Abstract
A 36-year-old male presented with a secondary, but anti-neutrophil cytoplasmic antibody (ANCA) (proteinase-3) positive, vasculitis with renal insufficiency due to a pauci-immune necrotizing glomerulonephritis. An infective process was initially excluded by blood cultures and an echocardiogram prior to immunosuppression. The patient's condition failed to improve and re-evaluation confirmed infective endocarditis requiring valve replacement. Subsequent tissue cultures identified Bartonella henselae. Antibiotic treatment led to full resolution of physical, biochemical and immunological markers. This is the first case of B. henselae endocarditis-associated ANCA positivity with a pauci-immune glomerulonephritis. It demonstrates the importance of revisiting standard investigations in patients not improving expectantly on conventional therapy.
Keywords: antineutrophil cytoplasmic antibodies, Bartonella henselae, cat-scratch disease, culture-negative endocarditis
Introduction
A variety of conditions may cause true- and false-positive anti-neutrophil cytoplasmic antibody (ANCA) results thus complicating identification of primary and secondary vasculitis [1, 2]. Malignancy, inflammation and infections, including infective endocarditis (IE), may not only be associated with anti-PR3/MPO positivity, but may also mimic the clinical picture of small-vessel vasculitis (SVV) [3].
The identification of secondary vasculitis is not academic: immunosuppression is likely to exacerbate any underlying disease.
We report the first case of ANCA-positive SVV secondary to Bartonella henselae. This case illustrates the importance of a methodical approach to the exclusion of secondary vasculitis and vigilance in patients not responding to conventional therapy.
Case report
A 36-year-old male presented with a cough responsive to doxycycline. Eight weeks later, he developed arthralgia, myalgia and an acral purpuric rash. He described a fever, epistaxes and weight loss of 10 kg over 3 months. He had occasional macroscopic haematuria. Systematic enquiry was otherwise unremarkable. There was no history of recent dental procedure, foreign travel or of prescribed/recreational drug use. He owned a cat (Felis catus).
The patient was normotensive and afebrile. He had non-blanching, palpable purpura on his legs with no other systemic signs. His jugular venous pressure was 3 cm, and he had pitting oedema. He had a grade 2/6 ejection systolic murmur. Chest, abdominal and neurological examinations were normal.
Serum biochemistry showed a creatinine of 186 µmol/L (eGFR 37 mL/min). The haemoglobin was 104 g/L with normal indices. The white cell count (WCC) was 10.7 × 109/L with a normal differential. The CRP was 13 mg/L (normal <10 mg/L). Immune serology showed a positive rheumatoid factor of 664 U/mL (normal 14–60 U/mL) and a PR3-specific ANCA of 26 U/mL (normal <6 U/mL). There was neither serum nor urine paraprotein and hepatitis serology was negative. Urinalysis demonstrated blood and protein. The chest roentgenogram was normal. An echocardiogram showed a bicuspid aortic valve with moderate regurgitation and no vegetations. Blood cultures were negative.
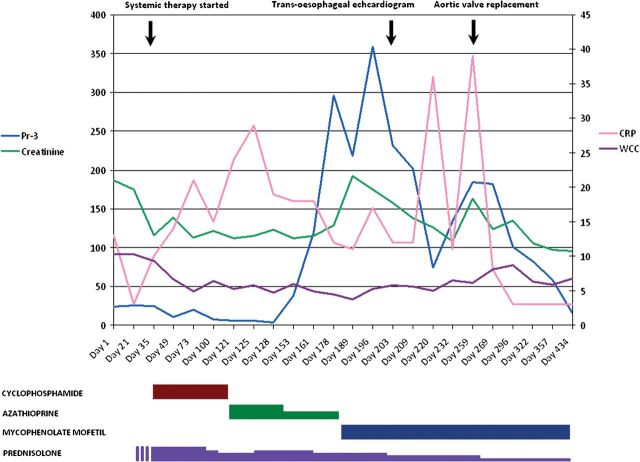
A renal biopsy showed pauci-immune necrotizing glomerulonephritis—consistent with SVV—and the patient was treated with prednisolone and intravenous cyclophosphamide (Figure 1).
Fig. 1.
Timeline of immunosuppressive therapy in relation to disease activity and clinical events.
The serum creatinine reduced to 113 µmol/L and ANCA normalized to 3.2 U/mL over 4 weeks, albeit with a weak positive indirect immunofluorescence result. These improvements paralleled a clinical improvement with resolution of the rash and myalgia.
Four months after induction, the patient was changed to azathioprine plus oral prednisolone. Within 2 weeks, the rash recurred accompanied by haemoptysis. The anti-PR3 increased to 38 U/mL. Azathioprine was stopped because of neutropaenia. Recovery of the WCC was associated temporally with an increased ANCA to 119 U/mL, and the patient was treated with mycophenolate mofetil (MMF) and an increased dose of prednisolone.
The patient's condition declined with persistent systemic symptoms, worsening renal function and a further increase in ANCA to 359 U/mL.
Echocardiography showed a significant deterioration in the aortic valve competence with a vegetation on one cusp. Multiple blood cultures were negative, and the patient was treated with broad-spectrum antibiotics (benzyl penicillin, gentamicin and flucloxacillin) for culture-negative endocarditis. Five days later, our patient had successful placement of a mechanical aortic valve. Steroids and MMF were continued.
Culture of the excised aortic valve was positive for B. henselae. Bartonella serology on stored serum samples from presentation was strongly positive (titre >1:128), indicating extant infection.
