Abstract
Introduction
Acute kidney injury (AKI) is a common clinical problem associated with adverse outcomes. This study identifies the incidence of AKI in two UK district general hospitals' without on-site renal services and assesses AKI management and level of nephrologist input.
Methods
The AKIN classification was used to identify 1020 AKI patients over 6 months. Data were collated on patient demographics, AKI management and referral to nephrology and intensive care services. Short/long-term renal outcomes were investigated. Patients were followed up for 14 months post-discharge.
Results
Incidence of hospital-based AKI was 6.4%. Mean patient age was 73 years. There was 28.1% acute in-hospital mortality with a further 21.6% 14-month mortality. Only 8.3% of patients were referred to nephrology services for in-hospital review, and only 8.1% had outpatient nephrology follow-up. Compliance with the AKI National Confidential Enquiry into Patient Outcomes and Deaths (NCEPOD) recommendations was poor with 32.8% of patients having renal imaging and 15% of patients having acid–base status assessed. NCEPOD compliance improved with nephrology input. Patients referred to nephrology were likely to be younger with pre-existing CKD and severe AKI. 10.5% of AKI episodes were unrecognized. Forty percent of those with unrecognized AKI, (compared with 15% of recognized AKI) developed de novo or progression of pre-existing CKD.
Conclusion
AKI in DGHs is mostly managed without nephrology input. There are significant shortcomings in AKI recognition and management in this setting. This is associated with poor mortality and long-term CKD. This study supports a need to improve the teaching and training of front-line medical staff in identifying AKI. Additionally, implementation of AKI e-alert systems may encourage early recognition and provide a prompt for renal referral.
Keywords: acute kidney injury, outcomes
Introduction
Acute kidney injury (AKI) is common in hospitalized patients and is associated with serious long-term adverse implications on patient outcome [1–4]. Clinically, AKI can easily be recognized through monitoring of urine output and analysis of simple serum biochemistry. In the UK, however, the AKI National Confidential Enquiry into Patient Outcomes and Deaths (NCEPOD) report published in 2009 reported major deficiencies in recognition and clinical care of patients with AKI [5]. In spite of this report and despite significant advances in health care, recent studies have, if anything, demonstrated an increasing incidence of AKI [2, 6–10]. At present, the reason for the rise of AKI in hospitalized patients and the influence of the publication of the AKI NCEPOD report in the management of AKI patients is not apparent. It is also not entirely clear whether involvement of specialist nephrology services improves outcomes in AKI. In this study, we identify and characterize a cohort of patients with AKI in two district general hospitals in the UK over a 6-month period. We study AKI management in these patients according to a number of NCEPOD criteria and investigate the level of nephrology input into management of patients with AKI.
Methods
The study was conducted over 6 months in two large district general hospitals (DGHs) in the UK. These hospitals have no on-site nephrology unit. Data were collected from electronic records of all adult medical and surgical patients admitted to the two DGHs that form the Aneurin Bevan Health Board in South-East Wales. These are Neville Hall Hospital (500 beds, 8 Critical Care beds) and Royal Gwent Hospital (774 beds, 16 Critical Care beds). Combined they serve an estimated population of 639 000. These hospitals also do not provide cardiothoracic surgery, neurosurgery, plastics or transplantation surgery.
Two nephrologists reviewed electronic biochemistry records of all medical and surgical admissions to these hospitals. All patients admitted between 11 July 2011 up to and including 15 January 2012 were included, unless already on maintenance renal replacement therapy. Admissions through general practice, accident and emergency and hospital outpatient clinics were included. AKI was defined according to the AKI Network classification, and creatinine criteria were used for identification of AKI [11]. Baseline serum creatinine (sCr) values for patients admitted with evidence of AKI on their serum biochemistry were determined through review of all sCr values taken from the patient over the preceding 12-month period. (All GP practices and peripheral hospitals within the Aneurin Bevan trust utilize a central laboratory for biochemical processing and hence all community and hospital blood tests were available for scrutiny in this assessment.) Baseline sCr for patients who developed AKI during their hospital stay was taken as sCr on admission, and confirmed to be representative of true baseline by review of 12 months prior results. Where no baseline sCr was available (49 patients), the percentage increase that defines AKI was calculated using the upper limit of normal laboratory reference range for sCr in males and females, respectively. In addition, for patients with unknown baseline values, we were able to chart the sCr after resolution of AKI, which further enabled approximation of baseline sCr and confirmation of true AKI. This method of identification of baseline sCr levels has been recommended in the recently published Kidney Disease Improving Global Outcomes (KDIGO) AKI guidelines [12].
