Abstract
Recent studies have demonstrated much larger variability in the total number of nephrons in normal populations than previously suspected. In addition, it has been suggested that individuals with a low nephron number may have an increased lifetime risk of hypertension or renal insufficiency, emphasizing the importance of evaluating the nephron number in each individual. In view of the fact that all previous reports of the nephron number were based on analyses of autopsy kidneys, the identification of surrogate markers detectable in living subjects is needed in order to enhance understanding of the clinical significance of this parameter. In this review, we summarize the clinicopathological factors and findings indicating a reduction in the nephron number, focusing particularly on those found at the time of a preserved renal function.
Keywords: birth weight, CKD, nephron number, renal biopsy
Introduction
Most types of chronic renal diseases are potentially progressive and irreversible, and once renal functional impairment is established, it is often difficult to retard further disease progression [1, 2]. Therefore, it is important to identify subjects at risk of future progression to renal failure as early as possible and take preventative measures before apparent renal dysfunction becomes established [3].
Recent autopsy studies have demonstrated that the total number of nephrons in general populations varies significantly even in the absence of specific renal diseases [4–7]. Such potential differences in the nephron number at the time of a preserved renal function may represent differences in the functional reserve of the kidneys and are currently recognized to be one cause of divergent renal outcomes between individuals with similar clinical background characteristics at the diagnosis of renal disease [8–10]. Therefore, the development of methods to non-invasively estimate the individual total nephron number would be very useful for evaluating the future progression of renal disease. However, at present, the detailed stereology-based analysis of autopsy kidneys is the only available method for accurately determining the human nephron number [11–13]. In addition, due to its microscopic size, it remains difficult to identify the individual fine structure and assess the total number of nephrons in living human subjects using commonly available clinical imaging studies. Therefore, for clinical assessments, it is necessary to integrate information regarding the origin and/or outcome of reductions in the nephron number.
In this review, we summarize currently available markers of a low nephron number that are detectable as clinicopathological information in living human subjects. As a result of current clinical needs, we particularly focused on factors and/or findings indicating nephron number reduction at the time of a preserved renal function.
The human nephron number: the relationship between a low nephron number and the risk of renal diseases
The human nephron number can be estimated by the stereology-based analysis of autopsy kidneys [11–13]. Recent studies have demonstrated much larger variability in the total nephron number in normal populations than previously suspected. As shown in Table 1, the variations among reported autopsy series are up to 8-fold. Of note, it has consistently been reported that there is a close relationship between the birth weight and the total nephron number [4, 5]. Recent investigations have revealed that nephron number increases by ∼260 000 per kg increase in birth weight [4]. The correlation between the birth weight and total nephron number is likely based on the fact that the final number of human nephrons is determined at 34–36 weeks of gestation and does not increase thereafter [17, 18].
Table 1.
Stereology-based estimation of human nephron number
| Reference | Population | n (male) | Mean age (year) | BSA (m2) | Mean kidney weight (g) | Nglom per kidney (mean) | Nglom per kidney (range) | Nglom per kidney (fold) |
|---|---|---|---|---|---|---|---|---|
| Nyengaar and Bendtsen [14] | Danish | 37 (19) | 58 | 1.7 | 131 | 617 000 | 331 000–1 424 000 | 4.3 |
| Keller et al. [15] | German | 20 (18) | 46 | 2.1 | 179 | 1 074 414 | 531 140–1 959 914 | 3.7 |
| Hughson et al. [4] | African Americans | 37 (21) | 29 | – | – | 959 306 | ||
| Caucasians | 19 (15) | 29 | – | – | 869 959 | 227 327–1 825 380a | 8 | |
| Hoy et al. [16] | Australian Aborigines | 17 (11) | 39 | 1.7 | 160 | 683 174 | 364 262–1 129 233 | 3.1 |
| Australian non-Aborigines | 24 (21) | 47 | 2.0 | 209 | 885 318 | 380 517–1 493 665 | 3.9 | |
| McNamara et al. [6] | Senegalese | 28 (14) | 35 | 1.7 | 119 | 925 485 | 536 171–1 394 010 | 2.6 |
| Cumulative | 182 (119) | 41 | 1.8 | 155 | 851 338 | 227 327–1 959 914 | 8.6 |
BSA, body surface area; Nglom, total number of glomeruli.
aRange among population of both African Americans and Caucasians.
A low birth weight can result from intrauterine growth restriction or preterm birth. In general, a low-birth-weight infant is defined as an individual born weighing <2500 g, while a preterm birth infant is defined as an individual born before 36 weeks of gestation. Sutherland et al. [19] conducted a study of the autopsied kidneys of preterm human neonates born between 24 and 35 weeks of gestation who lived for 2–68 days after birth. Compared with the gestational control tissues, the preterm kidneys exhibited a reduced width of the nephrogenic zone and a greater percentage of morphologically abnormal glomeruli. These observations suggest that preterm kidneys have fewer functional nephrons. This hypothesis is further supported by a series of experimental studies, including analyses using a baboon model of preterm birth [20].
