Abstract
The identification of large numbers of tubuloreticular inclusions (TRIs) in renal biopsies may be useful to raise diagnostic suspicion for certain clinical entities, particularly autoimmune diseases and viral infections. We report a case of a 65-year-old female with a 2-week history of malaise, massive proteinuria and lower extremity edema of acute onset. A renal biopsy was performed and the diagnosis of non-human immunodeficiency virus (HIV) tip-located, early focal segmental glomerulosclerosis (FSGS) was established. The electron microscopy examination was remarkable for the presence of diffuse foot process effacement and frequent TRIs in the endothelial cells of the glomerular capillary loops, endothelium of arterioles and cytoplasm of fibroblasts in the interstitium, highly suggestive of an underlying etiology. Patient clinical and laboratory workup revealed the absence of an autoimmune disease but the presence of a subclinical cytomegalovirus (CMV) infection. Therefore, we highlight that the identification of TRIs is a useful indicator of systemic interferon activity. In the present case, the unusual location of numerous TRIs was associated with a subclinical CMV infection in an immunocompetent patient.
Keywords: CMV infection, FSGS, interferon footprints, tubuloreticular structures
Introduction
Tubuloreticular inclusions (TRIs) are distinctive intracellular structures seen at ultrastructural examination, principally within the cytoplasm of endothelial cells and lymphocytes. Their presence has been shown to be a marker of systemic stimulation by interferons. The identification of large numbers of TRIs in renal biopsies may be useful to raise diagnostic suspicion of autoimmune diseases and viral infections. The presence of TRIs in the collapsing variant of focal segmental glomerulosclerosis has been strongly linked to HIV infection/AIDS. The occurrence of TRIs in other forms of FSGS and the diagnostic implications is less well recognized. We present an interesting case of a patient with massive proteinuria and nephrotic syndrome with a tip-variant FSGS associated with a viral infection.
Case report
Clinical history
A 61-year-old Chinese female presented to the emergency room with a 2-week history of malaise and lower extremity edema of acute onset. She denied any history of arthralgias, skin rash or alopecia. She denied being recently exposed to Chinese herbal teas containing Panax ginseng. She had no family history of kidney or autoimmune disease. At the time of presentation, her only medications were calcium and vitamin D supplements for osteoporosis. Review of systems was irrelevant.
In the emergency room, BP was 155/90 mmHg, HR 81, RR 18, oxygen saturation 97%, temperature 36.6°C and weight 50 kg. The physical exam was unremarkable except for bilateral pitting edema up to the mid-thigh.
Initial laboratory data
Urinalysis by dipstick resulted in 4+ protein, no blood and negative urine microscopy. There was mild normocytic anemia (Hb 11.7 g/dL), low white cell count (2.8 × 103/mm3), lymphopenia (0.84 × 103/mm3) and normal platelet count. The sCr was 0.84 mg/dL (74 μmol/L): random glucose 104 mg/dL (5.8 mmol/L), albumin 1.8 g/dL, and cholesterol 324 mg/dL (8.3 mmol/L). The electrolytes were normal and hepatic transaminases, alkaline phosphatase and bilirubin were unremarkable. A 24-h urine collection revealed protein 16.7 g. Anti-nuclear antibody was negative, C3 and C4 normal, anti-double-stranded DNA negative and serum protein electrophoresis was normal. Serology for hepatitis B and C was normal. Abdominal ultrasound showed kidneys of normal size and echo texture without intra-abdominal abnormalities. Serum samples for viral screening (including HIV and CMV) were taken and results were pending. A kidney biopsy was performed.
Kidney biopsy
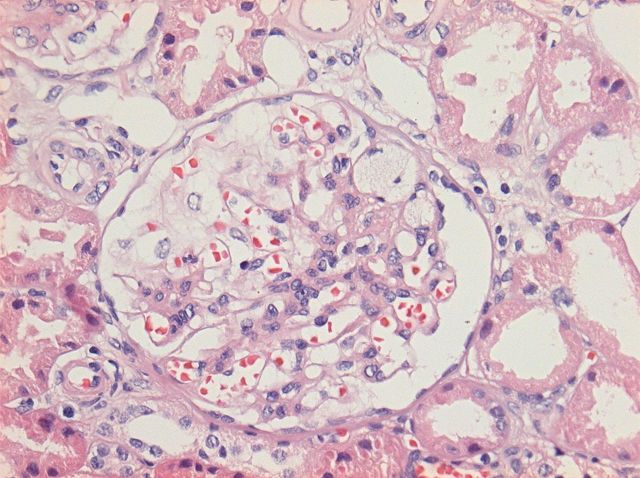
Four cores of the renal cortex were examined by a light microscopy. A sample contained 16 glomeruli, none of which were globally sclerosed. Glomeruli were slightly enlarged with prominent podocytes. Areas of early segmental sclerosis located at the tip of the glomeruli were present in two glomeruli, one of which included intraluminal foam cells (Figure 1). The tubulointerstitium was well preserved with mild tubulointerstitial fibrosis and tubular atrophy affecting <20% of the tissue. No arteriolar hyalinosis or arterial sclerosis was identified.