The patient was changed over to a combination of gentamicin and doxycycline, and he completed 6 weeks of this. Systemic symptoms resolved with a reduction in CRP (45 to 3 mg/L) and ANCA (359 to 15 U/mL). Maintenance immunosuppression was reduced to prednisolone 5 mg and MMF 500 mg twice daily.
Discussion
ANCA serology is an important screening investigation for primary vasculitis with renal disease [4]. Although high concentrations of ANCA are reliably associated with SVV, a renal biopsy is necessary to prove SVV. ANCA positivity is seen in several inflammatory and infective conditions. IE may mimic primary vasculitis both serologically and clinically [3, 5]. Renal histology in IE typically shows an immune-mediated focal segmental or endoproliferative glomerulonephritis and clandestine IE cases may be exposed by this investigation[3, 6].
Cat-scratch disease has been described since the early 20th century. The causative organism, B. henselae, proved as elusive as its host and was only identified in 1988. The clinical course of the disease is characteristically benign starting with a papule at the site of inoculation within 3–8 days. Regional, tender, lymphadenopathy develops 2 weeks later and typically resolves in 1–4 months. In children, immunosuppressed and, rarely, immunocompetent individuals, systemic disease may include ocular, hepatic, splenic and central nervous system involvement. IE secondary to B. henselae, and other species in the genus, is very rare and is a particular diagnostic challenge. It classically presents as a culture-negative endocarditis. The six species of Bartonella spp. are fastidious and even specialized laboratory conditions struggle to yield a positive culture. Only 25% of cases are associated with positive cultures. Over 95% of cases of IE are caused by either Bartonella quintana or, more rarely, B. hensalae and >60% of cases involve the aortic valve with over 55% of these having a pre-existing valvular abnormality: the majority of patients require surgical intervention with valve replacement [7]. Antibiotic treatment is indicated only in systemic disease or in patients with severe symptoms. Rifampicin, ciprofloxacin, gentamicin and trimethoprim/sulfamethoxazole are considered most effective. Penicillins are generally ineffective.
We report the first case of B. henselae-associated IE presenting with ANCA-positive SVV. Although our patient did not report any of the primary features of cat-scratch disease described above, it is almost certain that the disease would have completed its course subclinically before his presentation to us. Despite preliminary investigations to rule out conventional IE early on in the course of the disease, the diagnosis of an underlying secondary vasculitis initially eluded us. In particular, the pauci-immune renal histology did not suggest a secondary endoproliferative process that would increase the suspicion of an atypical IE. It is likely that immunosuppression resulted in more rapid progression of the aortic valve disease necessitating aortic valve surgery but also giving an opportunity to make a definitive diagnosis. The patient's grumbling disease activity, despite good doses of conventional immunosuppression (sufficient to cause neutropaenia), should have been a reasonable indication for a second survey to exclude other disease processes.
This is the only case of B. henselae IE associated with ANCA-positive pauci-immune glomerulonephritis recorded. Bartonella quintana caused a similar clinical presentation with cytoplasmic ANCA positivity in a single case report [8]. However, the renal biopsy showed focal glomerular sclerosis and immunohistochemistry showed complement deposition. This patient underwent aortic valve replacement for antibiotic-refractory endocarditis.
The relationship of Bartonella infection to vasculitis in our patient is supported by the temporal association of two rare conditions, the improvement in our patient's clinical condition and disease activity assessments after successful treatment of the valve infection and by serology at presentation. In the medium term, immunosuppression will be reduced/stopped subject to clinical and serological monitoring.
This case illustrates the importance of screening for IE in patients with apparent primary SVV. The possibility of an atypical IE should be re-considered in all patients with an incomplete response to therapy.
Conflict of interest statement
None declared. I confirm that this paper has not been published in part or whole in the past.
References
- 1.Solberg IC, Lyrgen I. Predictive value of serologic markers in a population-based Norwegian cohort with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:406–414. doi: 10.1002/ibd.20781. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ. Antineutrophil cytoplasmic antibodies and related diseases: a review. AJKD. 1990;6:517–529. doi: 10.1016/s0272-6386(12)80521-x. [DOI] [PubMed] [Google Scholar]
- 3.Chirinos JA, Corrales-Medina VF, Garcia S, et al. Endocarditis associated with ANCA: a case report and review of the literature. Clin Rheumatol. 2007;26:590–595. doi: 10.1007/s10067-005-0176-z. [DOI] [PubMed] [Google Scholar]
- 4.Harper L, Savage CO. Pathogenesis of ANCA-associated systemic vasculitis. J Pathol. 2000;190:349–259. doi: 10.1002/(SICI)1096-9896(200002)190:3<349::AID-PATH524>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 5.Beauvillain C, Delneste Y. Antineutrophil cytoplasmic antibodies: how should the biologist manage them? Clin Rev Allergy Immunol. 2008;35:47–58. doi: 10.1007/s12016-007-8071-9. [DOI] [PubMed] [Google Scholar]
- 6.Choi HK, Lamprecht P, Niles JL, et al. Subacute bacterial endocarditis with positive cytoplasmic ANCA and anti PR-3 antibodies. Arthritis Rheum. 2000;43:226–231. doi: 10.1002/1529-0131(200001)43:1<226::AID-ANR27>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Fournier PE, Lelievre H, Eykyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella Quintana and Bartonella Henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 2001;80:245–251. doi: 10.1097/00005792-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama H, Sahara M. Infective endocarditis by Bartonella quintana masquerading as antineutrophil cytoplasmic antibody associated small vessel vasculitis. Cardiology. 2009;114:208–211. doi: 10.1159/000228645. [DOI] [PubMed] [Google Scholar]