Abstract
Background
Low serum sodium (Na) has been associated with decreased body mass index and increased cardiovascular mortality in haemodialysis (HD) patients. We examined the relationship between serum Na and selected nutritional parameters of protein energy wasting that are not affected from the hydration status in a cohort of HD patients.
Methods
Triceps skinfold thickness (TSF), mid-arm circumference (MAC), mid-arm muscle circumference (MAMC), handgrip strength (HGS) and subjective global assessment (SGA) were assessed in maintenance HD patients using standard techniques. MAMC was calculated with the formula MAMC (cm) = MAC (cm) −3.142 × TSF cm. Pre-dialysis serum Na values from routine monthly laboratory measurements were averaged for the last 6 months prior to the nutritional assessment.
Results
Altogether 172 patients with anthropometric data were included in the final analysis. Mean age was 66 ± 14, females 62 (36%) and diabetics 48 (28.9%). Patients with pre-dialysis serum Na below the mean value (136.2 mEq/L) had lower MAMC, HGS, SGA scores and albumin levels (23.50 ± 3.16 cm versus 24.58 ± 3.71 cm, P = 0.048; 21.7 ± 13.6 kg versus 28.0 ± 12.4 kg, P = 0.030; 5.1 ± 1.2 versus 5.7 ± 1.0, P = 0.012 and 31.65 ± 4.73 mg/L versus 32.25 ± 3.91 mg/L, P = 0.022, respectively) and higher interdialytic weight gains. Pre-dialysis serum Na correlated positively with MAMC, handgrip and SGA (Pearson's correlation r = 0.165, P = 0.031, r = 0.237, P = 0.022 and r = 0.195, P = 0.011, respectively).
Conclusion
This study demonstrates that low serum sodium is associated with protein energy wasting and increased interdialytic weight gain in HD patients.
Keywords: hyponatraemia, interdialytic weight gain, malnutrition, MAMC, thirst
Introduction
Recent studies have revealed an association between lower pre-dialysis serum sodium levels and increased all-cause and cardiovascular mortality in haemodialysis (HD) patients [1, 2]. In these studies, hyponatraemia was also associated with low serum albumin, low body mass index and high interdialytic weight gain.
Protein energy wasting and malnutrition are common in patients with chronic kidney disease stage 5. It has been found to be present in 30–40% of patients [3] and has been associated with increased cardiovascular mortality [4] and in particular sudden cardiac death [5] in dialysis patients. Nutritional status is best assessed using a combination of measures on a regular basis as recommended by national guidelines [6]. Upper arm anthropometry measurements [7], handgrip strength (HGS) [8] and subjective global assessment (SGA) [9] are clinically useful measures of nutritional status and better surrogates of malnutrition and protein energy wasting compared with body mass index in maintenance HD patients as they are independent of hydration status. Though protein energy malnutrition and hyponatraemia are both related to low-grade systemic inflammation, the association between them has not been investigated.
The aim of our study was to investigate the relationship between serum Na levels and protein energy wasting as measured by upper arm anthropometry, HGS and SGA in a cohort of HD patients.
Materials and methods
Study population
We studied 182 HD patients treated across dialysis units affiliated with St George's Healthcare NHS Trust and had a nutritional assessment completed (SGA, MAC, TSF, HGS) in 2010 and/or 2011. All subjects received 4-h bicarbonate-based dialysis treatment thrice weekly according to the local protocols and national guidelines and had been established with HD for 6 months. All data analysed were collected as part of routine clinical audit.
Data collection
Subjective global assessment
SGA was used to evaluate the overall protein energy nutritional status of each patient. It comprises six assessments, three based on body weight change, food intake and gastrointestinal symptoms, and three based on physical assessments looking at muscle mass, subcutaneous fat and presence of oedema. An overall subjective score is given for each patient (between 1 and 7: score 1–2 = severe malnutrition, 3–5 = mild to moderate malnutrition and 6–7 = normal nutritional state to mild malnutrition).
Anthropometric measurements
A single mid-upper arm circumference (MAC) was obtained from the non-fistula arm of each patient. The lateral part of the upper arm was marked at the midpoint between the acromion process and the end of the humerus. The circumference was measured to the nearest millimetre at the marked midpoint.
