Abstract
Background
Patients undergoing hemodialysis frequently report pain with multifactorial causes, not limited to that experienced directly from hemodialysis treatment. Their pain may be nociceptive, neuropathic, somatic or visceral in nature. Despite this, pain in this population remains under-recognized and under-treated. Although several tools have been used to measure pain in patients undergoing hemodialysis as reported in the literature, none of them have been validated specifically in this population. The objective for this review was to compare and contrast these pain assessment tools and discuss their clinical utility in this patient population.
Methods
To identify pain assessment tools studied in patients undergoing hemodialysis, a literature search was performed in PubMed and Medline. An expert panel of dialysis and pain clinicians reviewed each tool. Each pain assessment tool was assessed on how it is administered and scored, its psychometric properties such as reliability, validity and responsiveness to change, and its clinical utility in a hemodialysis population. Brief Pain Inventory, McGill Pain Questionnaire, Pain Management Index, Edmonton Symptom Assessment System, Visual Analogue Scale and Faces Pain Scale were evaluated and compared.
Results
This assessment will help clinicians practicing in nephrology to determine which of these pain assessment tools is best suited for use in their individual clinical practice.
Keywords: dialysis, hemodialysis, pain, pain assessment, pain measurement
Introduction
Patients undergoing hemodialysis frequently report pain [1–3], and their pain tends to be moderate to severe in nature [1]. Despite this, there is evidence to show that pain in this population is inadequately managed. In a cohort of Canadian hemodialysis patients, 75% were found to have ineffective pain control [1]. The Dialysis Outcomes and Practice Patterns Study (DOPPS) found that analgesic use decreased in spite of increasing prevalence of pain, which suggests possible under-prescribing [4]. Furthermore, a recent systematic review found that the rate of effective pain control in patients with end-stage renal disease varies from 17 to 38%, with up to 84% of patients with significant pain receiving no analgesia [5]. These studies highlight the need for timely identification, assessment and management of pain in patients undergoing hemodialysis. Potential barriers to adequate pain management and assessment include patient under-reporting of pain, fear of addiction and adverse effects, lack of staff time and training and language [6].
It is important to note that pain experienced by the hemodialysis population tends to be multifactorial—including nociceptive, somatic, visceral, neuropathic and complex regional pain syndromes [1]. The etiology of pain in this group is not restricted to pain related to the hemodialysis treatment alone [1], but may also be a consequence of primary renal disease (e.g. polycystic kidney disease), renal failure (e.g. renal osteodystrophy, calciphylaxis) or other comorbidities such as diabetes, arthritis or vascular disease. Therefore, routine use of pain assessment tools that evaluate characteristics and severity of pain along with the functional disability caused by pain is crucial in developing effective pain management strategies and to monitor the safety and effectiveness of pain treatments [7].
As reported in the literature, several pain assessment tools have been used in the dialysis population to measure pain including the Brief Pain Inventory (BPI) [8–10], McGill Pain Questionnaire (MPQ) [1, 8, 11–14], Pain Management Index (PMI) [1, 11] and Edmonton Symptom Assessment System (ESAS) [14, 15]. Of these, the only tool that has been validated in hemodialysis population is the modified ESAS (mESAS) [15, 16], which includes a visual analoge scale (VAS) to assess pain as a part of a more comprehensive global patient assessment. In order to select an appropriate pain assessment tool for implementation into clinical practice, this paper seeks to compare and contrast the content, ease of administration and psychometric properties of these selected pain assessment tools and discuss their clinical utility in patients undergoing hemodialysis.
