Abstract
Background
Inflammation impairs erythropoiesis, iron availability and is associated with a higher mortality risk in patients with end-stage renal disease. We studied the associations between Delta-He [the difference between the reticulocyte haemoglobin content (Ret-He) and erythrocyte haemoglobin content], a suggested marker of iron availability, and markers of inflammation, iron status, response to erythropoiesis-stimulating agents (ESAs) and mortality in prevalent peritoneal dialysis (PD) patients.
Methods
Eighty-two PD patients were followed weekly for 12 weeks with an additional follow-up of 36 months. Delta-He, Ret-He and high-sensitivity C-reactive protein (hs-CRP) were measured weekly and interleukin-6 (IL-6) and iron markers every fourth week. Mortality risk was assessed by Cox proportional hazards model adjusting for potential confounding factors. The relationships between ESA response, inflammatory markers, iron markers and Delta-He were evaluated in the PD patients. The relationship between Delta-He and iron markers was analysed in 87 healthy subjects.
Results
Delta-He correlated with IL-6 (rho = 0.48, P < 0.001), hs-CRP (rho = 0.36, P < 0.001) and ESA hyporesponsivess index (EHRI; rho = −0.44, P < 0.001) in the PD patients. Delta-He did not correlate with iron markers in PD patients nor in healthy subjects. The mean Delta-He levels were significantly different between the tertiles of EHRI (P < 0.01). Delta-He was associated with all-cause mortality risk in PD patients after adjusting for age, gender, hs-CRP, comorbidity and nutritional status [OR 0.70 (0.51–0.96), P < 0.05].
Conclusions
Delta-He independently predicts all-cause mortality in PD patients after adjusting for potential confounders and is a predictor of ESA response in PD patients.
Keywords: anaemia, CRP, erythropoietin, ESRD, IL-6, inflammation, iron, peritoneal dialysis
Introduction
A certain degree of inflammation is common in end-stage renal disease (ESRD) patients and is considered an important risk factor for mortality and cardiovascular events in this patient population [1–3]. Cardiovascular disease (CVD) underlies more than half of the mortality in dialysis patients [4].
During recent years, the predictive role of inflammatory markers on mortality and cardiovascular events in patients with ESRD has been extensively studied [5, 6]. Of several markers of inflammation, interleukin-6 (IL-6) has been shown to be one of the strongest predictors of mortality [7, 8].
Inflammation may not only influence the prognosis and risk of CVD, it may also cause a poor response to erythropoietin-stimulating agents (ESAs) [9–11], which in turn has been associated with a negative impact on survival in both haemodialysis (HD) and peritoneal dialysis (PD) patients [12, 13].
Delta-He is a potential marker of iron availability and, in addition, it has been suggested as a marker of inflammation [14, 15]. It is calculated as the difference between reticulocyte haemoglobin content (Ret-He) and erythrocyte haemoglobin content (RBC-He). As Ret-He reflects the incorporation of iron into the erythroid progenitor cells over the previous 2–4 days, it provides an estimate of functionally available iron [16, 17]. In contrast, RBC-He reflects iron incorporation into erythrocyte haemoglobin over the previous 90–120 days due to the longer life span of mature erythrocytes. Under normal conditions, Delta-He assumes a positive value. This is due to the greater haemoglobin content of reticulocytes compared with that of erythrocytes, owing to the gradual decline in haemoglobin content as the erythrocyte ages [14]. As iron homeostasis and inflammation are intimately related through the action of hepcidin, an acute inflammatory response suppresses iron availability to the erythropoietic progenitor cells by retaining iron in the macrophages and inhibiting iron absorption in the gut [18]. The subsequent redistribution of plasma iron rapidly induces hypofaerremia and also a decrease in Ret-He, leading to a drop in Delta-He [14].
Information on Delta-He in the chronic kidney disease population is scarce. In this observational study, we study whether Delta-He can be used in PD patients to predict clinical outcome and ESA response.
Materials and methods
This observational study with longitudinal follow-up was aimed at evaluating the variation of inflammatory markers in all prevalent PD patients who were being controlled at the Karolinska University Hospital and Danderyds Hospital in the Stockholm region. Patients were recruited from March 2008 to April 2011. Only patients on PD for >3 months were considered for inclusion. A total of 163 patients who were receiving regular PD treatment were screened for inclusion, 66 of which refused to participate or were precluded to entry this study, 13 did not start the study due to various reasons (including transfer to HD, transplantation and mortality) and 2 were excluded because of missing laboratory measurements. The remaining 82 patients (54 men, median age 64 [range 25th to 75th percentile, 55–76] years) were included in the study and were followed with blood samples obtained at weekly or monthly intervals during a period of 3 months.