The tricep skinfold thickness was measured by pinching the fold of skin and subcutaneous tissue (without underlying muscle) formed over the tricep muscle with the thumb and index finger at the mid-point of the upper arm. The thickness of the fold was measured using steel callipers to the nearest millimetre.
The mid-arm muscle circumference (MAMC) was derived from MAC and TSF as follows: MAMC (cm) = MAC (cm) −3.14 × TSF (cm). Results from anthropometry measures are reported in centimetres.
Techniques used for these two measurements were carried out according to Bishop et al. [10] as recommended by the British Dietetic Association.
HGS was measured using a dynamometre. The subjects were instructed to apply as much handgrip pressure as possible using the non-fistula hand. Measurements were repeated three times and the average score was recorded.
Observer variability and inter-observer error
SGA and anthropometry measurements were taken by the same dietitian. To test the standardization and reliability of the anthropometric measurements the inter observer error was assessed according to target values described by Zerfas [11]. The aim was to achieve a ‘fair’ to ‘good’ accuracy for arm circumference and skinfold measurements and this was achieved.
Weight laboratory and demographic data
Dry weight was defined by the attending physician in the electronic patient record. IDWG was defined as the difference between the pre-dialytic weight and weight at the end of the previous dialysis session. The ratio of IDWG and patient dry weight was defined as IDWG%. Weight and blood pressure data were averaged from three consecutive dialysis session starting from the beginning of the week closest to the anthropometric measurements.
Pre-dialysis serum sodium values from routine monthly laboratory measurements were averaged for 6 months prior to the measurement. Serum C-reactive protein (CRP) and serum albumin were measured from the closest to the anthropometric measurements pre-dialysis routine bloods. Demographic data were extracted from the electronic patient records. Patients on antidiabetic medications or with fasting plasma glucose ≥7.0 mmol/L and/or A1C ≥6.5% (48 mmol/mol) were classified as diabetics.
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics 19. The T-test (two sided, independent) was used for comparison of numerical means and chi-square test was used for comparison of categorical values; P < 0.05 was considered statistically significant. Comparisons were performed on data dichotomized using the mean value of pre-dialysis serum sodium (Na < 136.18 mEq/L). A Pearson correlation was used to measure the strength of the relationships between variables and a Pearson's partial correlation was used when controlling the effect of other variables.
Results
Altogether 172 patients had anthropometric data and were included in the final analysis. One hundred and fifty-one patients had SGA data and 84 handgrip data. Mean age was 66 ± 14, females 62 (36%), diabetics 48 (28.9%), 34 (21%) Asians, 56 (34%) Blacks, 56 (32.6%) Caucasians and 26 (15%) of other ethnical background. Baseline data are presented in Table 1.
Table 1.
Baseline characteristics
| Age | 66.1 ± 14 |
|---|---|
| Female | 62 (36%) |
| Dialysis vintage (months) | 30 |
| Diabetes mellitus | 48 (28.9%) |
| Ethnicity | |
| Asian | n = 34(19.8%) |
| Blacks | n = 56 (32.6%) |
| Caucasians | n = 56 (32.6%) |
| Other | n = 26 (15%) |
| TSF (cm) | 1.97 ± 0.83 |
| MAC (cm) | 30.3 ± 4.53 |
| MAMC(cm) | 24.1 ± 3.52 |
| Handgrip (kg) | 25 ± 13 |
| SGA | 5.4 ± 1.2 |
| Dry weight (kilograms) | 74.4 ± 17.54 |
| IDWG (kilograms) | 1.78 ± 1.04 |
| IDWG % | 2.45 ± 1.452 |
| Pre-HD SBP (mmHg) | 144.7 ± 34.9 |
| Pre-HD DBP (mmHg) | 68.8 ± 12.1 |
| Post-HD SBP (mmHg) | 136.1 ± 29.5 |
| Post-HD DBP (mmHg) | 64.2 ± 12 |
| Dry weight (kg) | 73.62 ± 15.4 |
| Albumin (g/L) | 32.53 ± 4.46 |
| C-reactive protein (mg/L) | 21.98 ± 40.8 |
| Serum Na (mEq/L) | 136.2 ± 2.25 |
Data are expressed as means ± SD for numerical data.