Importantly, it has been suggested that a low birth weight, in association with a low nephron number, is a plausible risk factor for progression to renal failure in adult life [21]. The progressive loss of functioning nephrons is the hallmark of most types of renal disease. Therefore, increased severity and acceleration of renal disease is likely when the number of nephrons is already reduced prior to disease onset. On the other hand, studies have also suggested relationships between birth weight and the incidence of hypertension/cardiovascular diseases, which are closely related to the pathogenesis of chronic kidney disease (CKD) [22]. Therefore, the concept of ‘nephron number reduction in individuals with a normal renal function’ is fully consistent with that of CKD, which aims to prevent health hazards by observing renal and cardiovascular complications, not only in patients with end-stage kidney disease (ESKD), but also in subjects at risk of ESKD, from the time of a normal renal function. Therefore, the hypothesis of ‘developmental origins of health and disease’, first described by Barker et al., is attracting much attention from nephrology researchers, as this theory has the potential to elucidate the complex pathophysiological cascade of CKD [23–25].
Clinical surrogates for a low nephron number
As shown in Table 2, clinical surrogates for a low nephron number include parameters related to the origin and/or the outcome of nephron number reduction.
Table 2.
Clinical surrogates for a low nephron number
| Surrogate marker | Status, finding |
|---|---|
| Maternal | |
| Nutrition | Low protein intake, low calorie intake, low or high salt intake, iron deficiency, zinc deficiency, vitamin A deficiency |
| Behavior | Alcohol consumption, smoking |
| Medication use | Corticosteroids, RAAS inhibitors |
| Birth weight | Low birth weight |
| Neonatal | |
| Birth weight | Low birth weight |
| Birth period | Prematurity |
| Gene polymorphism | RET 1476A polymorphism, PAX2 AAA haplotype etc. |
| Kidney size | Small kidney size |
| Adult | |
| Race | Australian Aborigines |
| Gender | Female gender |
| Age | Elderly |
| Height | Low height |
| Blood pressure | Presence of essential hypertension |
| Urinary finding | Presence of proteinuria (albuminuria) |
| Response to therapy | Transient decrease of GFR in response to RAAS inhibitors |
RAAS, renin–angiotensin–aldosterone system; GFR, glomerular filtration rate.
Maternal nutrition and behavior
Maternal malnutrition during gestation has been shown to be associated with a low birth weight and low nephron number [26, 27]. In particular, it has been demonstrated that protein restriction reduces the nephron endowment during nephrogenesis in animals [28, 29]. Likewise, it is known that vitamin A deficiency influences kidney development and is related to a low nephron number [30, 31]. Other factors that have been found to be related to alterations in the nephron number in animals include a low or high salt intake and iron or zinc deficiency [32–34]. Maternal diabetes has also been shown to be associated with a risk of renal agenesis and dysplasia in animals [35]. In addition, maternal smoking and alcohol consumption are related to a low nephron number as well as defects in nephrogenesis in animal experiments [36, 37]. The use of medications by a pregnant female may also influence fetal kidney development. For example, it has been shown that the maternal use of steroids or renin–angiotensin–aldosterone system (RAAS) inhibitors affects fetal renal development [38, 39].
Fetal birth weight
Maternal social, environmental and nutritional factors during pregnancy are quite important in determining the ultimate number of fetal nephrons. In particular, because a low birth weight has been established to be an indicator of an insufficient gestational environment, it is the most reliable and useful parameter of a low nephron number [4, 5]. In addition, a recent study showed an odds ratio for a low-birth-weight infant of 1.8 among mothers born with a low birth weight, indicating the potential role of genetic factors other than fetal environmental factors in the occurrence of a low birth weight [40].
Genetic factors
Specific monogenic mutations in certain genes have been shown to be responsible for phenotypes of congenital abnormalities of the kidneys and urinary tract. Many of these phenotypes involve alterations in the nephron endowment, recognized as apparent renal malformations, agenesis or hypodysplasia. More subtle changes are inherited as variants in the DNA sequence, termed genetic polymorphisms. For example, the RET 1476A polymorphism [41] or PAX2AAA haplotype [42] are frequently identified in the general population. These gene polymorphisms have been shown to be associated with a 10% reduction in kidney size for each genotype without apparent morphological abnormalities in the kidneys, with a 23% reduction in kidney size in the presence of both genotypes [41].
Race and gender
Comparisons of the mean total nephron numbers in the African Americans and the Caucasian Americans did not show differences among the races [4]. It has been shown that the nephron number in certain Australian aboriginal communities is significantly low compared with that observed in other populations [16]. Of note, this population is well known for an extremely high incidence of CKD [43, 44].
Female gender has been shown to be associated with 12% fewer nephrons than that observed in males [5, 45]. This finding is consistent with the fact that females tend to have a lower birth weight than males in the general population.
Age
Among the physiological factors that can occur during an individual's lifetime, aging is probably the most important factor influencing the nephron number [46, 47]. As an individual ages, the nephron number progressively decreases due to ischemia as a result of intrarenal atherosclerosis, even in the absence of any specific renal diseases. An estimate from one study demonstrated a decrease in the nephron number of 4500 per year associated with normal aging [48]. As a result, the kidney size decreases to ∼50–60% of that observed at the maximal level on average [49].
Adult height and body weight
The total nephron number has been shown to be strongly related to height in adults, a characteristic predicted by birth weight [50]. A low adult height has also been reported to be associated with the incidence of hypertension and diabetic nephropathy [51, 52]. Although adult obesity is associated with glomerular hypertrophy in general, its relationship with the total nephron number is unclear.
Kidney size
The nephron number exhibits a weak correlation with kidney size until 3 months after birth [41, 42]. The total nephron number in adults has been shown to be correlated with kidney weight in adult human autopsy series [14]. However, the relationship between the total number of nephrons and the kidney size measured on imaging analyses remains undetermined, although a relationship between the renal size on ultrasonography and the incidence of hypertension or slope of the renal function has been reported [53–55].