Data were collated on age, sex, admission method, admitting specialty, length of hospital stay, intensive care unit (ITU) admission and length of ITU stay, in-hospital renal recovery and in-hospital mortality. Data were also collected on referral to renal services, days to renal review and/or transfer to renal unit. Recovery from an AKI episode was defined as achievement of a creatinine no longer in keeping with AKI definition (according to AKIN criteria). Patients were defined as having missed/unrecognized AKI if they were discharged from hospital with worsening renal function and with no AKI acknowledged in their hospital discharge letter.
Long-term mortality outcomes were assessed over 14 months from date of discharge. Development of de novo CKD and progression of pre-existing CKD was defined as an eGFR decline of >15% or fall in eGFR of >5 mL/min/year according to UK CKD guidelines and NICE CKD guidelines [13, 14]. CKD was identified either from electronic clinical letters, and/or from blood tests indicating that their baseline eGFRs were <60 mL/min according to the NICE CKD guidelines [14].
Statistical analysis was carried out using SPSS 20. Student's t-test, Pearson’s χ2 test and one-way ANOVA were used for analysis of normally distributed data. Otherwise, Mann–Whitney U-test, Wilcoxon signed-rank test and Kruskal–Wallis test were used. Continuous variables were described using mean and SD or median with interquartile range (IQR). P-values of <0.05 were deemed statistically significant.
Results
Patient characteristics
During the stated 6 months, there were 15 976 admissions to the two DGHs. Of these, 1020 patients with AKI were identified according to the AKIN criteria, giving an overall AKI incidence of 6.4%. The incidence of AKI admitted under medical specialties was 7% and under surgical specialties was 4.2%. Median number of in-patient days for the AKI admissions was 9 days (IQR 16 days, range 0–177 days). Six hundred and eighty-six (67.25%) patients developed AKI in the community and were admitted to hospital with AKI. The remaining 334 (32.75%) acquired AKI during hospitalization. The incidence of AKI was equal in males and females with 515 (50.5%) episodes in men and 505 (49.5%) episodes in women. Three hundred and twenty-three (31.7%) had acute on chronic kidney injury. These data are outlined in Table 1.
Table 1.
Patient demographics, AKI characteristics and in-hospital outcomes
| Sex, n (%) | Pre-existing CKD | ||
| Male | 515 (50.5) | Total, n (%) | 323 (31.7) |
| Female | 505 (49.5) | ||
| Age (mean ± SD) | |||
| CKD 1 and 2 | 17 (5.3) | ||
| All AKI | 73 ± 16.4 | CKD 3a | 62 (19.2) |
| Male | 74.2 ± 14.3 | CKD 3b | 145 (44.9) |
| Female | 76.3 ± 15.0 | CKD 4 | 92 (28.5) |
| CKD 5 | 7 (2.2) | ||
| Hospital, n (%) | Admissions method, n (%) | ||
| RGH | 521 (51.1) | GP | 614 (60.2) |
| NHH | 499 (48.9) | A&E | 363 (35.6) |
| Transfer/booked | 27 (2.6) | ||
| Via clinic | 16 (1.6) | ||
| Specialty, n (%) | AKI stage, n (%) | ||
| Medical | 876 (85.9) | Stage 1 | 464 (45.5) |
| Surgical | 144 (14.1) | Stage 2 | 304 (29.8) |
| Stage 3 | 252 (24.7) | ||
| In-hospital outcomes, n(%) | |||
| Recovery | 476 (46.7) | RRT | 9 (0.9) |
| CKD progression | 59 (5.8) | Death | 287 (28.1) |
| New CKD label | 80 (7.8) | Unrecognized AKI | 107 (10.5) |
| Unknown | 2 (0.2) | ||
CKD, chronic kidney diseas; GP, general practice; A&E, accident and emergency; RRT, renal replacement therapy.