DBP, diastolic blood pressure; HD, haemodialysis; IDWG, interdialytic weight gain; IDWG%, IDWG/dry weight, kg (kilograms); MAC, mid-arm circumference (cm); MAMC, mid-arm muscle circumference, MAC (cm) – 3.14 × TSF (cm); SGA, subjective global assessment; SBP, systolic blood pressure; TSF, triceps skinfold thickness (cm).
Patients with pre-dialysis serum Na less than the mean value (136.2 mEq/L) had lower MAMC, HGS and SGA scores and albumin levels and higher IDWG and IDWG%. There was no statistical significant difference in TSF and CRP between the two groups (Table 2).
Table 2.
Comparison between clinical and anthropometric parameters according to serum Na; dichotomized data according to mean pre-dialysis serum Na (136.2 mEq/L)
| Group 1 (n = 71) Na < 136.2 mEq/L |
Group 2 (n = 101) Na > 136.2 mEq/L |
P-value | |
|---|---|---|---|
| Serum Na (mEq/L) | 134.1 ± 1.8 | 137.7 ± 1.2 | N/A |
| Females (% of n) | 48 | 32 | 0.023 |
| Age | 68.5 ± 12.9 | 64.5 ± 15.2 | 0.046 |
| Diabetes (% of n) | 35.9 | 28.6 | 0.300 |
| MAMC (cm) | 23.50 ± 3.16 | 24.58 ± 3.71 | 0.048 |
| Handgrip strength(kg) | 21.7 ± 13.6 | 28.0 ± 12.4 | 0.030 |
| Triceps skin fold (cm) | 1.98 ± 0.78 | 1.95 ± 0.86 | 0.781 |
| SGA | 5.1 ± 1.2 | 5.7 ± 1.0 | 0.012 |
| Dry weight (kg) | 71.8 ± 14.0 | 75.0 ± 16.3 | 0.220 |
| Pre-HD SBP | 147.2 ± 44 | 142.3 ± 20.7 | 0.367 |
| Post-HD SBP | 139.2 ± 33.5 | 135.3 ± 24.2 | 0.437 |
| Pre-HD DBP | 67.9 ± 13.4 | 69.6 ± 11.1 | 0.427 |
| Post-HD DBP | 63.3 ± 16.2 | 65 ± 12.4 | 0.335 |
| IDWG (kg) | 2.0 ± 1.2 | 1.64 ± 2.0 | 0.022 |
| IDWG% | 2.88 ± 1.69 | 2.21 ± 1.19 | 0.007 |
| Albumin (g/L) | 31.65 ± 4.73 | 32.25 ± 3.91 | 0.022 |
| CRP (mg/L) | 24.06 ± 45.51 | 20.34 ± 36.94 | 0.576 |
Data are expressed as means ± SD for numerical values.
DBP, diastolic blood pressure; HD, haemodialysis; IDWG, interdialytic weight gain; IDWG%, IDWG/dry weight, kg (kilograms); MAC, mid-arm circumference (cm); MAMC, mid-arm muscle circumference, MAC (cm) – 3.14 × TSF (cm); n, number of subjects; SGA, subjective global assessment; N/A, not applicable; SBP, systolic blood pressure; TSF, triceps skinfold thickness (cm). Shaded areas highlight statistical significant differences.
Pre-dialysis serum Na correlated positively with MAMC, handgrip and SGA (Pearson correlation r = 0.165, P = 0.031, r = 0.237, P = 0.022 and r = 0.195, P = 0.011, respectively) but did not correlate with CRP or serum albumin. Scatterplots of correlations between pre-dialysis serum Na and MAMC and pre-dialysis serum Na and handgrip strength are shown in Figure 1. After controlling for CRP, the correlation persisted for SGA (r= 0.272, P = 0.045) but not for MAMC and handgrip. After controlling for age, sex and the presence of diabetes mellitus the correlation persisted for SGA (r = 0.315, P = 0.015) but not for MAMC and handgrip.
Fig. 1.