Hypertension
It was recently reported that the nephron number in patients with essential hypertension is lower than that observed in age-matched subjects without hypertension in an analysis of autopsy kidneys among a German population with sudden death [15]. In addition, a low nephron number is associated with hypertension in Australian Aborigines and Caucasian Americans. However, the relationship between a low birth weight and hypertension is not apparent in African Americans [5]. The precise mechanisms involved in increases in blood pressure in individuals with a low nephron endowment have not yet been fully elucidated. One possible effect of a low nephron endowment is a reduction in the number of sodium transporters as well as alterations in renal sodium handling, which may influence blood pressure control [56].
Proteinuria
In a study of Australian Aborigines, the odds ratio in subjects with a low birth weight was 2.8 (95% CI 1.26–6.31) for the appearance of microalbuminuria in comparison with those without a low birth weight. This result suggests that a low birth weight is associated with both the initiation and progression of CKD [57].
In routine clinical examinations, it is technically difficult to distinguish between proteinuria associated with primary immunological or non-immunological glomerular injury and proteinuria induced by the secondary compensatory failure of glomeruli due to a reduction in the functional nephron number. Therefore, the appearance of proteinuria per se is not always a marker of nephron number reduction. This finding is more probable when proteinuria is detected together with microscopic hematuria or various casts in urinary sediment.
Response to therapy
In a post hoc analysis of the Reduction of End Points in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan trial, it was demonstrated that a transient fall in glomerular filtration rate (GFR) following treatment with an angiotensin receptor blocker (losartan) in patients with diabetic nephropathy is associated with a subsequent reduction in the slope of the renal function [58]. These results suggest that the progression of renal tissue injury is attenuated, at least in part, by the amelioration of glomerular hypertension in patients with a transient decrease in GFR. Perhaps the kidneys in patients exhibiting a relatively large decrease in GFR following the initiation of the losartan therapy are pathophysiologically more dependent on the RAAS and therefore more sensitive to RAAS inhibitors. Previous studies have shown that experimental reductions in the nephron number are closely associated with glomerular enlargement together with increased activation of the intrarenal RAAS [59, 60]. Therefore, it is conceivable that maladaptive glomerular injury due to a subtle reduction in the nephron number is involved in the pathogenesis of certain renal diseases, such as diabetic nephropathy. Therefore, a transient decrease in GFR and subsequent reduction in proteinuria following the administration of RAAS inhibitors is a candidate surrogate marker of a relative reduction in the nephron number, even at the time of a preserved renal function.
Histopathological surrogates of a low nephron number on renal biopsy specimens
As shown in Table 3, the following factors may be associated with a reduction in the nephron number at the time of a preserved renal function. However, because renal biopsies are generally performed in patients with signs of renal injury, such as persistent urinary abnormalities, it is difficult to evaluate these histopathological factors in clinically ‘normal’ patients without any signs of renal injury.
Table 3.
Histopathological surrogates for a low nephron number on a biopsy
| Surrogate marker | Status, finding |
|---|---|
| Segmental glomerulosclerosis | Present together with glomerular hypertrophy, perihilar variant |
| Glomerular volume | Increase in glomerular volume |
| Glomerular capillary number | Increase in glomerular capillary number |
| Glomerular density | Low glomerular density |
Glomerular hypertrophy
In autopsy studies, it has been clearly demonstrated that the total nephron number is inversely correlated with the glomerular volume [4–7]. This finding indicates the presence of renal compensatory changes in order to maintain the GFR at the ideal level in response to nephron number reduction. Under such compensatory processes, the GFR of single nephrons increases among the remaining glomeruli and the glomeruli become enlarged. Therefore, glomerular hypertrophy is a finding indicative of a relative reduction in the nephron number. Consistent with this idea, previous reports have demonstrated a relationship between the detection of glomerular hypertrophy on a diagnostic renal biopsy and the subsequent progression to renal failure [61–65].
Currently, the glomerular size can be evaluated by measuring the maximal glomerular diameter or the glomerular surface area and estimating the glomerular volume based on these data using biopsy or autopsy specimens [61–66]. However, no consensus exists regarding the size of glomeruli defining glomerular hypertrophy and/or glomerulomegaly [67]. Accordingly, glomerular enlargement can be evaluated only when the glomerular size is compared among the specimens of certain patient cohorts.
Renal tubules are also postulated to become enlarged in response to reductions in the nephron number. However, it is technically difficult to estimate the tubular size on biopsies because the axis of the slices of the specimens is not always constant. In addition, the size of the tubules largely depends on each nephron segment, which further complicates quantitative evaluations.
Glomerular capillary number
In a rat heminephrectomy model, it was recently demonstrated that the glomerular capillary number increases during the process of compensatory glomerular enlargement [68]. Consequently, certain angiogenesis-promoting factors are secreted to increase the glomerular capillary number and maintain the total glomerular filtration rate under conditions of nephron number reduction. Consistent with this idea, increases in the glomerular volume in this animal model are abolished by treatment with a neutralizing antibody to vascular endothelial growth factor (VEGF) [69, 70]. The increased secretion of angiogenesis-promoting factors, including VEGF, has also been reported in tissue microarray analyses of renal biopsies in patients with obesity-related glomerulopathy, whose glomeruli are markedly increased in size [71]. These results suggest that an increase in the glomerular capillary number is indicative of a relative decrease in the nephron number.