Fig. 1.
Detail of a glomerulus showing a segmental area of scarring located at the tip with adhesions to the Bowman's capsule. Foam cells are present within the sclerosed capillary loops. The remaining glomerular tuft shows open capillary loops without endocapillary hypercellularity (HE staining and ×200).
Immunofluorescence showed positivity for IgM and C3 in areas of segmental scarring in one glomerulus. There was no significant staining for IgG, IgA, C1q, kappa or lambda. Numerous albumin droplets were present within the tubular epithelium.
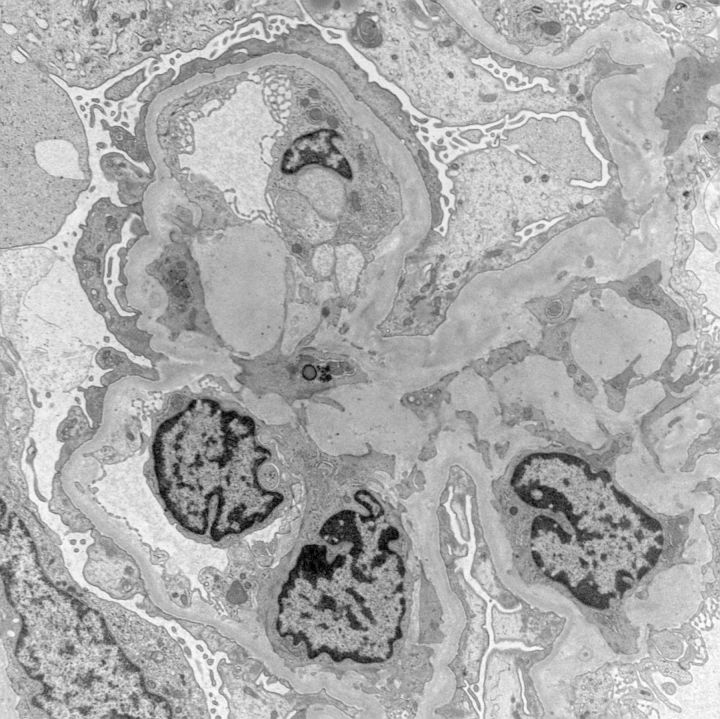
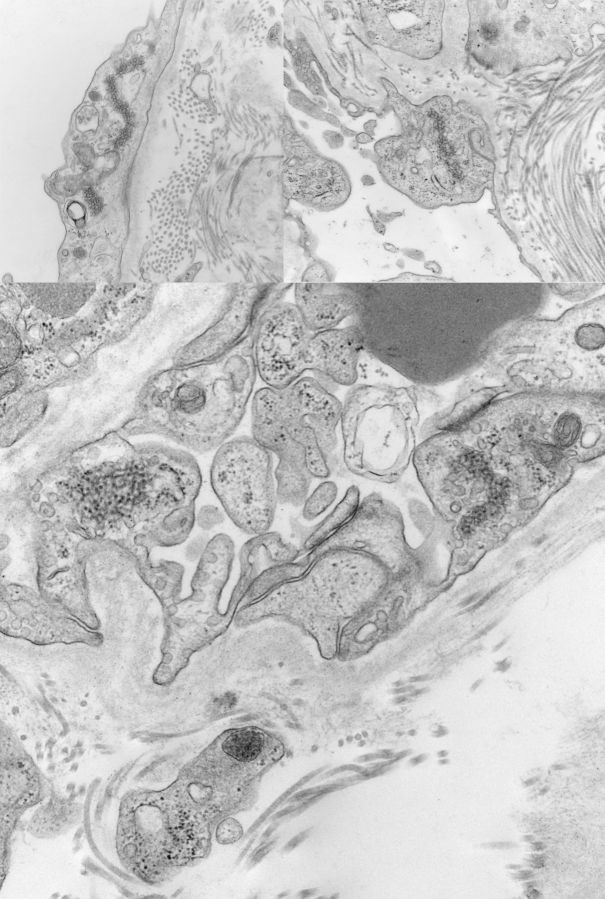
Electron microscopy was performed on two glomeruli and revealed extensive damage to the podocyte with marked foot-process effacement (Figure 2) and microvillus degeneration. Of interest, numerous TRIs were identified within glomerular endothelial cells, the endothelium of interstitial peritubular capillaries, as well as in interstitial cells (Figure 3). Focal endothelial cell swelling within capillary loops was also noted. Mesangial areas were unremarkable. No immune-type electron-dense deposits were seen. The glomerular basement membranes were of normal thickness.