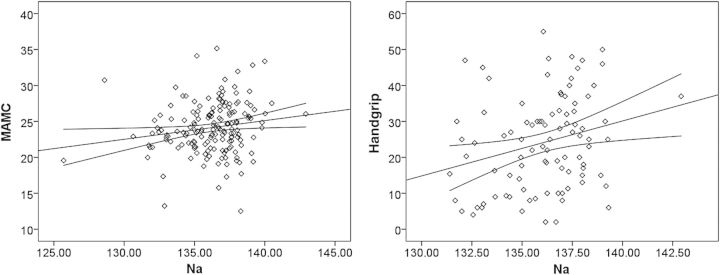
Scatter diagram showing the relationship between pre-dialysis serum Na (mEq/L) and mid-arm muscle circumference (MAMC) (A, left panel) and between pre-dialysis serum Na (mEq/L) and handgrip strength (kg) (B, right panel). Regression lines are plotted with their 95% confidence intervals.
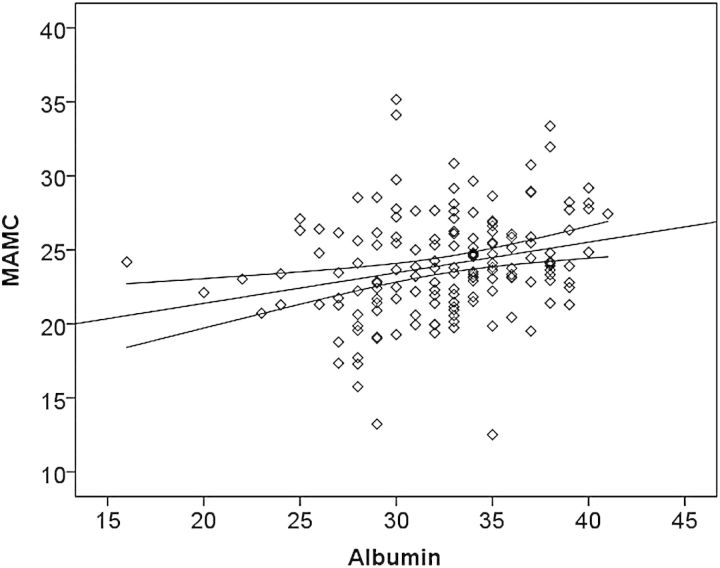
Serum albumin correlated positively with MAMC (Figure 2), handgrip and SGA (r= 0.254, P = 0.001, r= 0.283, P = 0.010 and r= 0.0194, P = 0.016, respectively) and inversely with CRP (r= −0.363, P = 0.000).
Fig. 2.
Scatter diagram showing the relationship between pre-dialysis serum albumin (g/L) and mid-arm muscle circumference (MAMC) (cm). Plotted regression line with its 95% confidence intervals.
Discussion
This study demonstrates a positive association between low serum sodium and decreased lean body mass. The relationship was attenuated after adjusting for CRP and remained significant only for SGA. We also showed that there is an inverse relationship between serum sodium and interdialytic weight gains.
Different possible mechanisms can explain the observed association between hyponatraemia and muscle wasting in dialysis patients.
Inflammation is a potential common pathogenetic pathway for the development of protein energy wasting and hyponatraemia. Inflammatory signalling in the hypothalamus has been suggested to mediate wasting in chronic diseases associated with low grade inflammation [12]. On the other hand hyponatraemia is a common finding in inflammation; an interaction between interleukin-6 and vasopressin-induced antidiuresis has been suggested as the underlying mechanism based on animal experimental data [13]. However, it should be highlighted that this mechanism cannot explain inflammatory hyponatraemia in HD patients as they are largely anuric and antiduresis cannot induce hyponatraemia. Homeostasis of serum sodium in dialysis patients is primarily regulated by the mechanism of thirst. A potential simultaneous inflammatory activation of the hypothalamic receptors that control thirst can be assumed as a cause of hyponatraemia in these patients. However, elucidating the potential link between inflammation and hyponatraemia in dialysis patients is challenging as many additional factors may affect the serum sodium levels. Our observations were limited to a small cohort of patients and although serum albumin was lower in the group of patients with lower sodium levels, the difference in CRP levels did not reach statistical significance and we did not find a direct correlation between Na and CRP or albumin in the total study population.
