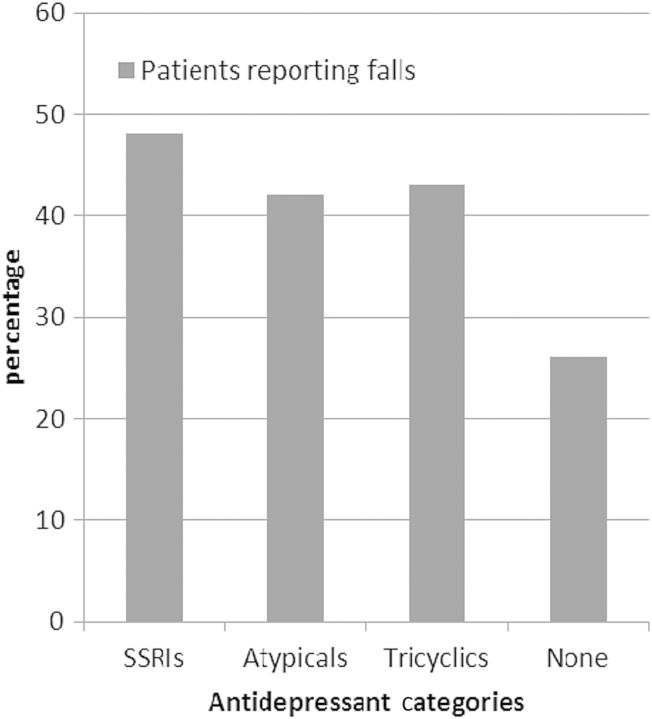
Figure 1 shows the proportion of study participants who reported falls within the four CES-D score/antidepressant categories. Approximately one-third of participants had an elevated CES-D score and/or had antidepressant medications prescribed. Participants in this combined grouping, compared with participants in the reference category of non-elevated CES-D score and no prescribed antidepressants, were over 80% more likely to be fallers (OR: 1.83, 95% CI 1.23–2.74, P = 0.003). Figure 2 shows that the proportion of patients who experienced falls was similar across the three antidepressant categories (48% for those with SSRIs prescribed, 42% for those with atypical antidepressants prescribed and 43% for those with tricyclic antidepressants prescribed).
Fig. 1.
Percentage of participants who reported falls, by CES-D score 18+/<18 and prescribed/no prescribed antidepressant(s). Participants in the three elevated CES-D score and/or prescribed antidepressant categories, compared with the reference category of non-elevated CES-D score and no prescribed antidepressants, were over 80% more likely to be fallers (OR: 1.83, 95% CI 1.23–2.74, P = 0.003).
Fig. 2.
Percentage of participants who reported falls, by types of antidepressant medication prescription and no antidepressant medication prescription. Examples of participants' prescribed SSRIs include escitalopram, fluoxetine, sertraline and paroxetine. Examples of participants' prescribed atypical antidepressants include bupropion, mirtazapine, trazodone and venlafaxine. Examples of participants' prescribed tricyclic antidepressants include amitriptyline, doxepin and nortriptyline. Twenty-eight percent of patients for whom an SSRI was prescribed were also prescribed a medication in another antidepressant category.
Discussion
This study, consistent with previous research (Table 1), found that falls are likely to occur annually among 25% or more of HD patients, even in a cohort that is not primarily elderly. Figure 3 shows the rate of falls by age group of study participants. Falls were more common among patients aged 45–64 years than those aged 65–74 years and were almost as common as in patients aged 75+.
Fig. 3.
Fall rates by age category of study participants.
Evidence for many of the fall risks that have been identified among HD patients mirrors findings from falls research in the general population. Having experienced a prior fall is a well-recognized precursor to falling [4, 7, 25], and more than half of the fallers in our study experienced multiple falls over 12 months. Fall risk has been shown to increase with increasing comorbidity burden, medication burden and age, although HD patients in a wide age range may experience falls [8]. Whether men or women who are undergoing HD have a greater risk for falls is less clear. Cognitive status is a key factor in the individual's inability to compensate for physical decline and instability and is therefore implicated in the etiology of falls [26], but cognitive status has not been routinely controlled in prior studies of falls among dialysis patients.