Demonstrates the demographics, characteristics and in-hospital outcomes of all patients identified as having AKI between 11 July 2011 and 15 January 2012.
AKI characteristics and outcomes
A total of 45.5% patients had Stage 1 AKI, 30% had Stage 2 AKI and 25% had AKI Stage 3. 46.7% of AKI episodes recovered to baseline sCr. A total of 13.6% developed either de novo CKD or progression of pre-existing CKD, while 0.9% were discharged from hospital requiring RRT. Of the patients with AKI, 28.1% died within their hospital admission and 10.5% of AKI episodes were identified as unrecognized (Table 1). Post-discharge, a further 21.6% of patients died within 14 months of their AKI episode, giving an overall 14-month mortality of 49.7% (n = 507).
Involvement of nephrology services in AKI management
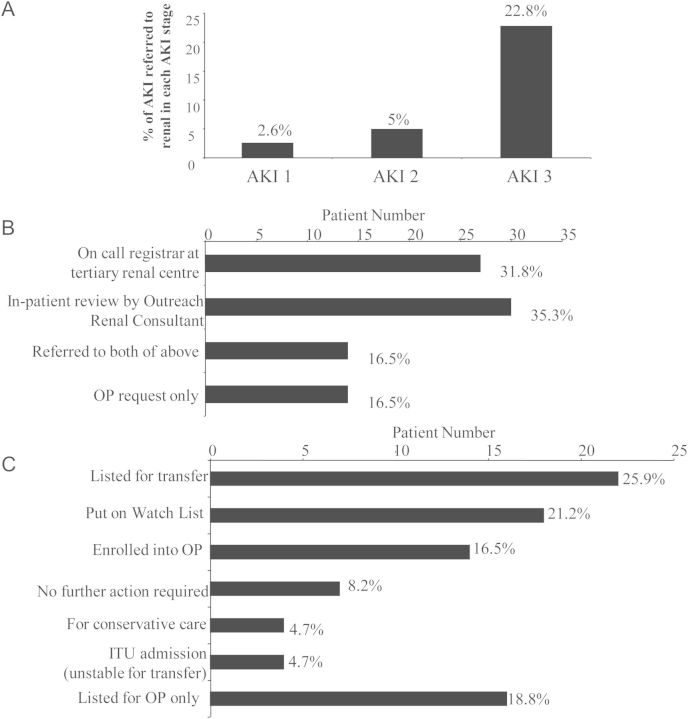
Only 85 (8.3%) patients were referred to nephrologists. Of these, 12 (14%) were Stage 1 AKI, 15 (18%) were Stage 2 AKI and 58 (68%) were AKI Stage 3. Only 22.8% of Stage 3 AKI and 5% of Stage 2 were referred to nephrology (Figure 1A). Patients referred to nephrology services were statistically more likely to be younger, with pre-existing CKD and more severe AKI in association with a higher mean peak creatinine and potassium. Those referred were also more likely to require ITU and had longer hospital stays (Table 2).
Fig. 1.
(A) Demonstrates the percentages of patients in Stages 1, 2 and 3 AKI referred to nephrology services. Referral includes telephone discussion with renal team regarding the patient, in-patient review or transfer to renal unit. Patients sustaining AKI Stage 3 were significantly most likely to be referred (Pearson’s χ2 test, P < 0.001). (B) Demonstrates percentages of patients referred to nephrology services categorized according to method of referral. OP, outpatient. (C) Demonstrates the outcome once patients have been referred to nephrology. Percentage of patients in each category is denoted with each individual bar. Patients ‘listed for OP only’ were not reviewed by a renal physician during their in-patient stay.