Materials and methods
To identify pain assessment tools studied in patients undergoing hemodialysis, a literature search was performed in PubMed and Medline using the MeSH terms ‘pain’, ‘assessment’ and ‘dialysis or hemodialysis’. Clinical trials and reviews were retrieved as sources of information. The reference lists of retrieved articles were also reviewed. This led to the identification of four multi-item tools: BPI, MPQ, PMI and ESAS. A mESAS has been validated for global assessment in patients undergoing hemodialysis. The PMI reflects the congruence between a patient's reported level of pain assessed by a variety of pain assessment tools and the potency of prescribed analgesics [16]. It was, therefore, excluded from this review. After discussion with experts including dialysis pharmacists (M.B. and K.C.) and a pain pharmacist (L.M.), it was decided that the BPI and MPQ would be included in our review based on guideline recommendations, ease of use in practice and evidence for reliability and validity. The VAS and Faces Pain Scale (FPS) were also subsequently included in our review because their pictorial bases of pain assessment lend well to their use and administration in a multilingual and multicultural population of varying literacy levels. Therefore, the pain assessment tools that were identified for review include VAS, FPS, BPI and MPQ. Although the mESAS has been validated in hemodialysis, pain assessment using a VAS represents only one part of this global assessment tool.
Once the assessment tools to be reviewed were finalized, another literature search was performed in Medline using the MeSH terms ‘pain assessment’ or ‘pain measurement’, in combination with the titles of individual pain assessment tools, e.g. ‘pain assessment.mp. or exp Pain Measurement’ [and] ‘Brief Pain Inventory’. This search was limited to studies published in English. Clinical trials and reviews that investigated properties of selected pain assessment tools were retrieved. The reference lists of retrieved articles were also reviewed. This review contains a summary of each pain assessment tool, which includes a brief description of how it is administered and scored, its psychometric properties such as reliability, validity and responsiveness to change and its clinical utility in a hemodialysis population.
Results
Visual Analoge Scale
Description. The VAS is a 1D tool that assesses global pain intensity [17] and is widely used for both clinical and research purposes. It consists of a simple horizontal or vertical line usually 100 mm in length, as shown in Figure 1 [18]. It is anchored at its extremes by two verbal descriptors—‘no pain’ at one end, and ‘worst pain possible’ at the other [19, 20].
Fig. 1.

Visual Analog Scale. Adapted from Haefeli et al. [18].
Number of items on scale: one.
Administration. This tool is comprised of a self-completed questionnaire where patients are asked to place a line perpendicular to the VAS line at a point representing their global pain intensity [20]. It requires minimal instruction and can be completed within 1min [21].
Scoring. The distance from the anchor of ‘no pain’ to the indicated mark is measured using a metric ruler [20]. The reported score may range from 0 to 100 mm, with a higher number indicating higher intensity of pain. Scores of 0–4 mm indicate no pain, 5–44 mm mild pain, 45–74 mm moderate pain and scores ≥75 mm indicate severe pain [22].
Types of pain assessed. Acute, chronic, nociceptive and neuropathic [17, 23, 24].
Psychometric information. The VAS has shown good reliability [23] but it tends to be lower among illiterate patients as demonstrated in one study in patients with arthritis [24]. It has shown excellent validity in patients with acute or chronic, cancer- or noncancer-related pain [17]. It has also shown high correlation rates with other pain scales [23, 25]. The horizontal and vertical versions of VAS are also highly correlated [26]. It is a sensitive tool to measure changes in intensity of pain [25]. According to patient-reported pain relief, changes of 10 mm or 15% on the VAS are correlated with ratings of ‘little relief’, and changes of 20–30 mm or 35–40% with rating of ‘some relief’ [22].
Clinical utility. The VAS is a rapid assessment tool and is adaptable to a wide range of settings, including hemodialysis. The graphic basis of VAS makes it a simple tool to use in a multicultural and multilingual population. However, limitations include: no information about other dimensions of pain, current pain treatments and their effectiveness, no differentiation between different types of pain and no information regarding functional interference. Older pain patients may have difficulty in understanding how to use this tool [20, 27] and may need additional supervision to assist in its completion.
How to obtain. VAS is readily available in public domain with no associated cost [21, 28], and can also be easily created.
Faces Pain Scale
Description. FPS is a 1D tool that assesses global pain intensity with the help of facial expressions. It consists of seven line-drawn, gender-neutral faces presented in a horizontal format [29]. To avoid confounding of affective distress with pain intensity [30], a revised version FPS-R was engineered [31] with faces without smiles and/or tears. The FPS-R also reduced the number of faces to six to allow metric scoring [31] as shown in Figure 2 [32] and is available in many languages [32]. This review will focus on FPS-R.