Fig. 2.
Ultrastructural examination of the glomeruli reveals diffuse foot-process effacement. Capillary loops exhibit mild endothelial cell swelling with the absence of normal fenestra. Prominent endothelial cells contain tubuloreticular inclusions. There is widespread podocyte damage with focal microvillus transformation (electron microscopy photograph, magnification ×8500).
Fig. 3.
Ultrastructural examination of three different areas of the tubulointerstitium reveals numerous tubuloreticular inclusions in the cytoplasm of the endothelial cells lining the peritubular capillaries. Pericapillary bundles of collagen are present (electron microscopy photographs, magnification ×43 000).
A diagnosis of focal and segmental glomerulosclerosis located at the tip was performed with a note suggesting the work up of the patient for viral infection or autoimmune disease.
Clinical follow-up
Results of viral screening were received. HIV and parvovirus B19 were negative. CT scan of the thorax, abdomen and pelvis was negative for malignancy. Oral prednisone 40 mg daily and diuretics were started. Response to therapy was rapid. Serum albumin rose from a nadir of 1.3–2.6 g/dL after 2 weeks of treatment to 3.4 g/dL after 1 month. Urinary protein-to-creatinine ratio fell from 16.35 g/g (1848 mg/mmol) prior to treatment to 0.54 g/g (62 mg/mol) after 1 month. There was rapid and complete resolution of edema, and diuretics were discontinued. Polymerase chain reaction (PCR) for CMV DNA (part of the virological work-up) was noted to be positive (445 copies/mL) soon after prednisone was started. Since the count was low in the absence of symptomatic CMV disease, and the patient was improving clinically, corticosteroids were continued and CMV activity monitored every 15 days. The CMV PCR initially increased to 3480 but then fell to <100 copies of DNA per mL 1 month after prednisone was started. The patient has never manifested clinical evidence of active CMV disease. The patient was given prednisone (12 weeks), with a slow taper (12–16 weeks).
Discussion
TRIs/structures were first described in the renal biopsies of patients with SLE [1] considered to be viral particles because of their resemblance to paramyxovirus [1, 2]. The term ‘tubuloreticular structures’ was first suggested by A.J. Dalton in 1971 [2]. This distinctive 23–25 nm subcellular organelle is outlined by anastomosing tubular structures. Commonly located in the cisternae of the endoplasmic reticulum, they have also been identified in the cytoplasm, perinuclear spaces and nuclei of cells [2]. Various morphologies (loose interwoven tubules to very orderly patterns of paracrystalline conformation) have been described. TRIs are seen mainly in endothelial cells and lymphocytes of many tissues including the kidney, liver, lungs, skin, muscle and eyes [2–4].
TRIs are frequently identified in patients with autoimmune diseases (SLE, DLE, chronic polymyositis, dermatomyositis, scleroderma, Sjögren's) and viral infections (HIV, hepatitis B and C) [2]. In addition, its presence has been observed in tumors, especially sarcomas (like Kaposi's) [5], other diseases (infectious mononucleosis, histiocytosis X) and renal allografts. TRIs are almost always seen in the tissues of patients with HIV/AIDS, sometimes even before the diagnosis/manifestation of the disease [6].
The mechanisms by which TRIs form have been elucidated through the study of lymphoblastic cell lines in response to elevated alpha- and beta-interferon levels [7] or in response to exogenous gamma-interferons [8]. Interferon levels display a log linear relationship to the number of TRIs based on the dose and duration of exposure [7]. The presence of TRIs is a marker of systemic stimulation by endogenous or exogenous interferons, as interferon ‘footprints’ [3].