Table 2.
Patient demographic data comparing referred and non-referred AKI
| Referred to renal (n = 85) | Nonreferred (n = 935) | P value | |
|---|---|---|---|
| Age (mean ± SD) | 68.5 ± 17.3 | 75.8 ± 14.3 | <0.001 |
| Male, % (n) | 55.3 (47) | 50.1 (468) | 0.36 |
| Pre-existing CKD, % (n) | 57.6 (49) | 29.3 (274) | <0.001 |
| AKI at point of admission, % (n) | 83.5 (71) | 65.8 (615) | 0.001 |
| AKI Severity, % (n) | |||
| Stage 1 | 14.1 (12) | 48.3 (452) | 0.001 |
| Stage 2 | 17.6 (15) | 30.7 (287) | 0.013 |
| Stage 3 | 68.2 (58) | 21 (196) | 0.001 |
| Length of in-patient stay (mean days ± SD) | 19.5 ± 18.2 | 15 ± 19.2 | 0.03 |
| ITU admission, % (n) | 21.2 (18) | 5.0 (47) | <0.001 |
| Biochemistry and radiology | |||
| Imaging performed, % (n) | 82.4 (70) | 29.1 (272) | <0.001 |
| Acid–base status check, % (n) | 58.5 (50) | 11 (103) | <0.001 |
| Peak creatinine (mean ± SD) | 534.6 ± 301.2 | 235.4 ± 131.4 | <0.001 |
| Potassium at peak Cr (mean ± SD) | 5.1 ± 1.1 | 4.6 ± 0.9 | <0.001 |
The table demonstrates patient demographics and AKI characteristics for all the identified AKIs between June 2011 and January 2012, categorized according to whether patients were referred to nephrology services or not. Comparison is also made between admission to ITU and compliance with NCEPOD recommendations between these two groups. P-value for non-normally distributed continuous variables (age, length of stay, peak Cr) are calculated using Mann–Whitney U-test. Normal data (peak potassium) are compared using Student’s t-test. P values reported for categorical variables using Pearson's χ2 test/Fisher's exact test where appropriate. A P-value of <0.05 was deemed as statistically significant.
Referral outcomes
A third of patients referred to nephrologists were referred via telephone to the registrar at the tertiary renal unit, while a third were referred for in-patient review by the outreach Nephrology Consultant. Sixteen percent were referred through both methods and a further 16.5% only referred for outpatient renal review (Figure 1B). Outcomes of nephrology referrals are demonstrated in Figure 1C. A total of 25.9% of referrals were listed for transfer to the renal unit. A total of 21.2% were put on the renal unit's ‘Watch List’. This list comprises a group of patients who are not listed for transfer but are monitored by nephrologists remotely and telephone advice is given to medical teams under which the patient is admitted. These patients may be listed for transfer if their renal parameters worsen. A total of 16.5% were listed for outpatient review following in-patient review, and 4.7% were deemed palliative and suitable for conservative care. A total of 18.8% were listed for outpatient review following discharge.
Acute RRT
Twenty-five patients required RRT for AKI. Fourteen percent (n = 12) of these had haemofiltration, while 12% (n = 10) had haemodialysis. 3.5% (n = 3) had both haemofiltration and haemodialysis. Overall, 65 patients (6.4%) were referred to ITU for management, of these 17 patients were admitted for only renal support while awaiting transfer to the renal unit.
Referral waiting times
The mean number of days between date of AKI onset and referral to nephrology was 3.5 ± 6.4 days. The mean number of days between referral and in-patient nephrology review was 2.9 ± 3.9 days. Waiting times for transfer to the renal unit was 2.2 ± 3.7 days. Minimum wait for transfer was 0 days and maximum wait was 14 days. A proportion of patients were transferred to ITU for renal support prior to transfer to the renal unit. For these, the mean wait on ITU prior to transfer was 1.4 ± 1.7 days. The minimum wait on ITU was 0 days and maximum wait 5 days.