Focal segmental glomerulosclerosis
Focal segmental glomerulosclerosis (FSGS) is a renal pathological finding that is believed to result from glomerular podocyte injury [72]. The mechanisms underlying glomerular podocyte injury include those induced by immunological injury, as well as transformation by direct viral infection, drug-induced cytotoxic effects and/or non-immunological hemodynamic or pressure loading [73]. Under conditions of nephron number reduction, FSGS may be established as a result of glomerular scarring due to the failure to maintain the physiological function when stressors on glomerular podocytes induced by excessive glomerular enlargement exceed a certain upper limit.
Such hemodynamic changes may also be found in subjects with massive obesity in which a ‘mismatch’ between body size and the nephron number exists. Indeed, glomerulomegaly and FSGS are characteristic histopathological findings in patients with obesity-related glomerulopathy and are occasionally found in massively obese individuals [74–76]. In this disease entity, the glomerular filtration rate increases to meet the increased metabolic demands of the kidneys [77, 78]. Accordingly, massive obesity may be a finding indicative of a relatively low nephron number. Likewise, the detection of FSGS in very-low-birth-weight infants or body builders with a large muscle mass is consistent with the ‘mismatch’ hypothesis [79, 80].
Histopathologically, FSGS induced by hemodynamic stress is often associated with the perihilar variant, which accompanies segmental sclerotic lesions near the vascular pole of the glomeruli [72]. In electron microscopic studies, the frequency of podocyte foot process effacement is relatively low compared with that observed in patients with FSGS induced by other mechanisms [75].
Glomerular density
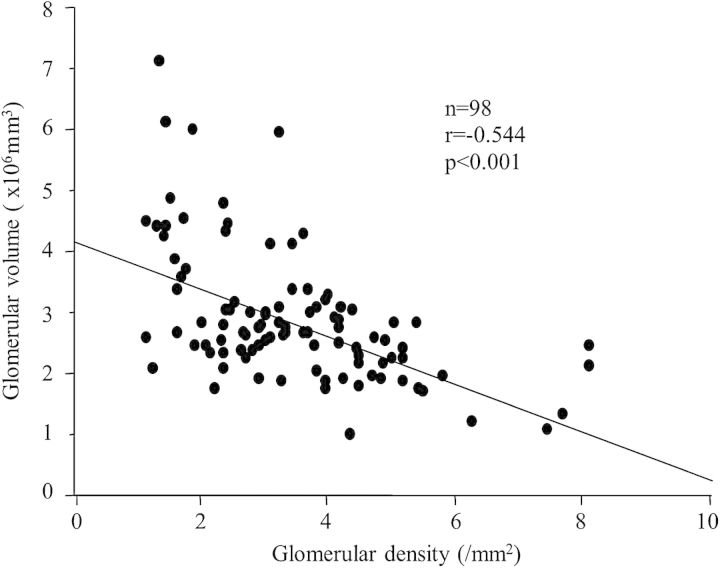
We recently demonstrated that the individual glomerular density (the number of non-sclerotic glomeruli per renal cortical area on a needle biopsy specimen) exhibits significant variation in patients with various primary glomerular diseases, although all biopsies were performed when the renal function was within the normal range [81–83]. The maximal differences in glomerular density were 6.8-fold among 98 patients with IgA nephropathy (IgAN), 4.3-fold among 65 patients with idiopathic membranous nephropathy (IMN) and 4.3-fold among 50 patients with minimal change disease (MCD). Figure 1A shows the representative renal biopsy findings in a patient with a high glomerular density and Figure 1B shows a patient with a low glomerular density. Of note, the glomerular density was found to be inversely correlated with the mean glomerular volume in these patient cohorts (Figure 2). Furthermore, a low glomerular density at the time of a preserved renal function is a plausible independent predictor of progression in patients with IgAN or IMN. The analyses of patients with MCD revealed that the patients with a low glomerular density tended to have similar clinicopathological characteristics to patients with a histological diagnosis of FSGS. During the initial treatment with corticosteroids in the patients with MCD, the number of patients achieving complete remission was significantly lower among the subjects with a low glomerular density than among those with a high glomerular density. These results suggest that the glomerular density on renal biopsy specimens is an important determinant of the variability in glomerular size and can influence the clinical phenotype, such as the response to therapy and the long-term renal outcome. Based on these findings, we suggest that the glomerular density observed on renal biopsy specimens reflects, at least in part, the personal number of nephrons in an individual. In support of this idea, a previous report showed that the glomerular density is correlated with the birth weight, a known factor related to the total nephron number [84].
Fig. 1.
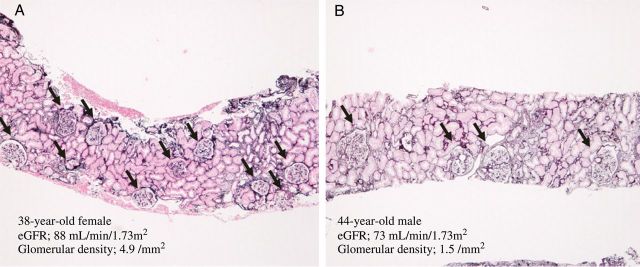
Representative renal biopsy findings in light microscopy. The representative renal biopsy findings in a patient with a high glomerular density (A) and a patient with a low glomerular density (B) are shown. Both the patients were diagnosed with IgA nephropathy. Arrows indicate non-sclerotic glomeruli. Periodic acid-methenamine silver stain (50×).
Fig. 2.