All patients were treated with PD using biocompatible solutions (Physioneal, Nutrineal, Extraneal, Gambrosol Trio or BicaVera) in different combinations. Sixty-three patients were treated with continuous ambulatory PD using 1.5–2.5 L fill volume and three to five exchanges/day, whereas 19 were treated with automated peritoneal dialysis. Weekly Kt/V was 2.18 (1.84–2.47). Residual glomerular filtration rate, estimated from the average of renal creatinine and urea clearance in a 24-h urine collection, was 2.5 (1.2–3.9) mL/min/1.73 m2 body surface area. The preceding treatment time on dialysis, dialysis vintage, was 28 (7–101) months. Most of the patients were on antihypertensive medications as well as phosphate and potassium binders, diuretics and vitamin supplementation in accordance with the clinical judgment of the treating physician. Seventy-seven patients received an individual ESA dosage (Aranesp, Eprex, Neorecormon or Mircera) with the aim of maintaining the haemoglobin level between 100 and 120 g/L. A factor of 200 was used to convert darbapoetin to the equivalent epoetin dose. Iron substitution was administered when required in order to maintain target haemoglobin values; 26 PD patients received additional iron supplementation.
All subjects gave written informed consent, and the regional ethical committee approved the protocols.
All samples were obtained early in the morning and after 12 h of fasting. The tubes were immediately placed on ice until the assay was performed in the laboratory. Biochemical measurements included plasma albumin, haemoglobin, parathyroid hormone (PTH), high-sensitivity C-reactive protein (hs-CRP), complete blood count (CBC), Ret-He and Delta-He, and were measured every week until study week 12. IL-6, serum ferritin and plasma iron were measured every fourth week until study week 12.
Ret-He and Delta-He were analysed with Sysmex XE-5000, an automated haematology analyser. With the flow cytometry technique, this instrument independently measures the volume and haemoglobin content of reticulocytes and mature erythrocytes, providing the mean haemoglobin content for reticulocytes (Ret-He; e denotes equivalent) and erythrocytes (RBC-He; e denotes equivalent) respectively, expressed in pg [17]. The Delta-He is defined as Ret-He minus RBC-He and is automatically calculated by the analyser [14]. The haemoglobin concentration was measured on Sysmex XE 5000. Biochemical parameters including plasma albumin, hs-CRP, plasma iron and plasma transferrin were analysed using Modular P (Roche Diagnostics). PTH and serum ferritin were measured using Modular E (Roche Diagnostics). S-IL-6 was analysed using Immulite (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA).
Comorbidity was assessed according to Davies et al. [19]. Scoring comorbidity includes chronic and active conditions with poor survival prognosis in seven domains. The comorbid score is the number of domains affected (minimum score, 0; maximum, 7). Comorbidity was graded as low (score = 0; i.e. no comorbidity), medium (score, 1 or 2) or high risk (score ≥3).
ESA hyporesponsivess index (EHRI), defined as the weekly ESA dose/kg/haemoglobin level (g/L), was calculated based on baseline ESA doses. The baseline EHRI was compared with baseline Delta-He in order to evaluate the relationship of Delta-He to ESA response. The interrelationship between baseline values of Delta-He, IL-6, hs-CRP, Ret-He, plasma albumin, plasma iron and serum ferritin, respectively, was assessed. PD patients treated with ESA (n = 77) were divided into tertiles based on their EHRI values. Baseline values of Delta-He, IL-6, hs-CRP, Ret-He, plasma iron, serum ferritin and PTH were compared between the EHRI tertiles.
Delta-He and hs-CRP were also analysed at all the sampling occasions in a mixed model in order to assess clinical and laboratory factors affecting Delta-He.
For comparative purposes, a group of healthy subjects (n = 87) were recruited from third year students at the Karolinska Institutet medical school. After attaining informed consent, laboratory measurements of Delta-He, Ret-He, CBC, plasma albumin, plasma iron and serum ferritin were obtained after overnight fasting and used for comparative analyses.
Non-normally distributed variables were expressed as median and interquartile range (25th and 75th percentiles), and normally distributed variables were expressed as mean ± SD as appropriate. A P-value <0.05 was considered to be statistically significant. Spearman's rank correlation was used to determine correlations with continuous variables. Stepwise multivariate regression analysis was used to assess the predictors for Delta-He levels.