Post-discharge follow-up
Only 8.1% of AKI patients were reviewed by nephrologists post-discharge. The majority of these were those that had been referred to nephrology services while an in-patient. A total of 34.2% had no follow-up planned, while 22.3% were followed-up by their GP or another hospital specialty. 62.5% were discharged from hospital with an eGFR of <60 mL/min/1.73 m2. However, only 13.3% of these patients were referred for outpatient nephrology review.
NCEPOD compliance
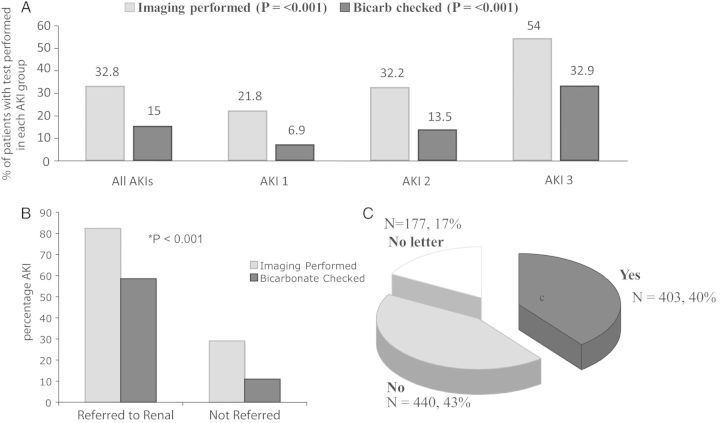
Among the recommendations outlined in the AKI NCEPOD report are appropriate assessment of acid–base status and renal imaging [5]. Only 32.8% of AKI's in this study had renal imaging performed, with only half of all AKI Stage 3 undergoing renal imaging (Figure 2A). Of those imaged (70 patients), 20.9% had some degree of obstructive pathology identified. Only 15% of AKI patients had acid–base status assessment performed (median serum bicarbonate 18 mmol/L, IQR 15–22), with only a third of AKI Stage 3 having this checked. Referral to nephrology was associated with marked improvement in NCEPOD compliance (Figure 2B). Only 40% of AKI hospital discharges had AKI mentioned in discharge documentation to primary care (Figure 2C).
Fig. 2.
(A) Demonstrates percentages of patients that had renal imaging and acid–base status analysis for all patients with AKI, dividing according to AKI stage. NCEPOD compliance increased with increasing AKI severity for both investigative parameters (Pearson’s χ2 test, P < 0.001). (B) Demonstrates percentages of patients that had renal imaging and acid–base status assessment divided according to whether they were referred or not to nephrology services. Renal referral was associated with improved NCEPOD compliance across both investigative parameters (Fisher's exact test, P < 0.001). (C) Demonstrates percentages of patients that had AKI documented as part of their in-patient admission on their discharge documentation to the GP.
Unrecognized AKI
One hundred and seven patients (10.5%) with AKI were discharged from hospital with missed/unrecognized AKI. Fifty-two percent (n = 56) of these patients were re-hospitalized within 6 months, with a total of 93 re-hospitalization events. 23.3% (n = 25) had pre-existing CKD and 75% (n = 80) had AKI apparent on admission bloods. 21.5% (n = 23) were AKI Stage 2 and 6.5% (n = 7) were AKI Stage 3. One patient with missed AKI had a serum potassium level >6 mM and three patients had haemolysed serum potassium samples, which were not repeated. Table 3 compares data in patients with recognized versus unrecognized AKI. Patients with unrecognized AKI were more likely to be admitted under surgical specialties, were less likely to have pre-existing CKD and were more likely to have less severe AKI. Moreover, they had shorter hospital admissions. A total of 32.7% of patients with unrecognized AKI died within 14 months of admission. Of patients with recognized AKI, 16.5% developed CKD by 14 months. In comparison, 42% of patients with unrecognized AKI developed CKD by 14 months.