The relationship between the glomerular density and the mean glomerular volume in the IgA nephropathy patients with an estimated glomerular filtration rate of 60 mL/min/1.73 mm2 or more at biopsy. The glomerular density showed a close inverse correlation with the mean glomerular volume. The figure is used with permission [81].
On the other hand, there is an issue regarding the evaluation of the glomerular density on renal biopsy specimens with respect to the limitations of morphological measurements using small tissue specimens obtained via needle biopsies. In fact, our recent study using autopsy kidneys obtained from 89 individuals without apparent renal disease, which enabled the evaluation of a much larger number of glomeruli than that permitted using biopsy specimens, showed a maximal 3.5-fold variation in the analyses of both superficial and juxtamedullary cortices between individuals; this result was smaller than that observed using biopsy specimens [85]. In addition, it is uncertain whether the glomerular density observed on a renal biopsy specimen truly represents the total number of nephrons in the entire kidney, since data on the total cortical volume of the kidneys were not available in our study. Accurately determining the origin of the variation in glomerular density observed in renal biopsy specimens therefore requires further investigation.
Imaging assessments of the nephron mass and future perspectives
Although the least invasive and most standardized marker of kidney size in living human subjects is that measured on ultrasonography, the obtained value is 2D and dependent on the technique of the operator [86–88]. In contrast, X-ray computed tomography (CT) and/or magnetic resonance imaging (MRI) are 3D and can provide more accurate values of the human kidney volume [89–91]. However, in response to nephron number reduction, renal compensatory changes, including hypertrophy of the glomeruli and tubules, may occur; thus, the kidney does not necessarily exhibit a remarkable difference in size in the absence of apparent renal dysfunction. Therefore, it can be difficult to estimate the total nephron number using only information obtained from imaging studies in living human subjects.
A new method using MRI to measure the number of glomeruli and individual glomerular volume in perfused rat kidneys has recently been reported [92, 93]. This method is based on the observation that labeling glomeruli with cationic ferritin in vivo allows for whole-kidney detection of each labeled glomerulus using MRI. The total MRI-based count is lower than stereological counts; however, the error is within 10%. Therefore, this new MRI method has the potential to enable measurement of the total nephron number.
Multiphoton microscopy of the kidneys in animals is a powerful technique for renal research. This imaging technique enables the dynamic 4D analysis of organ structures in vivo. Recent advances in research have resulted in not only the ability to detect molecular changes in the glomerulus, but also monitor the progression of renal disease. For example, the labeled human monoclonal antibody (F1.1) directed against the NC1 domain of α3(IV) collagen can be used to assess structural changes in the glomerular basement membrane during the course of glomerular disease, which potentially allows for quantitative estimates of the structural viability of glomeruli [94].
Conclusions
Variations in the nephron number endowment among the general population may determine the future risk of renal failure. Therefore, evaluating the nephron number in each individual is currently one of the most important issues in clinical nephrology. Surrogates for the total nephron number include fetal environmental factors, genetic factors and the presence of certain clinical or histopathological findings at diagnosis. However, due to its complex and multifactorial nature, it is often difficult to evaluate the nephron number. Therefore, the establishment of new technology to accurately estimate the nephron number in living human subjects is required.
Acknowledgements
Part of this study was supported by the Japan Kidney Foundation Research Fund and a grant-in-aid for Scientific Research (C) (N.T.).
Conflict of interest statement. None declared.
References
- 1.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klahr S, Schreiner G, Ichikawa I. The progression of renal disease. N Engl J Med. 1988;318:1657–1666. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- 3.Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int. 2006;70:1694–1705. doi: 10.1038/sj.ki.5001794. [DOI] [PubMed] [Google Scholar]
- 4.Hughson M, Farris AB, 3rd, Douglas-Denton R, et al. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 5.Hughson MD, Douglas-Denton R, Bertram JF, et al. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69:671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 6.McNamara BJ, Diouf B, Hughson MD, et al. Renal pathology, glomerular number and volume in a West African urban community. Nephrol Dial Transplant. 2008;23:2576–2585. doi: 10.1093/ndt/gfn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoy WE, Bertram JF, Denton RD, et al. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens. 2008;17:258–265. doi: 10.1097/MNH.0b013e3282f9b1a5. [DOI] [PubMed] [Google Scholar]