Comparisons between two groups were analysed by the Wilcoxon test, the Fischer's exact test or the Mann–Whitney U-test. Spearman's rank correlation (rho) was used to determine correlations of Delta-He with other variables in PD patients. Comparisons between the three groups were analysed by Kruskal–Wallis, ANOVA and χ2 tests. Clinical outcome was assessed through receiver operator characteristic (ROC) curve analysis and the C-statistic. Cox regression analyses were performed since the assumption of proportionality of hazards was met for all covariates. Selection of the confounders (age, sex, comorbidities, nutritional status and inflammation) in these models was performed on the basis of presumed pathophysiological pathways. Data are presented in the form of hazard ratios (HRs) and 95% confidence intervals (95% CIs).
The analysis of factors related to the variability of Delta-He was carried out using a linear mixed-effect model [20] that included both fixed effects and a random effect to account for intra-individual variations due to repeated observations of the same patients. The fixed effects specified in the model included baseline values (age, sex, vintage and comorbidity) and study week (0–12 weeks). F-tests were used to assess the significance of the fixed effects. We estimated intra-class correlation (ICC) for between- and within-individual variations for Delta-He. Total variation comprises the within- and between-subject variation, with the former further subdivided into analytic variation, arising from measurement error and true biological variation. The sources of variation in the analysis and their ICC, which quantify the percentage of total variation, are explained by between-subject variations. The remainder is explained by within-subject variation. All of the statistical analyses were performed in SAS version 9.3 (SAS Institute, USA).
Results
Of 82 patients enrolled in the study, 69 patients completed 12 study weeks.
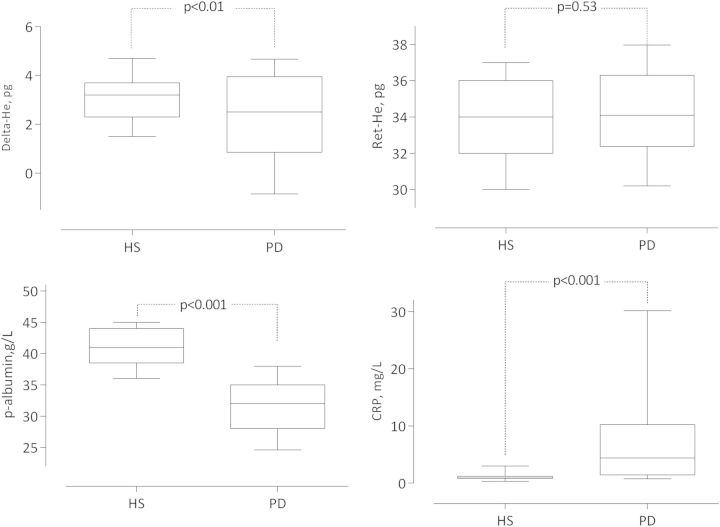
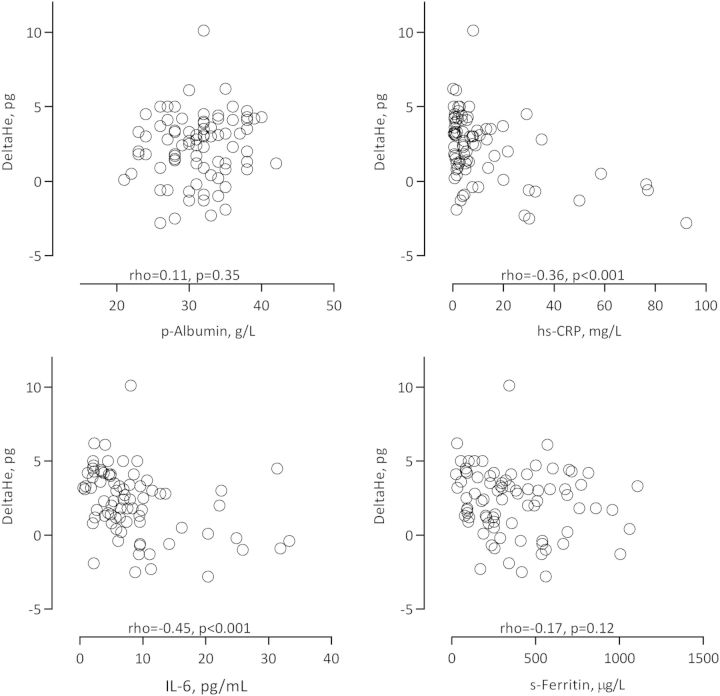
Baseline clinical and biochemical characteristics of PD patients and healthy subjects are summarized in Table 1. As shown in Figure 1, PD patients had lower levels of Delta-He and albumin, higher levels of CRP, whereas Ret-He did not differ between the two groups. Selected univariate Spearman rank correlations for PD patients are presented in Table 2 and Figures 2 and 3. Delta-He correlated positively with plasma iron, and inversely with the inflammatory markers and EHRI. Delta-He was not significantly associated with serum ferritin and plasma albumin. EHRI was significantly associated with IL-6 and Ret-He.