Table 3.
Unrecognized AKI
| Missed AKI (n = 107) | All other outcomes (n = 913) | P value | |
|---|---|---|---|
| Age (mean ± SD) | 76 ± 15.0 | 75.1 ± 14.7 | 0.479a |
| Male, % (n) | 48.6 (52) | 50.7 (463) | 0.679 |
| Hospital | |||
| RGH, % (n) | 52.3 (56) | 50.9 (465) | 0.783 |
| NHH, % (n) | 47.7 (51) | 49.1 (448) | |
| Admission route, % (n) | |||
| GP | 58.9 (63) | 60.4 (551) | 0.403 |
| A&E | 35.5 (38) | 35.6 (325) | |
| Transfer/other | 1.9 (2) | 2.5 (23) | |
| Clinic | 3.7 (4) | 1.3 (12) | |
| Specialty admitted under | |||
| Medicine, % (n) | 78.5 (84) | 86.7 (792) | 0.027 |
| Surgery, % (n) | 21.5 (23) | 13.3 (121) | |
| Pre-existing CKD, % (n) | 23.4 (25) | 32.6 (298) | 0.034 |
| AKI at point of admission, % (n) | 74.8 (80) | 66.4 (606) | 0.061 |
| AKI severity, % (n) | |||
| Stage 1 | 72 (77) | 42.4 (387) | <0.001 |
| Stage 2 | 21.5 (23) | 30.6 (279) | 0.033 |
| Stage 3 | 6.5 (7) | 27.1 (247) | <0.001 |
| Length of IP stay (mean days ± SD) | 6.5 ± 12.3 | 16.4 ± 19.5 | <0.001a |
| Long-term outcomes, % (n) | |||
| Died | 32.7 (35) | 52.2 (471) | 0.0002b |
| De novo CKD or progression of pre-existing CKD | 39.3 (42) | 16.5 (149) | <0.0001b |
| No CKD | 28 (30) | 31.3 (283) | 0.51b |
Demographics, characteristics and outcomes of recognized versus missed AKI.
aP values calculated using Mann–Whitney U-test.
bP values calculated using Fisher's exact test. All other P values calculated using Pearson’s χ2 test. P < 0.05 deemed as statistically significant.
AKI analyses by hospital
The incidence of AKI was greater in RGH than NHH (7.8 versus 5.6%, P < 0.001), with a greater proportion of HA-AKI which is reflective of RGH being the larger of the DGHs. However, there were no significant differences in AKI severity, NCEPOD compliance (as detailed above) or in-patient outcomes of AKI categorized as per Table 1. Specifically, there were equal proportions of missed AKI in both hospitals (10.7 and 10.2%, respectively). Renal referral rates were also equivalent (8.3 and 8.4%). There were no significant differences between hospitals in the long-term (14 months) renal outcomes defined as de novo CKD or progression of existing CKD (data not shown).
Discussion
AKI is a common clinical problem faced by a variety of specialists including general physicians, surgeons, intensivists and nephrologists. Several studies have demonstrated that AKI is associated with adverse patient outcomes including prolonged hospital stay, increased mortality and a heightened risk of developing CKD [3, 15–19]. Many of these risks persist well after hospital discharge, demonstrating high personal and health-care costs. AKI risk is heightened in the elderly and in individuals with increased comorbid illnesses [8]. With an ageing population and a rising burden of chronic disease, AKI and ensuing CKD will continue to represent a significant problem faced by many specialties. It is becoming increasingly vital to implement health strategies within hospital front-line and specialist services that are aimed at prevention and optimum management of this condition. This large study based in two DGHs in the UK identified an AKI incidence of 6.4%, of which approximately a third of patients had underlying CKD. Only 8.3% of AKI patients and 22.8% of patients with severe AKI were referred to nephrology. Thus, in the setting of a DGH with no dedicated in-patient nephrology service, AKI is almost entirely managed by general physicians and surgeons.