Independent of the influence of age, gender, cognitive function and comorbidity and medication burden, we observed significantly higher odds of falling in association with being assessed as frail. Consistent with the observed association of falls and frailty, patients who reported falling had lower mean (SD) serum creatinine values (and presumably less muscle mass) than those who did not report falling [8.8 (3.8.) versus 10.2 (3.6); P < 0.0001]. In addition, patients who sustained falls were more likely to have elevated depressive symptom scores and/or to have prescribed antidepressants. It is unclear whether the reason for the prescription of antidepressant medications, or the associated features of depression that warrant medications, or the actual drugs may portend risk.
Although poor balance is a well-recognized contributor to falling among the elderly [27], the total SPPB balance tests score was not significantly associated with falls among our study participants in the fully adjusted analysis, although an association was evident in the univariate analysis. Similarly, Rossier et al. [7] found that the Performance-Oriented Mobility Assessment (POMA) test, which evaluates position changes and gait maneuvers used during daily activities, did not independently predict severe falls in HD patients, although the authors observed a trend toward a lower POMA score among fallers when compared with non-fallers. Elements of the SPPB balance tests and the POMA test may be captured by the muscle strength and walk speed evaluations that are included in the Fried frailty assessment, and reduced cognitive function, for which dialysis patients are at increased risk, may be a key factor in the inability to compensate for balance vulnerabilities and subsequently fall.
Our study has several important strengths. Data were supplied by a large multi-center study cohort of over 700 patients. A large number of patient characteristics and treatment-related factors were carefully assessed, including performance-based measures of balance, muscle strength and gait speed. The fall prevalence that we observed was very similar to several 12-month prevalence estimates from other studies (Table 1).
However, although the ACTIVE-ADIPOSE cohort shares many similarities with the general ESRD population, the fact that participants were limited to seven outpatient clinics in the Atlanta area and seven outpatient clinics in the San Francisco Bay Area yielded a subset that was not highly representative of the national ESRD population. We also acknowledge that we did not obtain information about the circumstances during which falls occurred. We were able to confirm injurious falls that required hospitalization, but non-injurious falls may have been underreported.
Finally, the cross-sectional study design may also be viewed as a limitation. We stress that our data represent observed associations and that we cannot posit a clear temporal sequence between frailty status and falls, nor between depressive symptoms and falls. Falls may increase the likelihood of frailty and depressive symptoms, rather than vice versa. Both frailty and depressive symptoms are likely to evolve over a period of time, and measures obtained at only one point in time may be misleading. However, we suggest that identifying associations among falls, frailty and depressive symptoms is important because individuals who sustain a fall are at increased risk for a subsequent fall, and frailty status and depressive symptoms suggest potential opportunities for intervention.
We did not find an increased likelihood of falls in association with vintage, living alone or being non-English speaking, vision problems, HD prescription, average volume removed per HD treatment or average Kt/V, pre- or post-dialysis blood pressure or blood pressure changes or average serum bicarbonate level. The majority of all participants (76%) were receiving vitamin D therapy, but osteoporosis and vitamin D deficiency have been reported to affect weight-bearing lower limb antigravity muscles that are necessary for postural balance and walking [28], and significant associations of low serum 25 (OH) D concentration with falls and fall risk and the occurrence of falls have been shown [29, 30]. Other factors that may be important to investigate in relation to falls include nPCR, lowest intradialytic blood pressure and prescription of medications that may be associated with postural drop or altered mental status. We did investigate the association of falling with prescription of neuroleptics, narcotics and sleep medications, and no association with falling was evident for neuroleptics and narcotics. Study participants who reported falls were more likely to have sleep medications prescribed, but patients with sleep medications prescribed were also more likely to have elevated CES-D scores and/or to have an antidepressant prescribed, consistent with the well-recognized association between sleep disturbance and depression.
The frailty index, or selected indicators included in this index, could be used to screen for patients who are at increased risk for falls. Sourial et al. have recently shown that indicators of mobility, nutrition, physical activity and strength, in combination with cognition, improved the prediction of disability in older adults, beyond the contributions of age, sex and comorbidity. Although the improvement in prediction was modest, this ‘may still be worthwhile because while age, sex and the number of chronic diseases are not modifiable, frailty may be’ [31]. Similarly, depression management may be relevant for fall prevention [2, 32], although the role of antidepressants in fall risk remains unclear. Interestingly, in the MOBILIZE Boston study [33], researchers observed increased falling in association with depressed mood and antidepressants among participants who had no walking disability. In addition, longitudinal analyses of participants in the Women's Health Initiative Observational Study showed that depressive symptoms and antidepressant use were associated with incident frailty after 3 years of follow-up of >27 000 women aged ≥65 years who were classified as non-frail at their initial assessment [34].