In the kidney, TRIs are frequently seen in the glomerular endothelial cells of renal allografts and in the native kidneys of patients with autoimmune diseases [2], viral infections [7] and in those patients receiving interferon therapy [8]. Moreover, Chinese licorice root, Astragalus Henrii, Panax ginseng and other variants are shown to enhance the ability to resist microbial infections via TLR-4 that leads to high blood levels of interferons [9]. In the collapsing variant of FSGS, the identification of TRIs has been strongly associated with HIV infections [10].
The presence of TRIs is much higher in patients with HIV, systemic lupus erythematosus (SLE) and in those on interferon therapy (80–90%) compared with the general patient population (<5%) [8]. In patients with membranous nephropathy, the presence of TRIs has been associated with secondary forms (autoimmune disease, hepatitis, H. pylori infection, diabetes and lymphoma) [11]. To date, the presence of TRIs in the endothelial cells of interstitial capillaries has only been associated with HIV disease [10].
CMV is a highly prevalent, globally occurring infection that rarely elicits symptomatic disease in healthy/immunocompetent hosts. The most striking aspect of the immunobiology of CMV is the dimension of the cellular response elicited by the host where the virus efficiently adapts to its immune system. As a result, CMV is never eliminated from the infected immunocompetent individual, causing persistent asymptomatic infection [12]. CMV is able to infect several cell types, including endothelial cells, epithelial cells (plus retinal cells), smooth muscle cells, fibroblasts, leukocytes and dendritic cells. It has not been demonstrated in the podocyte but the mechanism for podocyte damage in cases of FSGS associated with CMV infection may be linked to the size of the CMV-specific T-cell response [12] and not as a result of a direct podocyte infection. The histological analysis of the present case revealed the presence of a tip-variant of FSGS. The possible relationship between minimal-change disease (MCD) and the tip-variant of FSGS has been recently noted by different authors [13]. MCD and the tip-variant of FSGS are very unique forms of podocytopathy that share, among other characteristics, the abrupt onset and the sensitivity to steroids. Almost 40 years ago, and based only on clinical observations, Shalhoub proposed that MCD is a systemic disorder of T cell function and cell-mediated immunity [14]. Although not fully understood, in support of this hypothesis, a number of functional assays for T cells have been proved to be abnormal in some patients with active MCD. Using more contemporary technology, it has been shown that T-cell genes are up-regulated during relapse of MCD, findings that may provide objective support for the old Shalhoub hypothesis [14]. Based on those findings, it is possible to propose that there may be a link between CMV infection in an immunocompetent host, T-cell response and podocyte damage. It has been demonstrated that in healthy subjects with CMV infection the frequencies of CMV-specific T cells are much higher than those observed for other human viruses (e.g. influenza, adenovirus, poxvirus, measles, mumps and other herpes viruses) and are only similar to those of HIV-specific T cells in active HIV infection [12]. This epiphenomenon may have relevance in the development of podocyte damage associated with CMV infection.
The diagnosis of viral-related nephropathies requires a high index of clinical suspicion [15]. CMV systemic infection is an uncommon cause of nephropathy.
The association of CMV systemic infection with FSGS was previously reported in the renal allograft in a patient with CMV viremia and nephrotic syndrome. Similar to the findings in our case, there were no CMV viral cytopathic changes, however, ultrastructural examination revealed numerous endothelial TRIs and severe foot process effacement [16]. There are also a few case reports of CMV infection being linked to collapsing glomerulopathy, the most aggressive variant of primary podocyte disease, in immunocompetent non-transplant patients [16]. The time course of the appearance of CMV-specific adaptive immune responses is difficult to follow in asymptomatic healthy subjects, since the time of the onset of primary infection typically goes unnoticed.
Conclusions
Our case highlights the finding of TRIs to link the possible association of CMV infection in an immunocompetent non-transplanted patient with massive proteinuria, subtle lymphopenia and a biopsy showing FSGS /diffuse podocyte damage. The marked presence of TRIs, not only in glomerular endothelial cells but also in the endothelium of interstitial capillaries and in interstitial cells, was particularly notable and suggestive of an underlying etiology. The identification of TRIs therefore served as an indicator of systemic interferon activity in the present case most likely due to CMV infection. CMV infection triggers an exaggerate T cell response that may be linked with the development of the podocyte injury.
In conclusion, we note that the presence of TRIs is an indicator of systemic interferon activity that may raise specific questions about the etiology of the glomerular disease.