The National Service Framework for Renal Services in the UK published in 2010 recommends that patients at risk of or with AKI should be promptly identified and given high-quality, clinically appropriate care in partnership with specialist renal teams [20]. Recently, KDIGO have published guidelines recommending that a nephrologist should follow-up survivors of AKI within 90 days of the AKI event [12]. In 2009, a national UK audit was performed evaluating recognition and management of AKI in patients who subsequently died [5]. This audit identified deficiencies in AKI management in ∼50% of cases. In this study, only a third of AKI patients had renal imaging performed during their admission and only half of the patients with severe AKI had renal imaging performed. Furthermore, in 20.9% of those imaged, urological pathology was identified. While it is beyond the scope of this study to evaluate management of individual AKI cases, this highlights the potential for missing reversible AKI causes amenable to radiologic intervention in two thirds of this patient cohort. Assessment of acid–base status was also poor, with only a third of severe AKI and 15% of AKI patients overall having this performed. Furthermore, only 8.1% patients were seen by nephrology post-discharge, despite a large proportion developing CKD. Patients who did have planned outpatient review were those that had been referred to nephrology during their admission, with a significant proportion of the remaining patients not having the episode of AKI documented as part of their hospital discharge letter to alert the community team to the patients heightened risk of CKD and death. Thus, according to Renal NSF and KDIGO recommendations, this study identifies significant shortcomings in AKI management. Furthermore, despite publication of the AKI NCEPOD audit 2 years prior to this study, little improvement has been made in basic management of patients with AKI.
According to the AKI NCEPOD enquiry, lack of specialist input was one of the factors that led to poor care delivery [5]. This was also a likely important factor in this study as NCEPOD compliance improved in patients that were referred to renal services. It also suggests that the NCEPOD enquiry has had little impact on care outside nephrology. Patients were more likely to be referred to nephrology if they had severe AKI, AKI on admission, pre-existing CKD or if they were transferred to ITU, indicating that, in these circumstances, doctors were more easily able to recognize importance of nephrology input. It was also clear that age plays a role in the decision for referral. It may have been appropriate to not refer some elderly patients if their care was palliative. However, as patients are surviving longer with multiple comorbid conditions, the importance of developing an optimum management approach in elderly patients with AKI becomes increasingly crucial. In the UK, the number of elderly patients on RRT programmes is increasing; thus, it may be considered as ageist to not offer the elderly the benefit of health care that may enhance recovery from AKI, limit progression to CKD and reduce mortality. Conversely, if nephrologists were to see all AKI inpatients and organize subsequent 90-day outpatient review for these patients, this would overwhelm nephrology services and impact on care delivery across other aspects of nephrology. Careful consideration needs to be given to a measured and appropriate reconfiguration of nephrology services to assist in optimal AKI management. Additionally, development of nephrology-led education and training programmes for front-line and general hospital teams, with the implementation of AKI care pathways as advised by KDIGO may be effective.
As kidney disease is generally silent, it can often go unnoticed without meticulous attention to blood results and urine output. In contrast to the CKD staging system, the AKI staging system has received little attention in clinical practice and appears to play only a small role in AKI recognition. This study identifies a concerning trend of patients discharged with unrecognized AKI. The need for clinicians to be vigilant of AKI is important for several reasons; it may be predictive of a longer in-hospital stay, it identifies the patient's heightened short and long-term mortality risk, and it identifies the increased risk of developing CKD. Patients with unrecognized AKI were more likely to be admitted under surgical specialties, specifically highlighting the need to focus AKI education/training in this discipline. Unrecognized AKI did not appear to impact on long-term mortality. However, it did impact significantly on CKD development, with over 40% of patients with unrecognized AKI developing either de novo CKD or as progression of pre-existing CKD. There is unequivocal evidence that CKD progression can be slowed by optimal management of hypertension and proteinuria [21]. Thus, this work illustrates the need for improving AKI awareness, and has prompted us to set-up an AKI alert system in both of these hospitals to improve recognition.
