Abstract
Background
Inflammatory markers vary considerably over time in haemodialysis (HD) patients, yet the variability is poorly defined. The aim of the study was to assess changes of plasma levels of pentraxin-3 (PTX-3), C-reactive protein (CRP), albumin and homocysteine (Hcy) over 3 months and the association between the changes in these biomarkers and mortality.
Methods
In 188 prevalent HD patients, inflammatory markers were measured at inclusion and after 3 months. Mortality was recorded during a median follow-up of 41 months. The changes of the biomarker levels were categorized according to change in tertile for the specific biomarker. The variation was calculated as the intra-class correlation (ICC). Mortality was analysed by Kaplan–Meier and Cox proportional hazards model. The predictive strength was calculated for single measurements and for the variation of each inflammatory marker.
Results
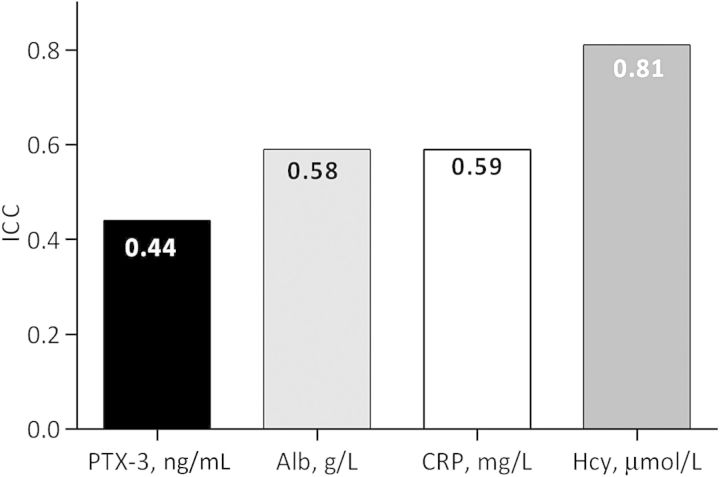
The intra-individual variation (low ICC) was largest for PTX-3 [ICC 0.44; 95% confidence interval (CI): 0.33–0.55], albumin (ICC 0.58; 95% CI: 0.49–0.67) and CRP (ICC 0.59; 95% CI: 0.51–0.68) and lowest for Hcy (ICC 0.81; 95% CI: 0.77–0.86). During follow-up, 88 patients died.
Conclusions
PTX-3 measurements are less stable and show higher variation within patients than CRP, albumin and Hcy. Persistently elevated PTX-3 levels are associated with high mortality. Moreover, in multivariate logistic regression we found that stable high PTX-3 adds to the mortality risk, even after inclusion of clinical factors and the three other biomarkers. The associations of decreasing albumin levels as well as low Hcy levels with worse outcome reflect protein-energy wasting.
Keywords: end-stage renal disease, HD, inflammation, pentraxin 3, variation
Introduction
The role of inflammatory biomarkers as predictors of morbidity and mortality in chronic kidney disease (CKD) patients is debated [1, 2]. CKD patients are repeatedly exposed to inflammatory stimuli, and serum levels of inflammatory markers show a substantial inter- and intra-individual variation over time [3, 4]. The variability of the inflammatory response is influenced by traditional risk factors such as age, body mass index (BMI), smoking [5], comorbidities, inter-current events and factors related to declining renal function and the dialysis procedure, i.e. bio-incompatibility, ultrafiltrate backflow and vascular access factors [6, 7]. Malnourished dialysis patients often have protein-energy wasting (PEW), a well-known complication of CKD described as a progressive loss of body mass and energy reserves [8].
Both pentraxin 3 (PTX-3) and C-reactive protein (CRP) are members of the pentraxin family of proteins considered to be markers of the acute phase of inflammation [9]. CRP is the most used inflammatory biomarker as a predictor of comorbidities and mortality in patients with CKD [10]. The relationship between longitudinal inflammatory variation and mortality risk is of interest, and recently a study of CRP variation over a 3-month period in prevalent haemodialysis (HD) patients show an association with a higher mortality risk [6, 11, 12]. In contrast to CRP that is synthesized in the liver, PTX-3 is produced in vascular endothelial cells, neutrophils and macrophages. Neutrophil granules serve as a reservoir of PTX-3 and can rapidly release PTX-3 in response to microbial recognition and inflammatory signals. PTX-3 can, therefore, directly reflect the inflammatory status in the vasculature [13]. Elevated PTX-3 levels reflect endothelial dysfunction in CKD patients [14].
As far as we know, the impact of longitudinal variation of PTX-3 on mortality has not been studied yet. The aim of the present study was to study changes of PTX-3 and other biomarkers over time and their association to mortality. We compare the mortality risk associated with changes in PTX-3 with a pure inflammatory marker (CRP), a negative acute-phase reactant (albumin) and the metabolic marker homocysteine (Hcy).