While all the components of the Fried frailty index provide important information, slowed or irregular gait, which is likely to reflect impaired energy, movement control and support, seems to be especially predictive of fall risk in older adults [35]. Slowed gait may be associated with a ‘vicious cycle of reduced physical activity and deconditioning that has a direct effect on health’. [36] Of note, 78% of HD patients classified as frail by the Fried index in our study population were defined as frail on the walk speed indicator [37]. Addressing low levels of physical activity, even with a simple walking program, is a logical target. Participation in self-selected exercise activities was shown in the Health, Aging and Body Composition study to be independently associated with delaying the onset and progression of frailty [38]. In addition, a recent Cochrane review concluded that exercise is moderately more effective than no therapy for reducing symptoms of depression [39]. Tai Chi, which has been shown to improve functional balance and reduce the frequency of falls as well as impact favorably on indicators of psychosocial well-being [25, 27], can be a useful option for dialysis patients [40]. Reducing the prevalence of falls among patients undergoing HD should have high priority as a marker of the quality of delivered care.
Conflict of interest statement
The results presented in this paper have not been published previously in whole or part, except in abstract format. The authors declare that they have no other relevant financial interests.
Acknowledgements
This study was supported by National Institutes of Health contract HHSN267200715004C, ADB No. N01-DK-7-5004 (Dr Kutner). The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
References
- 1.Roberts RG, Kenny RA, Brierley EJ. Are elderly haemodialysis patients at risk of falls and postural hypotension? Int Urol Nephrol. 2003;35:415–421. doi: 10.1023/b:urol.0000022866.07751.4a. [DOI] [PubMed] [Google Scholar]
- 2.Desmet C, Beguin C, Swine C, et al. the Universitė Catholique de Louvain Collaborative Group. Falls in hemodialysis patients: prospective study of incidence, risk factors, and complications. Am J Kidney Dis. 2005;45:148–153. doi: 10.1053/j.ajkd.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Cook WL, Jassal SV. Prevalence of falls among seniors maintained on hemodialysis. Int Urol Nephrol. 2005;37:649–652. doi: 10.1007/s11255-005-0396-9. [DOI] [PubMed] [Google Scholar]
- 4.Cook WL, Tomlinson G, Donaldson M, et al. Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol. 2006;1:1197–1204. doi: 10.2215/CJN.01650506. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Tomlinson G, Naglie G, et al. Geriatric comorbidities, such as falls, confer an independent mortality risk to elderly dialysis patients. Nephrol Dial Transplant. 2008;23:1396–1400. doi: 10.1093/ndt/gfm778. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Rahman EM, Yang G, Turgut F, et al. Long-term morbidity and mortality related to falls in hemodialysis patients: role of age and gender—a pilot study. Nephron Clin Pract. 2011;118:c278–c284. doi: 10.1159/000322275. [DOI] [PubMed] [Google Scholar]
- 7.Rossier A, Pruijm M, Hannane D, et al. Incidence, complications and risk factors for severe falls in patients on maintenance haemodialysis. Nephrol Dial Transplant. 2012;27:352–357. doi: 10.1093/ndt/gfr326. [DOI] [PubMed] [Google Scholar]
- 8.McAdams-DeMarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14:224. doi: 10.1186/1471-2369-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaubrun AC, Kilpatrick RD, Freburger JK, et al. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol. 2013;24:1461–1469. doi: 10.1681/ASN.2012090916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, Tangen CM, Walston J, et al. for the Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Ewing SK, Taylor BC, et al. for the Study of Osteoporotic Fractures Research Group . Frailty and risk of falls, fracture and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 12.Ensrud KE, Ewing SK, Taylor BC, et al. for the Study of Osteoporotic Fractures Research Group. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 13.Ensrud KE, Ewing SK, Cawthon PM, et al. for the Osteoporotic Fractures in Men Research Group . A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue Q-L, Walston JD, Fried LP, et al. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: the Women's Health and Aging Study. Arch Intern Med. 2011;171:1119–1121. doi: 10.1001/archinternmed.2011.252. [DOI] [PubMed] [Google Scholar]