Our study has a number of limitations. There was no information on aetiology of AKI or on the cause of death in our patient cohort. Patients on palliative care pathways were not excluded, although by definition they may not have been being biochemically monitored, and therefore should not impact significantly on our findings. In addition, as information was gathered retrospectively from an electronic database, information on differences in patient management could not be reliably obtained. Furthermore, although overall patient numbers were large, the cohort of patients referred to nephrology was small in comparison to the non-referred group, thus introducing difficulty in concluding any difference in outcomes, and hence not presented in this work. Further research should focus on elucidating specific differences in AKI care in those referred to renal services compared with those managed solely by DGH medics in order to fully understand any deficiencies in AKI management that might be improved by bettering education. Finally, these results, although maybe representative of other DGHs within the UK, are unlikely to be applicable to teaching hospitals with on-site specialist renal units.
In conclusion, this study shows that AKI in DGHs is managed by medical and surgical specialties with limited nephrology input. It highlights serious shortcomings in AKI recognition and management and highlights an urgent need for clear guidance for colleagues in all disciplines on recognition and management of AKI and appropriate referral to nephrology services. Implementation of AKI-eAlert systems may assist in this important reform.
Acknowledgements
Aneurin Bevan Health Board for assistance with information relating to patient admissions.
Conflict of interest statement. None declared.
References
- 1.Aitken E, Carruthers C, Gall L, et al. Acute kidney injury: outcomes and quality of care. QJM. 2013;106:323–332. doi: 10.1093/qjmed/hcs237. [DOI] [PubMed] [Google Scholar]
- 2.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 4.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 5.National Confidential Enquiry Into Patient Outcome and Death (NCEPOD) Adding Insult to Injury. London: NCEPOD; 2009. [Google Scholar]
- 6.Li PK, Burdmann EA, Mehta RL. Acute kidney injury: global health alert. Kidney Int. 2013;83:372–376. doi: 10.1038/ki.2012.427. [DOI] [PubMed] [Google Scholar]
- 7.Pannu N, James M, Hemmelgarn B, et al. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8:194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coca SG. Acute kidney injury in elderly persons. Am J Kidney Dis. 2010;56:122–131. doi: 10.1053/j.ajkd.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham KA, Thompson EB, Bodger K, et al. Inequalities in outcomes of acute kidney injury in England. QJM. 2010;105:729–740. doi: 10.1093/qjmed/hcs037. [DOI] [PubMed] [Google Scholar]
- 10.Meier P, Bonfils RM, Vogt B, et al. Referral patterns and outcomes in noncritically ill patients with hospital-acquired acute kidney injury. Clin J Am Soc Nephrol. 2011;6:2215–2225. doi: 10.2215/CJN.01880211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clinical Practice Guidelines for Acute Kidney Injury http://www.kdigo.org/clinical_practice_guidelines/AKI.php. (16 February 2014, date last accessed).
- 13. Joint Specialty of Royal College of Physicians and Renal Association: The UK CKD guidelines http://www.rcplondon.ac.uk/sites/default/files/documents/ckd_full_guide_navigable.pdf. (16 February 2014, date last accessed).
- 14. Chronic Kidney Disease: Early Identification and Management of Chronic Kidney Disease in Primary and Secondary Care. NICE http://www.nice.org.uk/CG73. (16 February 2014, date last accessed).
- 15.Bucaloiu ID, Kirchner HL, Norfolk ER, et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2011;81:477–485. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 16.Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu CY, Chertow GM, McCulloch CE, et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liangos O, Wald R, O'Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 20.Department of Health. National Service Framework for Renal Services http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Healthcare/Longtermconditions/Vascular/Renal/index.htm. (16 February 2014, date last accessed)
- 21.Lambers Heerspink HJ, Holtkamp FA, Parving HH, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330–337. doi: 10.1038/ki.2012.74. [DOI] [PubMed] [Google Scholar]