- 8.Ingelfinger JR. Disparities in renal endowment: causes and consequences. Adv Chronic Kidney Dis. 2008;15:107–114. doi: 10.1053/j.ackd.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Abitbol CL, Ingelfinger JR. Nephron mass and cardiovascular and renal disease risks. Semin Nephrol. 2009;29:445–454. doi: 10.1016/j.semnephrol.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Puelles VG, Hoy WE, Hughson MD, et al. Glomerular number and size variability and risk for kidney disease. Curr Opin Nephrol Hypertens. 2011;20:7–15. doi: 10.1097/MNH.0b013e3283410a7d. [DOI] [PubMed] [Google Scholar]
- 11.Bertram JF. Counting in the kidney. Kidney Int. 2001;59:792–796. doi: 10.1046/j.1523-1755.2001.059002792.x. [DOI] [PubMed] [Google Scholar]
- 12.Cullen-McEwen LA, Douglas-Denton RN, Bertram JF. Estimating total nephron number in the adult kidney using the physical disector/fractionator combination. Methods Mol Biol. 2012;886:333–350. doi: 10.1007/978-1-61779-851-1_30. [DOI] [PubMed] [Google Scholar]
- 13.Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999;10:1100–1123. doi: 10.1681/ASN.V1051100. [DOI] [PubMed] [Google Scholar]
- 14.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 15.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 16.Hoy WE, Hughson MD, Singh GR, et al. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70:104–110. doi: 10.1038/sj.ki.5000397. [DOI] [PubMed] [Google Scholar]
- 17.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol. 2010;298:F235–F247. doi: 10.1152/ajprenal.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinchliffe SA, Sargent PH, Howard CV, et al. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64:777–784. [PubMed] [Google Scholar]
- 19.Sutherland MR, Gubhaju L, Moore L, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. 2011;22:1365–1374. doi: 10.1681/ASN.2010121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland MR, Gubhaju L, Black MJ. Stereological assessment of renal development in a baboon model of preterm birth. Am J Nephrol. 2011;33(Suppl 1):25–33. doi: 10.1159/000327073. [DOI] [PubMed] [Google Scholar]
- 21.Lackland DT, Bendall HE, Osmond C, et al. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med. 2000;160:1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 22.White SL, Perkovic V, Cass A, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 23.Barker DJ, Osmond C, Golding J, et al. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 25.Ritz E, Amann K, Koleganova N, et al. Prenatal programming-effects on blood pressure and renal function. Nat Rev Nephrol. 2011;7:137–144. doi: 10.1038/nrneph.2011.1. [DOI] [PubMed] [Google Scholar]
- 26.Langley-Evans SC, Langley-Evans AJ, Marchand MC. Nutritional programming of blood pressure and renal morphology. Arch Physiol Biochem. 2003;111:8–16. doi: 10.1076/apab.111.1.8.15136. [DOI] [PubMed] [Google Scholar]
- 27.Benz K, Amann K. Maternal nutrition, low nephron number and arterial hypertension in later life. Biochim Biophys Acta. 2010;1802:1309–1317. doi: 10.1016/j.bbadis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65:1339–1348. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 29.Almeida JR, Mandarim-de-Lacerda CA. Maternal gestational protein-calorie restriction decreases the number of glomeruli and causes glomerular hypertrophy in adult hypertensive rats. Am J Obstet Gynecol. 2005;192:945–951. doi: 10.1016/j.ajog.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Bhat PV, Manolescu DC. Role of vitamin A in determining nephron mass and possible relationship to hypertension. J Nutr. 2008;138:1407–1410. doi: 10.1093/jn/138.8.1407. [DOI] [PubMed] [Google Scholar]
- 31.Merlet-Bénichou C, Vilar J, Lelièvre-Pégorier M, et al. Role of retinoids in renal development: pathophysiological implication. Curr Opin Nephrol Hypertens. 1999;8:39–43. doi: 10.1097/00041552-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Koleganova N, Piecha G, Ritz E, et al. Both high and low maternal salt intake in pregnancy alter kidney development in the offspring. Am J Physiol Renal Physiol. 2011;301:F344–F354. doi: 10.1152/ajprenal.00626.2010. [DOI] [PubMed] [Google Scholar]
- 33.Drake KA, Sauerbry MJ, Blohowiak SE, et al. Iron deficiency and renal development in the newborn rat. Pediatr Res. 2009;66:619–624. doi: 10.1203/PDR.0b013e3181be79c2. [DOI] [PubMed] [Google Scholar]
- 34.Tomat AL, Inserra F, Veiras L, et al. Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am J Physiol Regul Integr Comp Physiol. 2008;295:R543–R549. doi: 10.1152/ajpregu.00050.2008. [DOI] [PubMed] [Google Scholar]
- 35.Tran S, Chen YW, Chenier I, et al. Maternal diabetes modulates renal morphogenesis in offspring. J Am Soc Nephrol. 2008;19:943–952. doi: 10.1681/ASN.2007080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manalich R, Reyes L, Herrera M, et al. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58:770–773. doi: 10.1046/j.1523-1755.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 37.Gray SP, Kenna K, Bertram JF, et al. Repeated ethanol exposure during late gestation decreases nephron endowment in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2008;295:R568–R574. doi: 10.1152/ajpregu.90316.2008. [DOI] [PubMed] [Google Scholar]
- 38.Schreuder MF, Bueters RR, Huigen MC, et al. Effect of drugs on renal development. Clin J Am Soc Nephrol. 2011;6:212–217. doi: 10.2215/CJN.04740510. [DOI] [PubMed] [Google Scholar]