Fig. 2.
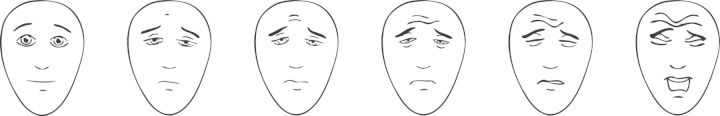
Faces Pain Scale—revised. Permission for reprint pending [32].
Number of items on scale. One.
Administration. This tool is a self-completed questionnaire where a patient is asked to select one of six faces that best represents the intensity of the patient's pain. It requires minimal instruction and can be completed within 1 min.
Scoring. Each face corresponds to a numeric score (0, 2, 4, 6, 8, 10) [32]. The numeric score corresponding to the face selected by the patient is recorded as a measure of pain intensity. The score may range from 0 to 10, with a higher number indicating higher intensity of pain.
Types of pain assessed. Acute and chronic pain, including noncancer-related pain [32–35].
Psychometric information. FPS-R has previously been validated in the pediatric pain population [32] but has also demonstrated strong reliability in adult populations with acute and chronic noncancer-related pain [30], including older adults with cognitive impairment [33]. FPS-R shows good validity and high correlation rates when compared with a variety of other pain assessment tools [30, 33, 34] including the VAS [33, 34]. An electronically administered version of the FPS-R has also been validated for use in hospitalized children [35] but remains to be validated in an older population. It has also been shown to have acceptable responsiveness to changes in pain intensity albeit lower when compared with other pain assessment tools like VAS [34]. This may be in part due to the smaller number of response options that FPS-R provides when compared with other pain assessment tools.
Clinical utility. Like the VAS, FPS-R is a rapid assessment tool and is adaptable to a wide range of settings. The graphic basis of FPS-R and availability of instructions in foreign languages makes it a simple tool to use in a multicultural and multilingual population, including older adults with cognitive impairment. An electronically administered version of the FPS-R is also available. These factors may help to ease the burden of administration. Limitations include: no information about other dimensions of pain, current pain treatments and their effectiveness, no differentiation between different types of pain and no information regarding functional interference. As previously mentioned, these form important considerations for clinicians to decide the type and aggressiveness of required pain management strategies.
How to obtain. FPS-R can be used in its unaltered form without prior permission for clinical, educational or research purposes [32]. Permission to use for other purposes and/or to make changes to FPS-R must be obtained from the International Association for Study of Pain, accessible online at www.painsourcebook.ca.
Brief Pain Inventory
Description. The BPI assesses multiple aspects of pain including pain intensity, interference from pain with daily activities, location of pain and effectiveness of treatments received for pain. Although it was previously validated only in cancer pain, it has now been validated in several chronic nonmalignant pain populations [36]. BPI is available in many versions, including the traditional longer form which includes more pain descriptors but is more cumbersome to use, and the BPI short form (SF-BPI) which is more widely used [37]. The SF-BPI also has the most number of foreign language translations [28, 37]. Our review will focus on the SF-BPI.
Number of items on scale. Fifteen [37].
Administration. This tool is administered as a self-completed questionnaire or an interview [37] which asks the patient to record the presence, distribution and severity of their pain. It provides an opportunity for the patient to record their worst, least and average pain typically over the prior week, and the level and type of functional disability caused by pain. It also allows supplemental information to be recorded like treatments being currently received for pain management and their effectiveness in relieving pain. The SF-BPI can be completed within 5 min [37].
Scoring. Four items in the pain intensity section of SF-BPI are scored from 0 (no pain) to 10 (worst pain possible), and the seven items in the functional interference section are scored from 0 (no interference) to 10 (complete interference). A pain severity score is calculated from the mean of four pain intensity items and a pain interference score is calculated from the mean of seven pain interference items [37]. These scores provide information about patient's pain along with single-item questions described above.
Types of pain assessed. Acute, chronic, nociceptive and neuropathic [28, 36, 38, 39].