Table 1.
Baseline clinical and biochemical characteristics of 82 PD patients and 87 healthy subjects
| Parameters | PD patients (n = 82) | Healthy subjects (n = 87) |
|---|---|---|
| Age (years) | 64 ± 14 | 26 (±5) |
| Male (%) | 66 | 33 |
| Female (%) | 44 | 67 |
| Davies score (%) | ||
| Low comorbidity risk | 28 | – |
| Medium comorbidity risk | 58 | |
| High comorbidity risk | 14 | |
| Diabetes mellitus (%) | 25 | – |
| History of cardiovascular disease (%) | 29 | – |
| Vintage (months)a | 28 (7–101) | – |
| Kt/Vurea | 2.2 (1.8–2.5) | – |
| Residual GFR (mL/min/1.73 m2) | 3 (1–4) | – |
| Haemoglobin (g/L) | 119 ± 11 | 137 ± 14 |
| P-albumin (g/L) | 32 ± 5 | 41 ± 3 |
| hs C-reactive protein (mg/L) | 4.1 (1.4–10.6) | – |
| Delta-He (pg) | 2.3 (0.9–3.9) | 3.1 (2.3–3.7) |
| Ret-He (pg) | 34.0 (32.4–36.3) | 33.8 (32.0–36.0) |
| S-ferritin (μg/L) | 299 (179–538) | 42 (23–82) |
| Transferrin saturation (%) | 26 (19–35) | – |
| EHRI (IU/kg/g/L Hb per week) | 0.65 (0.26–0.89) | – |
| P-interleukin-6 (pg/mL) | 6.5 (3.9–9.6) | NA |
| P-tumour necrosis factor (pg/mL) | 16.7 (14.7–18.6) | NA |
| P-parathyroid hormone (pg/mL) | 281 (146–443) | NA |
| Medication use (%) | ||
| Antihypertensive drugs | 91 | – |
| Statins | 49 | |
| Antiaggregants/anticoagulants | 49 | |
| Erythropoietin-stimulating agents | 93 | |
| Phosphate binders | 72 | |
| Vitamin D receptor activators | 83 | |
| Calcimimetics | 12 | |
| Immunosuppressant drugs | 13 | |
| Iron supplementation (intravenous or oral) | 32 | |
Categorical data are shown as percentage; continuous data as mean ± SD or median and interquartile range (25th and 75th percentiles) as appropriate.
NA, not available.
aVintage denotes preceding total time on PD.
Fig. 1.
Delta-He, Ret-He, albumin and CRP levels in 82 PD patients and 87 healthy subjects (HS). Data are presented as box plots with whiskers representing 10th and 90th percentiles. Mann–Whitney U-test assessed statistical differences.
Table 2.
Univariate Spearman rank correlations for PD patients
| Ret-He | Plasma iron | Transferrin saturation (%) | IL-6 | hs-CRP | EHRI | |
|---|---|---|---|---|---|---|
| Delta-He (pg) | — | 0.43** | 0.32* | −0.45** | −0.36** | −0.44** |
| Ret-He (pg) | 0.37** | 0.32* | −0.150.19 | −0.200.07 | −0.36* | |
| Plasma iron (µmol/L) | 0.86** | −0.54** | −0.58** | −0.38** | ||
| Transferrin saturation (%) | −0.38** | −0.42** | −0.210.06 | |||
| Plasma IL-6 (pg/mL) | 0.71** | 0.32* | ||||
| hs-CRP (mg/L) | 0.35* |
P-values are provided in superscript; *P < 0.01; **P < 0.001.
Fig. 2.
Correlations, expressed as univariate Spearman rank rho values, between Delta-He and plasma albumin, CRP, IL-6 and ferritin concentrations in 82 PD patients.