- 39.Singh RR, Cullen-McEwen LA, Kett MM, et al. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J Physiol. 2007;579(Pt 2):503–513. doi: 10.1113/jphysiol.2006.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins JW, Rankin KM, David RJ. Low birth weight across generations: the effect of economic environment. Matern Child Health J. 2011;15:438–445. doi: 10.1007/s10995-010-0603-x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Quinlan J, Hoy WT, et al. A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol. 2008;19:2027–2034. doi: 10.1681/ASN.2007101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinlan J, Lemire M, Hudson T, et al. A common variant of the PAX2 gene is associated with reduced newborn kidney size. J Am Soc Nephrol. 2007;18:1915–1921. doi: 10.1681/ASN.2006101107. [DOI] [PubMed] [Google Scholar]
- 43.Hoy WE, Wang Z, VanBuynder P, et al. The natural history of renal disease in Australian Aborigines. Part 1. Changes in albuminuria and glomerular filtration rate over time. Kidney Int. 2001;60:243–248. doi: 10.1046/j.1523-1755.2001.00792.x. [DOI] [PubMed] [Google Scholar]
- 44.Hoy WE, Wang Z, VanBuynder P, et al. The natural history of renal disease in Australian Aborigines. Part 2. Albuminuria predicts natural death and renal failure. Kidney Int. 2001;60:249–256. doi: 10.1046/j.1523-1755.2001.00793.x. [DOI] [PubMed] [Google Scholar]
- 45.Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 46.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 2012;82:270–277. doi: 10.1038/ki.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoy WE, Hughson MD, Bertram JF, et al. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol. 2005;16:2557–2564. doi: 10.1681/ASN.2005020172. [DOI] [PubMed] [Google Scholar]
- 49.Gourtsoyiannis N, Prassopoulos P, Cavouras D, et al. The thickness of the renal parenchyma decreases with age: a CT study of 360 patients. AJR Am J Roentgenol. 1990;155:541–544. doi: 10.2214/ajr.155.3.2117353. [DOI] [PubMed] [Google Scholar]
- 50.Eide MG, Øyen N, Skjaerven R, et al. Size at birth and gestational age as predictors of adult height and weight. Epidemiology. 2005;16:175–181. doi: 10.1097/01.ede.0000152524.89074.bf. [DOI] [PubMed] [Google Scholar]
- 51.Sichieri R, Siqueira KS, Pereira RA, et al. Short stature and hypertension in the city of Rio de Janeiro, Brazil. Public Health Nutr. 2000;3:77–82. doi: 10.1017/s1368980000000094. [DOI] [PubMed] [Google Scholar]
- 52.Rossing P, Tarnow L, Nielsen FS, et al. Short stature and diabetic nephropathy. BMJ. 1995;310:296–297. doi: 10.1136/bmj.310.6975.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh GR, Hoy WE. Kidney volume, blood pressure, and albuminuria: findings in an Australian aboriginal community. Am J Kidney Dis. 2004;43:254–259. doi: 10.1053/j.ajkd.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Singh GR, White AV, Hoy WE. Renal ultrasound findings in an Australian Aboriginal population with high rates of renal disease. Nephrology (Carlton) 2005;10:358–361. doi: 10.1111/j.1440-1797.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- 55.Kariyanna SS, Light RP, Agarwal R. A longitudinal study of kidney structure and function in adults. Nephrol Dial Transplant. 2010;25:1120–1126. doi: 10.1093/ndt/gfp654. [DOI] [PubMed] [Google Scholar]
- 56.Walker KA, Cai X, Caruana G, et al. High nephron endowment protects against salt-induced hypertension. Am J Physiol Renal Physiol. 2012;303:F253–F258. doi: 10.1152/ajprenal.00028.2012. [DOI] [PubMed] [Google Scholar]
- 57.Hoy WE, Rees M, Kile E, et al. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int. 1999;56:1072–1077. doi: 10.1046/j.1523-1755.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 58.Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–287. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 59.Anderson S, Meyer TW, Rennke HG, et al. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–619. doi: 10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdi R, Brenner BM. Impact of renin angiotensin system blockade on renal function in health and disease: an end or a beginning? Semin Nephrol. 2004;24:141–146. doi: 10.1016/j.semnephrol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Fogo A, Hawkins EP, Berry PL, et al. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990;38:115–123. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- 62.Nishimoto K, Shiiki H, Nishino T, et al. Reversible glomerular hypertrophy in adult patients with primary focal segmental glomerulosclerosis. J Am Soc Nephrol. 1997;8:1668–1678. doi: 10.1681/ASN.V8111668. [DOI] [PubMed] [Google Scholar]
- 63.Nochy D, Heudes D, Glotz D, et al. Preeclampsia associated focal and segmental glomerulosclerosis and glomerular hypertrophy: a morphometric analysis. Clin Nephrol. 1994;42:9–17. [PubMed] [Google Scholar]
- 64.Tóth T, Takebayashi S. Glomerular hypertrophy as a prognostic marker in childhood IgA nephropathy. Nephron. 1998;80:285–291. doi: 10.1159/000045188. [DOI] [PubMed] [Google Scholar]
- 65.Kataoka H, Ohara M, Honda K, et al. Maximal glomerular diameter as a 10-year prognostic indicator for IgA nephropathy. Nephrol Dial Transplant. 2011;26:3937–3943. doi: 10.1093/ndt/gfr139. [DOI] [PubMed] [Google Scholar]
- 66.Tsuboi N, Utsunomiya Y, Koike K, et al. Factors related to the glomerular size in renal biopsies of chronic kidney disease patients. Clin Nephrol. 2013;79:277–284. doi: 10.5414/CN107817. [DOI] [PubMed] [Google Scholar]
- 67.Hughson MD, Hoy WE, Douglas-Denton RN, et al. Towards a definition of glomerulomegaly: clinical–pathological and methodological considerations. Nephrol Dial Transplant. 2011;26:2202–2208. doi: 10.1093/ndt/gfq688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nyengaard JR. Number and dimensions of rat glomerular capillaries in normal development and after nephrectomy. Kidney Int. 1993;43:1049–1057. doi: 10.1038/ki.1993.147. [DOI] [PubMed] [Google Scholar]