Psychometric information. The reliability of the SF-BPI has been demonstrated [38, 40] and ranges from 0.83 to 0.87 for pain severity and 0.84 to 0.93 for functional interference [40] in patients with osteoarthritis. Construct validity for the SF-BPI has been supported in assessment in chronic pain populations such as osteoarthritis [28, 36], multiple sclerosis [36] and diabetic neuropathy [36, 39] as well as acute postoperative pain [38]. It has also shown responsiveness in detecting improvement with analgesic use [40].
Clinical utility. The SF-BPI has been validated in the most number of foreign languages and hence has excellent utility in a multicultural and multilingual population. It has also been validated in cancer populations and importantly many noncancer chronic pain populations, including neuropathic pain. This is a crucial aspect for assessing pain in patients undergoing hemodialysis because of the diverse nature and etiology of their pain. It provides additional information in the form of current treatments being undertaken for pain management and their effectiveness, which makes it a useful follow-up assessment tool once treatments have been started. However, it takes longer to complete than the above tools. Moreover, it does not include items like the quality of pain experienced by patients, which may be essential in differentiating between the different etiologies of pain. Although it gathers information about the effectiveness of pain treatments being administered, it does not give patients an opportunity to comment on their tolerability. Finally, it has been shown [41] that administering a self-completed questionnaire without prior discussion about pain may hinder patients’ ability to assess their own pain, especially in older adults. Patients undergoing hemodialysis tend to be older and hence may require more assistance in completing this questionnaire.
How to obtain. The use or reproduction of BPI requires prior consent from its copyright holders [37]. Consent can be obtained by contacting Cleeland and associates via e-mail at symptomresearch@mdanderson.org or via mail through: the Department of Symptom Research, Attn: assessment Tools, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1450, Houston, TX 77030, USA. Permission to use BPI for nonfunded academic research or individual clinical practice is not associated with any charges. However, its use in commercial or funded academic research is associated with charges [37].
McGill Pain Questionnaire
Description. MPQ is a multidimensional tool for pain assessment, and primarily consists of three major classes of pain descriptors—sensory, affective and evaluative [42]. The traditional version of MPQ consists of 78 pain descriptors categorized into 20 groups. However, this can prove to be lengthy and inconvenient [43]. Our review will focus on the short form of MPQ, SF-MPQ, which consists of 15 pain descriptors (11 from sensory and 4 from evaluative aspects of pain), a VAS for assessing average pain intensity and a verbal descriptor scale for assessing current pain [42].
Number of items on scale. Fifteen.
Administration. It is administered as a self-completed questionnaire which asks the patient to rate each of the 15 pain descriptors as none, mild, moderate or severe. It also allows the patient to record their present and average pain intensity. The SF-MPQ can be completed within 5 min [43].
Scoring. Pain descriptors are self-rated by the patient according to intensity level (0 = none, 1 = mild, 2 = moderate and 3 = severe). Three pain scores are derived from the sum of the intensity rank values of the words chosen for sensory, affective and total descriptors and may range from 0 to 45 [43]. Scores on present pain intensity range from 0 to 5 [43] and on VAS as described above. A higher score indicates more pain.
Types of pain assessed. Acute, chronic, musculoskeletal, neuropathic and non-neuropathic [44–48].
Psychometric information. SF-MPQ demonstrates excellent reliability [49] in nonmalignant chronic pain populations including osteoarthritis [44] and neuropathic pain [45]. It has also shown to be a reliable tool when administered electronically [46]. Both concurrent and criterion validity for SF-MPQ has been shown when compared with the traditional longer version of MPQ [47]. It has also been shown to be responsive to change with pharmacological and nonpharmacological treatments [43] in different groups of patients [49], including patients with neuropathic [45] and musculoskeletal pain [48].
Clinical utility. SF-MPQ has been validated in many languages [45, 47, 50] and can hence be used in a multicultural and multilingual population. It has been shown that the meaning of pain descriptors and intensity ranking scale is easily understood by patients [43]. It has been validated in neuropathic and non-neuropathic chronic pain populations, including cancer- and noncancer-related pain. Hence, it can prove to be useful in patients undergoing hemodialysis who present with diverse etiologies and presentations of pain. Furthermore, the availability of a reliable electronically administered version of SF-MPQ has enhanced the convenience of its use and administration. However, it does not include information regarding functional interference caused by pain, treatments being received for pain or effectiveness and safety of these treatments. It also takes longer to complete when compared with the VAS and the FPS-R. It has been shown that lack of familiarity with the multiple pain descriptors in the questionnaire hinders the completion rate [44]. This may necessitate increased supervision and guidance among new users of SF-MPQ. Although patients are able to report the intensity of their pain, they are unable to report its frequency.