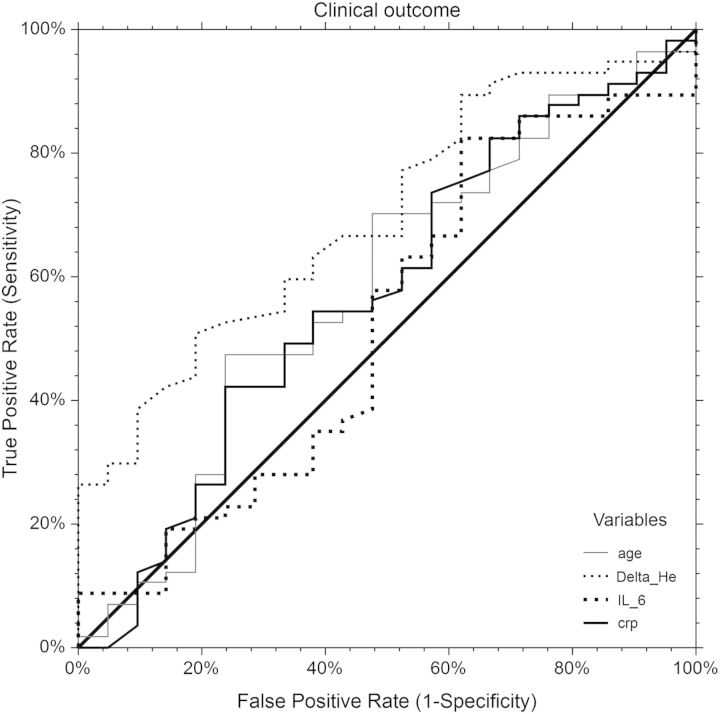
Fig. 3.
ROC curve analysis of all-cause mortality risk in relation to age, hs-CRP, Delta-He and IL-6 among 82 PD patients. All-cause mortality risk was assessed during follow-up period of up to 36 months.
In healthy subjects, Delta-He was not significantly associated with serum ferritin (rho = 0.07, P = 0.54) and plasma iron (rho = 0.02, P = 0.89).
The linear mixed model showed that time of sampling (study week; 1–12), hs-CRP and Davies comorbidity score may significantly influence the variability of Delta-He (Table 3). Delta-He had the lowest component of variability explained by between-subject variation (ICC for Delta-He 0.39, SE = 0.05) and thus, the highest within-subject variation of 61%, suggesting that Delta-He measurements are less stable within individuals.
Table 3.
Variance components analysed by a linear mixed model for Delta-He in weekly samples of PD patients (n = 69) obtained over a period of 12 weeks
| Variable | Coefficient | SE | Z-test | P-value |
|---|---|---|---|---|
| Age, years | 0.025 | 0.01 | 2.09 | 0.04 |
| Time (study week) | −0.06 | 0.02 | −3.06 | 0.00 |
| Subjective global assessment | 0.15 | 0.34 | 0.44 | 0.66 |
| Log hsC-reactive protein (mg/L) | −0.47 | 0.051 | −9.14 | 0.001 |
| Davies, medium risk | −1.4 | 0.38 | −3.6 | 0.001 |
| Davies, high risk | −1.7 | 0.38 | −3.62 | 0.001 |
| Gender | −0.18 | 0.36 | −0.50 | 0.61 |
| Intercept | 2.61 | 0.84 | 3.11 | 0.001 |
Delta-He and Ret-He were significantly different between the tertiles of EHRI, as reported in Table 4. IL-6, hs-CRP and plasma iron also differed significantly between the tertiles of EHRI. There were no significant differences for serum ferritin and PTH between the EHRI tertiles.
Table 4.
Laboratory parameters according to tertiles of EHRI (IU/kg/g/L Hb per week) in 77 PD patients
| Tertile 0 (n = 26) | Tertile 1 (n = 25) | Tertile 2 (n = 26) | P-values | |
|---|---|---|---|---|
| EHRI | 0.13 (0.23–0.34) | 0.46 (0.56–0.69) | 0.91 (1.15–1.46) | |
| Ret-He (pg) | 36.1 (33.5–37.1) | 33.5 (32.0–35.4) | 33.4 (31–35.2) | 0.007 |
| Delta-He (pg) | 3.4 (1.9–4.3) | 2.3 (0.8–4.0) | 1.2 (−0.5–2.9) | 0.002 |
| IL-6 (pg/mL) | 5.0 (3.0–8.4) | 5.6 (3.6–8.5) | 8.5 (5.7–14.3) | 0.030 |
| hs-CRP (mg/L) | 2.6 (0.9–6.8) | 1.7 (1.0–7.1) | 7.4 (3.5–20.5) | 0.009 |
| P-iron (µmol/L) | 14.5 (11.0–18.5) | 13.0 (9.5–18.0) | 11.5 (7.8–13.3) | 0.030 |
| S-ferritin (µg/L) | 255 (127–624) | 273 (154–473) | 382 (200–544) | 0.510 |
| PTH (pg/mL) | 267 (185–344) | 329 (129–450) | 235 (131–539) | 0.910 |
During the follow-up period of 36 months, 24 patients died. ROC curve analysis assessing markers in relation to mortality showed similar areas under the curve (AUCs) for Delta-He (AUC = 0.76) and IL-6 (AUC = 0.77). The ROC curve for the risk score models showed acceptable discriminant power with a C-statistic of 0.636 (95% CI 0.573–0.698). The cut-off for Delta-He was identified as 1.4 pg and had a value of 0.57 and 0.76 for specificity and sensitivity, respectively.