Also, CMV infection in healthy subjects is usually asymptomatic making the diagnosis unnoticed but activating innate immunity through type I IFN, and specific immunity thought an exaggerated T-cell response.
The T-cell response in CMV infection is only comparable with HIV infection and may be linked to podocyte damage as an epiphenomenon.
Acknowledgements
The authors would like to thank Maria Gliniecki and Lucy Andrighetti for their expertise in the electron microscopy tissue processing and excellent ultrastructural photographs.
References
- 1.Gyorkey F, Sinkovics JG, Min KW, et al. Systemic lupus erythematosus and myxovirus. N Engl J Med. 1969;280:33. doi: 10.1056/nejm196902062800620. [DOI] [PubMed] [Google Scholar]
- 2.Schaff Z, Heine U, Dalton AJ. Ultramorphological and ultracytochemical studies on tubuloreticular structures in lymphoid cells. Cancer Res. 1972;32:2696–2706. [PubMed] [Google Scholar]
- 3.Chander P, Soni A, Suri A, et al. Renal ultrastructural markers in AIDS-associated nephropathy. Am J Pathol. 1987;126:513–526. [PMC free article] [PubMed] [Google Scholar]
- 4.Maturi RK, Font RL. Ultrastructural features and prevalence of tubuloreticular structures in the ocular vasculature of patients with AIDS: a study of 23 cases. Br J Opthalmol. 1996;80:252–255. doi: 10.1136/bjo.80.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marquart KH. Occurrence of tubuloreticular structures and intracisternal paracrystalline inclusions in endothelial cells of tissue from different epidemiological types of Kaposi's sarcoma. Ultrastruct Pathol. 2005;29:85–93. doi: 10.1080/01913120590912205. [DOI] [PubMed] [Google Scholar]
- 6.Kostianovsky M, Kang YH, Grimley PM. Disseminated tubuloreticular inclusions in acquired immunodeficiency syndrome (AIDS) Ultrastruct Pathol. 1983;4:331–336. doi: 10.3109/01913128309140585. [DOI] [PubMed] [Google Scholar]
- 7.Grimley PM, Rutherford MN, Kang YH, et al. Formation of tubuloreticular inclusions in human lymphoma cells compared to the induction of 2′-5′-oligoadenylate synthetase by leucocyte interferon in dose-effect and kinetic studies. Cancer Res. 1984;44:3480–3488. [PubMed] [Google Scholar]
- 8.Markowitz GS, Nasr SH, Stokes MB, et al. Treatment with IFN-{alpha},-{beta}, or - {gamma} is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:607–615. doi: 10.2215/CJN.07311009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakaya T, Kita M, Kuriyama H, et al. Panax Ginseng induces production of proinflammatory cytokines via Toll-like receptors. J Interferon Cytokine Res. 2004;24:93–100. doi: 10.1089/107999004322813336. [DOI] [PubMed] [Google Scholar]
- 10.Laurinavicius A, Rennke HG. Collapsing glomerulopathy: a new pattern of renal injury. Semin Diagn Pathol. 2002;19:106–115. [PubMed] [Google Scholar]
- 11.Yang AH, Lin BS, Kuo KL, et al. The clinicopathological implications of endothelial tubuloreticular inclusions found in glomeruli having histopathology of idiopathic membranous nephropathy. Nephrol Dial Transplant. 2009;24:3419–3425. doi: 10.1093/ndt/gfp288. [DOI] [PubMed] [Google Scholar]
- 12.La Rosa C, Diamond DJ. The immune response to human CMV. Future Virol. 2012;7:279–293. doi: 10.2217/fvl.12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Agati Pathologic classification of focal segmental glomerulosclerosis. Sem Nephrol. 2003;23:117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 14.Cunard R, Kelly CJ. T cells and minimal change disease. J Am Soc Nephrol. 2002;13:1409–1411. doi: 10.1097/01.asn.0000016406.82019.b3. [DOI] [PubMed] [Google Scholar]
- 15.Avila-Casado C, Fortoul TI, Chugh SS. HIV-associated nephropathy: experimental models. Contrib Nephrol. 2011;169:270–285. doi: 10.1159/000320212. [DOI] [PubMed] [Google Scholar]
- 16.Grover V, Gaiki MR, DeVita MV, et al. Cytomegalovirus-induced collapsing focal segmental glomerulosclerosis. Clin Kidney J. 2013;6:71–73. doi: 10.1093/ckj/sfs097. [DOI] [PMC free article] [PubMed] [Google Scholar]