How to obtain. MPQ can be obtained at no charge from its developer via mail through Ronald Melzack, PhD, Department of Psychology, McGill University, 1205 Dr Penfield Avenue, Montreal, Quebec, Canada H3A 1B1, and online at www.qolid.org after paying a membership fee [51].
Discussion
Several tools that may be used for pain assessment in patients undergoing hemodialysis have been reviewed here. Each tool has its own strengths and limitations for use in this pain population. The VAS and FPS-R are 1D tools that assess global pain intensity. They are rapid assessment tools and easy to administer and score. Both are available with instructions in multiple languages, which make them useful in multicultural and multilingual population. However, neither is a comprehensive pain assessment tool because they lack information regarding other dimensions of pain, quality of pain and pain treatments being administered. In contrast, the SF-BPI and SF-MPQ are multidimensional, more comprehensive pain assessment tools but take longer to complete. Both are widely used for research and clinical purposes. They are also available in multiple languages for use in diverse populations. The SF-BPI, unlike SF-MPQ, allows patients to record information about pain treatments being administered and their effectiveness in relieving pain. This allows clinicians to both, monitor pain management and make changes to treatments as appropriate. On the other hand, SF-MPQ allows patients to record the quality of their pain unlike the SF-BPI. This may help clinicians differentiate between neuropathic and nociceptive pain, which will ultimately guide the choice of pain treatment.
This review is the first of its kind in nephrology to inform clinicians of the strengths and limitations of selected pain assessment tools for patients undergoing hemodialysis. Although none of these tools have been validated in patients undergoing peritoneal dialysis, their clinical utility can be hypothesized to be equally true in peritoneal dialysis. Clinicians can use this information to help them determine the most appropriate tool to implement in their individual clinical practices. It is hoped that with implementation of routine pain assessments, identification and treatment will improve for patients in pain undergoing hemodialysis.
Conflict of interest statement
None declared.
References
- 1.Davison SN. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42:1239–1247. doi: 10.1053/j.ajkd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Fainsinger RL, Davison SN, Brenneis C. A supportive care model for dialysis patients. Palliat Med. 2003;17:81–82. doi: 10.1191/0269216303pm666xx. [DOI] [PubMed] [Google Scholar]
- 3.Davison SN, Jhangri GS. The impact of chronic pain on depression, sleep and desire to withdraw from dialyisis. J Pain Symptom Manage. 2005;30:465–473. doi: 10.1016/j.jpainsymman.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Bailie GR, Mason NA, Bragg-Gresham JL, et al. Analgesic prescription patterns among hemodialysis patients in the DOPPS: potential for underprescription. Kidney Int. 2004;65:2419–2424. doi: 10.1111/j.1523-1755.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 5.Wyne A, Rai R, Cuerden M, et al. Opioid and benzodiazepine use in end-stage renal disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:326–333. doi: 10.2215/CJN.04770610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salisbury EM, Game DS, Al-Shakarchi I, et al. Changing practice to improve pain control for renal patients. Postgrad Med J. 2009;85:30–33. doi: 10.1136/pgmj.2008.071191. [DOI] [PubMed] [Google Scholar]
- 7.Glick N, Davison SN. Managing chronic pain in advanced chronic kidney disease, US. Nephrology. 2011;6:21–28. [Google Scholar]
- 8.Calls J, Rodríguez Calero M, Hernández Sánchez D, et al. An evaluation of pain in hemodialysis patients using different validated measurement scales. Nefrologia. 2009;29:236–243. doi: 10.3265/Nefrologia.2009.29.3.5120.en.full. [DOI] [PubMed] [Google Scholar]
- 9.Gamondi C, Galli N, Schönholzer C, et al. Frequency and severity of pain and symptom distress among patients with chronic kidney disease receiving dialysis. Swiss Med Wkly. 2013;143:w13750. doi: 10.4414/smw.2013.13750. [DOI] [PubMed] [Google Scholar]