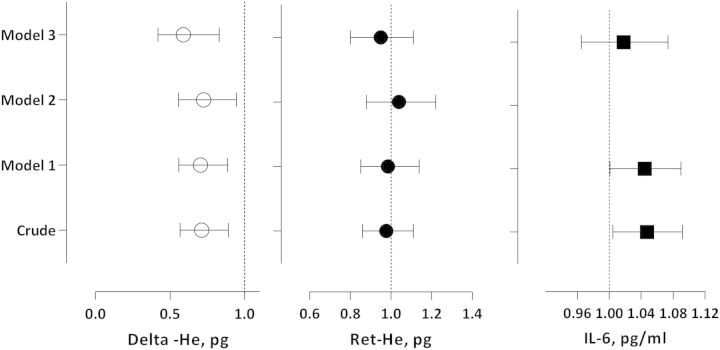
In a multivariate Cox regression analysis, baseline Delta-He value was able to independently predict mortality (Figure 4). In a model adjusting for age, gender, hs-CRP, Davies comorbidity score and SGA, Delta-He was independently associated with clinical outcome with a HR of 0.70 (95% CI 0.51–0.96). In crude analysis, Ret-He had a HR of 0.98 (95% CI 0.86–1.11) (Figure 4). Statistical significance was not present in crude or after multivariate adjustment. In crude analysis, IL-6 had a HR of 1.05 (95% CI 1.00–1.09) (Figure 4). However, statistical significance was lost after full multivariate adjustment.
Fig. 4.
Delta-He, Ret-He and IL-6 in relation to all-cause mortality risk in 82 PD patients. Associations are expressed as HRs with 95% CIs for all-cause mortality risk during up to 36 months of follow-up in the crude model and adjusted models where Model 1 = adjustment for age and gender; Model 2 = adjustment for Model 1 + hs-CRP (not done for IL-6); and Model 3= adjustments for Model 2 + Davies comorbidity score + subjective global assessment.
Discussion
Delta-He is based on two parameters; Ret-He and RBC-He, where the former reflects the rapid change in iron availability. The latter parameter, on the other hand, reflects the long-term iron availability. Acute inflammation rapidly induces a redistribution of plasma iron mediated by hepcidin, resulting in hypoferraemia with a consequent drop in Delta-He. Whereas the mean haemoglobin content of reticulocytes, measured as Ret-He, is readily affected by the rapid decrease in iron supply, the mean haemoglobin content of erythrocytes (RBC-He) is not affected correspondingly in the short run, because of the longer life span of red blood cells (90–120 days) when compared with the life span of reticulocytes. It is thus through the action of hepcidin and iron redistribution that Delta-He constitutes a marker of both erythropoiesis and inflammation. In absolute iron deficiency, however, low-to-normal values of Delta-He are expected because of the gradual decrease in both Ret-He and RBC-He over time, in contrast to the rapid change in Delta-He seen during an acute inflammatory response [14, 18].
In the current study of ESRD patients, Delta-He was negatively associated with inflammation (IL-6 and hs-CRP) and predicted the response to ESA treatment as shown by the significantly different Delta-He values of the EHRI tertiles in addition to a statistically significant association of baseline Delta-He to baseline EHRI. As shown in previous studies, inflammation is a well-known cause of ESA hyporesponsiveness [9–11].
Delta-He predicted all-cause mortality risk as well as did IL-6. We have shown that a single baseline measurement of Delta-He can independently predict all-cause mortality in PD patients. The association was robust as it remained after adjustment for age, sex, comorbidity and nutritional status; factors known to affect mortality rates in ESRD patients [21]. In the adjusted model, an increase of one unit of Delta-He was associated with a 30% reduction in the mortality risk. The ROC curve of Delta-He demonstrated an AUC of 0.76, explaining 26% of the mortality in the PD patients, and was comparable with that of IL-6.
This study is the first to show that Delta-He can be useful as a marker for risk stratification and ESA requirements in the dialysis population. A unique feature of the present study is the frequent measurement of parameters during 12 weeks of follow-up, allowing analysis based on the linear mixed model which showed that inflammation (hs-CRP) significantly affected the variability of Delta-He.
Several parameters of inflammation have been identified as risk markers for mortality in patients with ESRD [22]. Hitherto, IL-6 has shown the strongest predictive value for all-cause mortality in patients on maintenance dialysis [7, 8]. Inflammation as a risk marker for mortality has also been demonstrated in studies performed on PD-patients solely, in which CRP has been shown to be an independent risk factor of all-cause mortality and CVD [6, 23]. Despite the superiority of IL-6, CRP still remains the most commonly measured inflammatory parameter in the clinical setting due to its high availability, low cost and lack of diurnal variation [24].