A second potential common mediator of muscle wasting and hyponatraemia is angiotensin II. Angiotensin II receptors type 1 are expressed in skeletal muscle and regulate its function, whereas elevated angiotensin II levels have been implicated in skeletal muscle atrophy [14]. On the other hand elevated angiotensin II levels have been associated with polydipsia in HD patients [15]. Inflammation remains a potential mediator as angiotensin II induces a proinflammatory state [16].
The primary role of thirst in achieving Na homeostasis in HD patients is clinically important. Although compliance with a strict low-salt diet and reduced interdialytic fluid intake is of paramount importance in HD patients, a dysregulation of Na homeostasis may pose a challenge in achieving targets in a subgroup of malnourished and inflamed patients, or even lead to iatrogenic large interdialytic weight gain due to the large gradient between serum and dialysate Na [17]. Large interdialytic weight gain is not a uniform phenomenon and can represent two opposite ends of the spectrum of inflammation malnutrition syndrome: cachectic inflamed subjects with associated hyponatraemia on one side and well-nourished patients with high-salt intake between dialysis treatments patients. This heterogeneity associated with large interdialytic weight gain explains the discrepancy between studies examining the relationship between IDWG and nutritional status [18–20]. Pre-dialysis serum sodium can help differentiate between these two different groups of patients and identify the subgroup of patients where dietary changes are more likely to be effective.
The emerging role of serum sodium in mortality and interdialytic weight gains has added one more level uncertainty to the relationship between interdialytic weight gain and mortality. It has been suggested that large interdialytic weight gain has a causal role in increasing cardiovascular mortality due to the resulting cardiovascular stress with a mechanism similar to heart failure [21] but recent studies have shown that the association between IDWG and cardiovascular mortality does not persist after adjusting for pre-dialysis serum Na [1] while reducing the IDWG by decreasing the sodium in the dialysate has not been proven effective in improving the outcomes and has even been associated with increased risk of adverse outcomes in retrospective epidemiological studies [22]. However, to our knowledge, there are no prospective controlled data assessing the value of adjusting the Na dialysate to minimize IDWG in hyponatraemic patients. Large IDWG may represent an epihenomenon of an underlying inflammatory condition that manifests amongst others with hypothalamic inflammation, protein energy wasting and hyponatraemia.
Limitations
There are several limitations of this study that should be considered. Firstly, it is a retrospective study in a small number of haemodialysis patients. Serum sodium levels are affected by a number of additional factors. Our study was not prospectively controlled and our sample was not sufficient to investigate the relationship between serum sodium and inflammation. In addition our analysis was limited to routinely collected laboratory parameters and we did not measure more sensitive markers of inflammation. Secondly, not all of our patients underwent a nutritional assessment (patients not giving consent, patients unstable or due to language barrier) and within the assessed patients we did not have SGA and handgrip data for every patient. HGS measurements may not be possible for other reasons affecting the dexterity of the hand needed for the movement other than forearm muscle strength such as hand arthritis and neuropathic pain. Patients with poor compliance to medical treatment are more likely to decline nutritional assessment and this may introduce selection bias in the analysed population. Finally, we used anthropometric data but did not perform bioelectrical impedance analysis and dual energy X-ray absorptiometry which are considered more accurate methods for the determination of body composition.
Conclusion
This is the first study to our knowledge to associate serum sodium with practical, broadly used and well-validated anthropometric measures of lean body mass.
Conflict of interest statement
None declared.