- 15.Bowen ME. The relationship between body weight, frailty, and the disablement process. J Gerontol A Biol Sci Med Sci. 2012;67:618–626. doi: 10.1093/geronb/gbs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tom SE, Adachi JD, Anderson FA, Jr, et al. for the GLOW investigators. Frailty and fracture, disability, and falls: a multiple country study from the Global Longitudinal Study of Osteoporosis in Women. J Am Geriatr Soc. 2013;61:327–334. doi: 10.1111/jgs.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US. Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. , Chapter 9. [Google Scholar]
- 18.Buchner DM, Hornbrook MC, Kutner NG, et al. Development of the common data base for the FICSIT trials. J Am Geriatr Soc. 1993;41:297–308. doi: 10.1111/j.1532-5415.1993.tb06708.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurella M, Luan J, Yaffe K, et al. Validation of the kidney disease quality of life (KDQOL) cognitive function subscale. Kidney Int. 2004;66:2361–2367. doi: 10.1111/j.1523-1755.2004.66024.x. [DOI] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D scale: a new self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 21.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Biol Sci Med Sci. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 22.Taylor HL, Jacobs DR, Schuker B, et al. A questionnaire for the assessment of leisure-time physical activities. J Chronic Dis. 1978;31:745–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 23.Saum K-U, Müller H, Stegmaier C, et al. Development and evaluation of a modification of the Fried frailty criteria using population-independent cutpoints. J Am Geriatr Soc. 2012;60:2110–2115. doi: 10.1111/j.1532-5415.2012.04192.x. [DOI] [PubMed] [Google Scholar]
- 24.Hedayati SS, Bosworth HB, Kuchibhatla M, et al. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69:1662–1668. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Harmer P, Fisher KJ, et al. Tai Chi: improving functional balance and predicting subsequent falls in older persons. Med Sci Sports Exerc. 2004;36:2046–2052. doi: 10.1249/01.mss.0000147590.54632.e7. [DOI] [PubMed] [Google Scholar]
- 26.Martin KL, Blizzard L, Srikanth VK, et al. Cognitive function modifies the effect of physiological function on the risk of multiple falls—a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68:1091–1097. doi: 10.1093/gerona/glt010. [DOI] [PubMed] [Google Scholar]
- 27.Wolf SL, Barnhart HX, Kutner NG, et al. the Atlanta FICSIT group. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. J Am Geriatr Soc. 1996;44:489–497. doi: 10.1111/j.1532-5415.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Rahman EM, Turgut F, Turkmen K, et al. Falls in elderly hemodialysis patients. Q J Med. 2011;104:829–838. doi: 10.1093/qjmed/hcr108. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 30.Boudville N, Inderjeeth C, Elder GJ, et al. Association between 25-hydroxyvitamin D, somatic muscle weakness and falls risk in end-stage renal failure. Clin Endocrinol. 2010;73:299–304. doi: 10.1111/j.1365-2265.2010.03821.x. [DOI] [PubMed] [Google Scholar]
- 31.Sourial N, Bergman H, Karunananthan S, et al. Implementing frailty into clinical practice: a cautionary tale. J Gerontol A Biol Sci Med Sci. 2013;68:1505–1511. doi: 10.1093/gerona/glt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coupland C, Dhiman P, Morriss R, et al. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quach L, Yang FM, Berry SD, et al. Depression, antidepressants, and falls among community-dwelling elderly people: the MOBILIZE Boston study. J Gerontol A Biol Sci Med Sci. 2013;68:1575–1581. doi: 10.1093/gerona/glt084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakey SL, LaCroix AZ, Gray SL, et al. Antidepressant use, depressive symptoms, and incident frailty in women aged 65 and older from the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2012;60:854–861. doi: 10.1111/j.1532-5415.2012.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59:887–892. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kutner NG, Zhang R, Huang Y, et al. Risk factors for frailty in a large prevalent cohort of hemodialysis patients. Am J Med Sci. 2013 doi: 10.1097/MAJ.0000000000000250. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson MJ, Giuliani C, Morey MC, et al. for the Health, Aging and Body Composition Study Research Group. Physical activity as a preventative factor for frailty: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64A:61–68. doi: 10.1093/gerona/gln001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database of Systematic Reviews. 2013;(Issue 9) doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kutner NG. How can exercise be incorporated into the routine care of patients on dialysis? Int Urol Nephrol. 2007;39:1281–1285. doi: 10.1007/s11255-007-9281-z. [DOI] [PubMed] [Google Scholar]