An advantage of Delta-He compared with IL-6 is the low cost and the practical advantage of using the same blood sample as for a routine CBC, allowing simultaneous analysis of Delta-He as well as saving both money and patient blood needed for additional laboratory tests. In addition, Delta-He has the advantage that it can be measured automatically, enabling the provision of rapid results to the clinician [25, 26]. Both Ret-He and RBC-He have demonstrated high precision and good stability with minimal variations over time during storage, and apart from alpha- or beta thalassaemia or macrocytosis, Delta-He is exempt from significant interferences [17, 27]. However, even though a single baseline value of Delta-He was able to predict ESA hyporesponsiveness and mortality, Delta-He demonstrated high intra-individual variation, suggesting the need for follow-up testing in clinical praxis.
There are several limitations of the current study that need to be taken into account when interpreting the results. First, the observational design precludes conclusions regarding causality. A second limitation is the relatively small number of patients. Thirdly, a possible confounder is that some patients were treated with intravenous iron, which can affect Ret-He and thus, in theory, also affect Delta-He [28]. Finally, we analysed data on dialysis/plasma creatinine (D/P Cr) in a subset of patients (57 of the 82 patients in the study). We found no effect on outcome nor any correlation to Delta-He in this cohort.
Aside from these limitations, repeated measurements of parameters during a 12-week time period allowing assessment of factors linked to the variability of Delta-He represent a major strength of this study.
In this study comprising 82 PD patients, Delta-He was found to be associated with inflammation, response to ESA, and all-cause mortality risk. Thus, our findings suggest that Delta-He may have a role as a marker of mortality risk in this patient population. Considering the wide distribution of the instrument (Sysmex XE-5000) and the high availability to clinicians, measurements of Delta-He have the potential to be integrated into the routine follow-up of patients with minimal extra costs.
Risk assessment in ESRD patients is important, as the mortality rates are remarkably high. With the aid of risk markers such as Delta-He and IL-6, patients at higher risk of poor outcome may be identified, so that treatable risk factors may be eliminated in order to alleviate the total risk burden of the individual patient.
Conflict of interest statement
Baxter Novum is the result of a grant from Baxter Healthcare Corporation to the Karolinska Institutet. Kristin Danielson is employed by Takeda since September 2012. None of the other authors have any conflicts of interest to declare.
Acknowledgements
We are grateful to the patients involved in this study. We are also indebted to Åsa Lindé for exemplary data and sample collection, and to our research staff Annika Nilsson, Anki Emmoth, Ulrika Jensen, Björn Anderstam, Monica Ericsson and Ann-Christin Bragfors-Helin. We thank also Kjell Kalén and Agneta Collberg for analysing Delta-He in the laboratory. The observational 3-month study was supported by an unrestricted grant from Amgen.
References
- 1.Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom J, Lindholm B, Lacson E, Jr, et al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Semin Dial. 2000;13:163–175. doi: 10.1046/j.1525-139x.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 3.Iseki K, Tozawa M, Yoshi S, et al. Serum C-reactive protein (CRP) and risk of death in chronic dialysis patients. Nephrol Dial Transplant. 1999;14:1956–1960. doi: 10.1093/ndt/14.8.1956. [DOI] [PubMed] [Google Scholar]
- 4.Rostand SG. Coronary heart disease in chronic renal insufficiency: some management considerations. J Am Soc Nephrol. 2000;11:1948–1956. doi: 10.1681/ASN.V11101948. [DOI] [PubMed] [Google Scholar]
- 5.Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease—what have we learned in 10 years? Semin Dial. 2010;23:498–509. doi: 10.1111/j.1525-139X.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 6.Noh H, Lee S, Kang S, et al. Serum C-reactive protein: a predictor of mortality in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1998;18:387–394. [PubMed] [Google Scholar]
- 7.Tripepi G, Mallamaci F, Zoccali C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol. 2005;16(Suppl 1):S83–S88. doi: 10.1681/asn.2004110972. [DOI] [PubMed] [Google Scholar]