- 10.Golan E, Haggiag I, Os P, et al. Calcium, parathyroid hormone, and vitamin D: major determinants of chronic pain in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1374–1380. doi: 10.2215/CJN.00680109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barakzoy AS, Moss AH. Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17:3198–3203. doi: 10.1681/ASN.2006050477. [DOI] [PubMed] [Google Scholar]
- 12.Harris TJ, Nazir R, Khetpal P, et al. Pain, sleep disturbance and survival in hemodialysis patients. Nephrol Dial Transplant. 2012;27:758–765. doi: 10.1093/ndt/gfr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masajtis-Zagajewska A, Pietrasik P, Krawczyk J, et al. Similar prevalence but different characteristics of pain in kidney transplant recipients and chronic hemodialysis patients. Clin Transplant. 2011;25:E144–E151. doi: 10.1111/j.1399-0012.2010.01359.x. [DOI] [PubMed] [Google Scholar]
- 14.Binik YM, Baker AG, Kalogeropoulos D, et al. Pain, control over treatment, and compliance in dialysis and transplant patients. Kidney Int. 1982;21:840–848. doi: 10.1038/ki.1982.108. [DOI] [PubMed] [Google Scholar]
- 15.Davison SN, Jhangri GS, Johnson JS. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 2006;69:1621–1625. doi: 10.1038/sj.ki.5000184. [DOI] [PubMed] [Google Scholar]
- 16.Davison SN, Jhangri GS, Johnson JS. Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3189–3195. doi: 10.1093/ndt/gfl380. [DOI] [PubMed] [Google Scholar]
- 17.McCormack HM, Horne DJ, Heather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18:1007–1019. doi: 10.1017/s0033291700009934. [DOI] [PubMed] [Google Scholar]
- 18.Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15:S17–S24. doi: 10.1007/s00586-005-1044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 21.Burckhardt CS, Jones KD. Adult measures of pain: the McGill Pain Questionnaire (MPQ), Rheumatoid Arthritis Pain Scale (RAPS), Short-Form McGill Pain Questionnaire (SF-MPQ), Verbal Descriptive Scale (VDS), Visual Analog Scale (VAS), and West Haven-Yale Multidisciplinary Pain Inventory (WHYMPI) Arthritis Rheum. 2003;49:S96–S104. [Google Scholar]
- 22.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–414. doi: 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 23.Englbrecht M, Tarner IH, van der Heijde DM, et al. Measuring pain and efficacy of pain treatment in inflammatory arthritis: a systematic literature review. J Rheumatol Suppl. 2012;90:3–10. doi: 10.3899/jrheum.120335. [DOI] [PubMed] [Google Scholar]
- 24.Ferraz MB, Quaresma MR, Aquino LR, et al. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol. 1990;17:1022–1024. [PubMed] [Google Scholar]
- 25.McCarberg B, Stanos S. Key patient assessment tools and treatment strategies for pain management. Pain Practice. 2008;8:423–432. doi: 10.1111/j.1533-2500.2008.00223.x. [DOI] [PubMed] [Google Scholar]
- 26.Scott J, Huskisson EC. Vertical or horizontal visual analogue scales. Ann Rheum Dis. 1979;38:560. doi: 10.1136/ard.38.6.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters ML, Patijn J, Lamé I. Pain assessment in younger and older pain patients: psychometric properties and patient preference of five commonly used measures of pain intensity. Pain Med. 2007;8:601–610. doi: 10.1111/j.1526-4637.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 28.Williams DA, Arnold LM. Measures of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) Sleep Scale, and Multiple Ability Self-Report Questionnaire (MASQ) Arthritis Care Res. 2011;63(Suppl 11):S86–S97. doi: 10.1002/acr.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bieri D, Reeve R, Champion GD, et al. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation and preliminary investigation for ratio scale properties. Pain. 1990;41:139–150. doi: 10.1016/0304-3959(90)90018-9. [DOI] [PubMed] [Google Scholar]
- 30.Miró J, Huguet A, Nieto R, et al. Evaluation of reliability, validity, and preference for a pain intensity scale for use with the elderly. J Pain. 2005;6:727–735. doi: 10.1016/j.jpain.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Hicks CL, von Baeyer CL, Spafford P, et al. The Faces Pain Scale—revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 32.Faces Pain Scale—Revised. International Association for the Study of Pain. http://www.painsourcebook.ca. (30 July 2013, date last accessed) [Google Scholar]