References
- 1.Waikar SS, Curhan GC, Brunelli SM. Mortality associated with low serum sodium concentration in maintenance hemodialysis. Am J Med. 2011;124:77–84. doi: 10.1016/j.amjmed.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hecking M, Karaboyas A, Saran R, et al. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: The dialysis outcomes and practice patterns study (DOPPS) Am J Kidney Dis. 2012;59:238–248. doi: 10.1053/j.ajkd.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Ikizler TA, Hakim RM. Nutrition in end-stage renal disease. Kidney Int. 1996;50:343–357. doi: 10.1038/ki.1996.323. [DOI] [PubMed] [Google Scholar]
- 4.de Mutsert R, Grootendorst DC, Axelsson J, et al. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant. 2008;23:2957–2964. doi: 10.1093/ndt/gfn167. [DOI] [PubMed] [Google Scholar]
- 5.Drechsler C, Grootendorst DC, Pilz S, et al. Wasting and sudden cardiac death in hemodialysis patients: a post hoc analysis of 4D (die deutsche diabetes dialyse studie) Am J Kidney Dis. 2011;58:599–607. doi: 10.1053/j.ajkd.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Wright M, Jones C. Renal association clinical practice guideline on nutrition in CKD. Nephron Clin Pract. 2011;118(Suppl 1):c153–64. doi: 10.1159/000328067. [DOI] [PubMed] [Google Scholar]
- 7.Stosovic M, Stanojevic M, Simic-Ogrizovic S, et al. The predictive value of anthropometric parameters on mortality in haemodialysis patients. Nephrol Dial Transplant. 2011;26:1367–1374. doi: 10.1093/ndt/gfq497. [DOI] [PubMed] [Google Scholar]
- 8.Chang YT, Wu HL, Guo HR, et al. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant. 2011;26:3588–3595. doi: 10.1093/ndt/gfr013. [DOI] [PubMed] [Google Scholar]
- 9.de Mutsert R, Grootendorst DC, Boeschoten EW, et al. Subjective global assessment of nutritional status is strongly associated with mortality in chronic dialysis patients. Am J Clin Nutr. 2009;89:787–793. doi: 10.3945/ajcn.2008.26970. [DOI] [PubMed] [Google Scholar]
- 10.Bishop CW, Bowen PE, Ritchey SJ. Norms for nutritional assessment of American adults by upper arm anthropometry. Am J Clin Nutr. 1981;34:2530–2539. doi: 10.1093/ajcn/34.11.2530. [DOI] [PubMed] [Google Scholar]
- 11.Zerfas AJ. Checking Continuous Measures. 1. Manual for Anthropometry. Los Angeles, CA, USA: UCLA Division of Epidemiology, School of Public Health; 1985. [Google Scholar]
- 12.Braun TP, Marks DL. Pathophysiology and treatment of inflammatory anorexia in chronic disease. J Cachexia Sarcopenia Muscle. 2010;1:135–145. doi: 10.1007/s13539-010-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swart RM, Hoorn EJ, Betjes MG, et al. Hyponatremia and inflammation: the emerging role of interleukin-6 in osmoregulation. Nephron Physiol. 2011;118:45–51. doi: 10.1159/000322238. [DOI] [PubMed] [Google Scholar]
- 14.Sukhanov S, Semprun-Prieto L, Yoshida T, et al. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci. 2011;342:143–147. doi: 10.1097/MAJ.0b013e318222e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graziani G, Badalamenti S, Del Bo A, et al. Abnormal hemodynamics and elevated angiotensin II plasma levels in polydipsic patients on regular hemodialysis treatment. Kidney Int. 1993;44:107–114. doi: 10.1038/ki.1993.219. [DOI] [PubMed] [Google Scholar]
- 16.Zhong J, Guo D, Chen CB, et al. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57:314–322. doi: 10.1161/HYPERTENSIONAHA.110.164244. [DOI] [PubMed] [Google Scholar]
- 17.Munoz Mendoza J, Sun S, Chertow GM, et al. Dialysate sodium and sodium gradient in maintenance hemodialysis: a neglected sodium restriction approach? Nephrol Dial Transplant. 2011;26:1281–1287. doi: 10.1093/ndt/gfq807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Gomez JM, Villaverde M, Jofre R, et al. Interdialytic weight gain as a marker of blood pressure, nutrition, and survival in hemodialysis patients. Kidney Int Suppl. 2005:S63–S68. doi: 10.1111/j.1523-1755.2005.09314.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang SC, Chiang CK, Hsu SP, et al. Relationship between interdialytic weight gain and nutritional markers in younger and older hemodialysis patients. J Ren Nutr. 2008;18:210–222. doi: 10.1053/j.jrn.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Chen YW, Chen HH, Pan CF, et al. Interdialytic weight gain does not influence the nutrition of new hemodialysis patients. J Ren Nutr. 2012;22:41–49. doi: 10.1053/j.jrn.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–679. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecking M, Karaboyas A, Saran R, et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol. 2012;7:92–100. doi: 10.2215/CJN.05440611. [DOI] [PMC free article] [PubMed] [Google Scholar]