- 8.Goicoechea M, Quiroga B, García de Vinuesa S, et al. Intraindividual interleukin-6 variations on the cardiovascular prognosis of patients with chronic renal disease. Ren Fail. 2012;34:1002–1009. doi: 10.3109/0886022X.2012.696469. [DOI] [PubMed] [Google Scholar]
- 9.Panichi V, Rosati A, Bigazzi R, et al. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transplant. 2011;26:2641–2648. doi: 10.1093/ndt/gfq802. [DOI] [PubMed] [Google Scholar]
- 10.Won HS, Kim HG, Yun YS, et al. IL-6 is an independent risk factor for resistance to erythropoiesis-stimulating agents in hemodialysis patients without iron deficiency. Hemodial Int. 2012;16:31–37. doi: 10.1111/j.1542-4758.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 11.Wei M, Bargman JM, Oreopoulos DG. Factors related to erythropoietin hypo-responsiveness in patients on chronic peritoneal dialysis. Int Urol Nephrol. 2007;39:935–940. doi: 10.1007/s11255-007-9226-6. [DOI] [PubMed] [Google Scholar]
- 12.Duong U, Kalantar-Zadeh K, Molnar MZ, et al. Mortality associated with dose response of erythropoiesis-stimulating agents in hemodialysis versus peritoneal dialysis patients. Am J Nephrol. 2012;35:198–208. doi: 10.1159/000335685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Thamer M, Stefanik K, et al. Epoetin requirements predict mortality in hemodialysis patients. Am J Kidney Dis. 2004;44:866–876. [PubMed] [Google Scholar]
- 14.de Mast Q, van Dongen-Lases EC, Swinkels DW, et al. Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol. 2009;145:657–664. doi: 10.1111/j.1365-2141.2009.07664.x. [DOI] [PubMed] [Google Scholar]
- 15.Weimann A, Lun A, Lun S, et al. Leukocyte, neutrophil, immature granulocyte counts and interleukin-6 are superior to procalcitonin, C-reactive protein and delta-he for detection of mild inflammation: data from marathon runners producing mild systemic inflammation visible immediately after the run. LaboratoriumsMedizin. 2010;34:53–59. [Google Scholar]
- 16.Brugnara C, Laufer MR, Friedman AJ, et al. Reticulocyte hemoglobin content (chr): early indicator of iron deficiency and response to therapy. Blood. 1994;83:3100–3101. [PubMed] [Google Scholar]
- 17.Brugnara C, Schiller B, Moran J. Reticulocyte hemoglobin equivalent (ret he) and assessment of iron-deficient states. Clin Lab Haematol. 2006;28:303–308. doi: 10.1111/j.1365-2257.2006.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies SJ, Phillips L, Naish PF, et al. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17:1085–1092. doi: 10.1093/ndt/17.6.1085. [DOI] [PubMed] [Google Scholar]
- 20.Ockene IS, Matthews CE, Rifai N, et al. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem. 2001;47:444–450. [PubMed] [Google Scholar]
- 21.Qureshi AR, Alvestrand A, Divino-Filho JC, et al. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol. 2002;13(Suppl 1):S28–S36. [PubMed] [Google Scholar]
- 22.Carrero J, Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S49–S55. doi: 10.2215/CJN.02720409. [DOI] [PubMed] [Google Scholar]
- 23.Herzig KA, Purdie DM, Chang W, et al. Is C-reactive protein a useful predictor of outcome in peritoneal dialysis patients? J Am Soc Nephrol. 2001;12:814–821. doi: 10.1681/ASN.V124814. [DOI] [PubMed] [Google Scholar]
- 24.Carrero J, Yilmaz M, Lindholm B, et al. Cytokine dysregulation in chronic kidney disease: how can we treat it? Blood Purif. 2008;26:291–299. doi: 10.1159/000126926. [DOI] [PubMed] [Google Scholar]
- 25.Miwa N, Akiba T, Kimata N, et al. Usefulness of measuring reticulocyte hemoglobin equivalent in the management of haemodialysis patients with iron deficiency. Int J Lab Hematol. 2010;32:248–255. doi: 10.1111/j.1751-553X.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 26.Urrechaga E, Borque L, Escanero JF. Potential utility of the new Sysmex XE 5000 red blood cell extended parameters in the study of disorders of iron metabolism. Clin Chem Lab Med. 2009;47:1411–1416. doi: 10.1515/CCLM.2009.301. [DOI] [PubMed] [Google Scholar]
- 27.Sudmann AA, Piehler A, Urdal P. Reticulocyte hemoglobin equivalent to detect thalassemia and thalassemic hemoglobin variants. Int J Lab Hematol. 2012;34:605–613. doi: 10.1111/j.1751-553X.2012.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buttarello M, Pajola R, Novello E, et al. Diagnosis of iron deficiency in patients undergoing hemodialysis. Am J Clin Pathol. 2010;133:949–954. doi: 10.1309/AJCPQAX0JFHFS0OA. [DOI] [PubMed] [Google Scholar]