- 33.Ware LJ, Epps CD, Herr K, et al. Evaluation of the Revised Faces Pain Scale, Verbal Descriptor Scale, Numeric Rating Scale, and Iowa Pain Thermometer in older minority adults. Pain Manag Nurs. 2006;7:117–125. doi: 10.1016/j.pmn.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152:2399–2404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Wood C, von Baeyer CL, Falinower S, et al. Electronic and paper versions of a faces pain intensity scale: concordance and preference in hospitalized children. BMC Pediatr. 2011;11:87–95. doi: 10.1186/1471-2431-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkinson TM, Mendoza TR, Sit L, et al. The Brief Pain Inventory and its ‘pain at its worst in the last 24 hours’ item: clinical trial endpoint considerations. Pain Med. 2010;11:337–346. doi: 10.1111/j.1526-4637.2009.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brief Pain Inventory (BPI) The University of Texas MD Anderson Cancer Center. http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. (30 July 2013, date last accessed) [Google Scholar]
- 38.Mendoza TR, Chen C, Brugger A, et al. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin J Pain. 2004;20:357–362. doi: 10.1097/00002508-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Zelman DC, Gore M, Dukes E, et al. Validation of a modified version of the Brief Pain Inventory for painful diabetic peripheral neuropathy. J Vasc Nurs. 2005;23:97–104. doi: 10.1016/j.jvn.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Mendoza T, Mayne T, Rublee D, et al. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10:353–361. doi: 10.1016/j.ejpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 41.McDonald DD, Shea M, Fedo J, et al. Older adult pain communication and the Brief Pain Inventory Short Form. Pain Manag Nurs. 2008;9:154–159. doi: 10.1016/j.pmn.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 43.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 44.Grafton KV, Foster NE, Wright CC. Test-retest reliability of the Short-Form McGill Pain Questionnaire: assessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. Clin J Pain. 2005;21:73–82. doi: 10.1097/00002508-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Adelmanesh F, Jalali A, Attarian H, et al. Reliability, validity, and sensitivity measures of expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2) in Iranian patients with neuropathic and non-neuropathic pain. Pain Med. 2012;13:1631–1636. doi: 10.1111/j.1526-4637.2012.01517.x. [DOI] [PubMed] [Google Scholar]
- 46.Cook AJ, Roberts DA, Henderson MD, et al. Electronic pain questionnaires: a randomized, crossover comparison with paper questionnaires for chronic pain assessment. Pain. 2004;110:310–317. doi: 10.1016/j.pain.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Yakut Y, Yakut E, Bayar K, et al. Reliability and validity of the Turkish version short-form McGill pain questionnaire in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:1083–1087. doi: 10.1007/s10067-006-0452-6. [DOI] [PubMed] [Google Scholar]
- 48.Menezes CLC, Maher CG, McAuley JH, et al. The Brazilian-Portuguese versions of the McGill Pain Questionnaire were reproducible, valid, and responsive in patients with musculoskeletal pain. J Clin Epidemiol. 2011;64:903–912. doi: 10.1016/j.jclinepi.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Strand LI, Ljunggren AE, Bogen B, et al. The Short-Form McGill Pain Questionnaire as an outcome measure: test-retest reliability and responsiveness to change. Eur J Pain. 2008;12:917–925. doi: 10.1016/j.ejpain.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Lazaro C, Bosch F, Torrubia R, et al. The development of a Spanish questionnaire for assessing pain: preliminary data concerning reliability and validity. Euro J Psychol Assess. 1994;10:145–151. [Google Scholar]
- 51.Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res. 2011;63:S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]