- 69.Flyvbjerg A, Schrijvers BF, De Vriese AS, et al. Compensatory glomerular growth after unilateral nephrectomy is VEGF dependent. Am J Physiol Endocrinol Metab. 2002;283:E362–E366. doi: 10.1152/ajpendo.00007.2002. [DOI] [PubMed] [Google Scholar]
- 70.Schrijvers BF, Flyvbjerg A, Tilton RG, et al. Pathophysiological role of vascular endothelial growth factor in the remnant kidney. Nephron Exp Nephrol. 2005;101:e9–15. doi: 10.1159/000086034. [DOI] [PubMed] [Google Scholar]
- 71.Wu Y, Liu Z, Xiang Z, et al. Obesity-related glomerulopathy: insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology. 2006;147:44–50. doi: 10.1210/en.2005-0641. [DOI] [PubMed] [Google Scholar]
- 72.D'Agati VD. Pathobiology of focal segmental glomerulosclerosis: new developments. Curr Opin Nephrol Hypertens. 2012;21:243–250. doi: 10.1097/MNH.0b013e32835200df. [DOI] [PubMed] [Google Scholar]
- 73.D'Agati V. Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 74.Jennette JC, Charles L, Grubb W. Glomerulomegaly and focal segmental glomerulosclerosis associated with obesity and sleep apnea syndrome. Am J Kidney Dis. 1987;10:470–472. doi: 10.1016/s0272-6386(87)80196-8. [DOI] [PubMed] [Google Scholar]
- 75.Kambham N, Markowiz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 76.Tsuboi N, Utsunomiya Y, Kanzaki G, et al. Low glomerular density with glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc Nephrol. 2012;7:735–741. doi: 10.2215/CJN.07270711. [DOI] [PubMed] [Google Scholar]
- 77.Griffin KA, Kramer H, Bidani AK. Adverse renal consequence of obesity. Am J Physiol. 2008;294:F685–F696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 78.Tsuboi N, Utsunomiya Y, Hosoya T. Obesity-related glomerulopathy and the nephron complement. Nephrol Dial Transplant. 2013;28(Suppl 4):iv108–iv113. doi: 10.1093/ndt/gft258. [DOI] [PubMed] [Google Scholar]
- 79.Hodgin JB, Rasoulpour M, Markowitz GS, et al. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2009;4:71–76. doi: 10.2215/CJN.01700408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herlitz LC, Markowitz GS, Farris AB, et al. Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol. 2010;21:163–172. doi: 10.1681/ASN.2009040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsuboi N, Kawamura T, Koike K, et al. Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5:39–44. doi: 10.2215/CJN.04680709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsuboi N, Kawamura T, Miyazaki Y, et al. Low glomerular density is a risk factor for progression in idiopathic membranous nephropathy. Nephrol Dial Transplant. 2011;26:3555–3560. doi: 10.1093/ndt/gfr399. [DOI] [PubMed] [Google Scholar]
- 83.Koike K, Tsuboi N, Utsunomiya Y, et al. Glomerular density-associated changes in clinicopathological features of minimal change nephrotic syndrome in adults. Am J Nephrol. 2011;34:542–548. doi: 10.1159/000334360. [DOI] [PubMed] [Google Scholar]
- 84.Zidar N, Cör A, Premru Srsen T, et al. Is there an association between glomerular density and birth weight in healthy humans. Nephron. 1998;80:97–98. doi: 10.1159/000045141. [DOI] [PubMed] [Google Scholar]
- 85.Kanzaki G, Tsuboi N, Utsunomiya Y, et al. Distribution of glomerular density in different cortical zones of the human kidney. Pathol Int. 2013;63:169–175. doi: 10.1111/pin.12044. [DOI] [PubMed] [Google Scholar]
- 86.Dinkel E, Ertel M, Dittrich M, et al. Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr Radiol. 1985;15:38–43. doi: 10.1007/BF02387851. [DOI] [PubMed] [Google Scholar]
- 87.Emamian SA, Nielsen MB, Pedersen JF, et al. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. Am J Roentgenol. 1993;160:83–86. doi: 10.2214/ajr.160.1.8416654. [DOI] [PubMed] [Google Scholar]
- 88.Miletić D, Fuckar Z, Sustić A, et al. Sonographic measurement of absolute and relative renal length in adults. J Clin Ultrasound. 1998;26:185–189. doi: 10.1002/(sici)1097-0096(199805)26:4<185::aid-jcu1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 89.Glodny B, Unterholzner V, Taferner B, et al. Normal kidney size and its influencing factors - a 64-slice MDCT study of 1.040 asymptomatic patients. BMC Urol. 2009;23:9–19. doi: 10.1186/1471-2490-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheong B, Muthupillai R, Rubin MF, et al. Normal values for renal length and volume as measured by magnetic resonance imaging. Clin J Am Soc Nephrol. 2007;2:38–45. doi: 10.2215/CJN.00930306. [DOI] [PubMed] [Google Scholar]
- 91.Bakker J, Olree M, Kaatee R, et al. Renal volume measurements: accuracy and repeatability of US compared with that of MR imaging. Radiology. 1999;211:623–628. doi: 10.1148/radiology.211.3.r99jn19623. [DOI] [PubMed] [Google Scholar]
- 92.Beeman SC, Zhang M, Gubhaju L, et al. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol. 2011;300:F1454–7. doi: 10.1152/ajprenal.00044.2011. [DOI] [PubMed] [Google Scholar]
- 93.Heilmann M, Neudecker S, Wolf I, et al. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol DialTransplant. 2012;27:100–107. doi: 10.1093/ndt/gfr273. [DOI] [PubMed] [Google Scholar]
- 94.Chaudhary K, Kleven DT, McGaha TL, et al. A human monoclonal antibody against the collagen type IV α3NC1 domain is a non-invasive optical biomarker for glomerular diseases. Kidney Int. 2013;84:403–408. doi: 10.1038/ki.2013.99. [DOI] [PubMed] [Google Scholar]