Materials and methods
Subjects and study design
This study comprises individuals from the Mapping of Inflammatory Markers in Chronic Kidney Disease (MIMICK) cohort, the protocol of which has been described elsewhere in more detail [5]. In total, 188 HD patients who had two consecutive measurements of CRP, PTX-3, albumin and Hcy 3 months apart, were included. The study protocol was approved by the Ethics Committee of Karolinska Institutet, Stockholm, Sweden, and informed consent was obtained from each patients.
Comorbidity and nutritional status assessments
Comorbidities were classified according to Davies et al. [15] on a seven-point scale which is simplified into three risk categories (low, medium and high comorbidity risk). Nutritional status was assessed by the subjective global assessment (SGA), BMI, lean body mass index (LBMI) and fat body mass index (FBMI) [16]. SGA was trans-calculated to a three-point scale (1 = normal nutritional status, 2 = mild-to-moderate malnutrition and 3 = severe malnutrition) [17], and poor nutritional status was defined as an SGA > 1. BMI was calculated as body weight (kg) divided by the squared height (m). The skinfold thicknesses were assessed at four sites (biceps, triceps, subscapular and suprailiac) using a skinfold calliper (Cambridge Scientific Instruments, Cambridge, MA), and FBM was estimated from these data according to Durnin and Womersley [18].
Laboratory analysis
PTX-3: the plasma and serum were centrifuged (2500 g, 20 min, 4°C) within 30 min, and samples were kept frozen at −70°C if not analysed immediately. Plasma PTX-3 concentration was measured by using a commercially available enzyme-linked immunosorbent assay kit (Perseus Proteomics Inc., Tokyo, Japan).
High-sensitivity CRP was measured by an immunometric assay (Immulite, DPC, Siemens, CA), and serum albumin levels were determined by routine procedures at the Department of Clinical Chemistry, Karolinska University Hospital. To analyse total Hcy, 10 µL tri-n-butylphosphine was mixed in glass tubes with 100 µL plasma and kept for 30 min at +4°C in order to reduce thiols and to decouple protein-bound thiols. Then, the proteins were precipitated with 100 µL of 10% (vol/weight) trichloroacetic acid (TCA) containing 1 mM ethylenediamine tetraacetate and again resting 30 min at + 4°C pending centrifugation for 5 min at +4°C, 5100 g. The centrifugated filtered (0.22 µm UFC 30GVNB, Millipore) supernatant was stored at −70°C until analysed [19].
Statistical methods
The patients were grouped according to within which tertile (33rd and 66th percentiles) their baseline and 3-month levels of PTX-3, CRP, albumin and Hcy were. Four groups were created: (i) patients with a decrease in serum levels over the 3-month period (from high-to-middle, middle-to-low or high-to-low tertiles) were assigned as ‘decrease group’; (ii) patients with an increase (from low-to-middle, middle-to-high and low-to-high tertiles) were assigned as an ‘increase group’; (iii–iv) individuals with both values within the highest tertile or the lowest and middle tertile of distribution were assigned as ‘stable high group’ and ‘stable low group’, respectively. For PTX-3, the 33rd and 66th percentiles were 7.9 and 13.6 ng/mL for baseline measurements and 6.9 and 12.1 ng/mL for the 3-month measurements.
All values are expressed as mean ± standard deviation, median (10–90 percentiles) or percentage, as appropriate. Comparisons of nominal variables between groups were made by χ2 test. Differences between the four groups were analysed using the non-parametric one-way analysis of variance Kruskal–Wallis. We used differences over the 3-month period (ΔPTX-3, ΔCRP, Δalbumin and ΔHcy) for Spearman's rank correlation. To study within-subject variation for the markers, ICC was calculated from estimates of between-subject and within-subject variance and derived from a mixed model [20].
Survival was assessed through the Kaplan–Meier analysis and the Cox proportional hazards model. Age, sex, comorbidities and nutritional status were used as confounders in the multivariate Cox models. All variables were tested for the proportionality hazards assumption. The significance level in all analyses was set at a P-value of <0.05. Clinical outcome was expressed as hazard ratios (HRs) and 95% confidence intervals (95% CIs). All statistical analyses were performed using statistical software SAS version 9.4 (SAS Campus Drive, Cary, NC).
Results
Patients in the ‘PTX-3 stable high group’ had significantly lower albumin, BMI, LBMI, FBMI and higher Davies score compared with the ‘stable low PTX-3’ group (Table 1) indicating that patients with elevated PTX-3 were wasted and had more comorbid conditions. The patients in the stable high PTX-3 group had a significantly higher mortality rate than the stable low group. Age, sex, dialysis vintage, presence of CVD or PEW according to SGA showed no significant differences between groups. At baseline, patients with CVD, PEW or inflammation had significantly higher levels of PTX-3 and CRP, and significantly lower levels of albumin and Hcy. There were significant associations between PTX-3 and BMI (rho = −0.33, P < 0.001), LMBI (rho = −0.35, P < 0.001) and FBMI (rho = −0.26, P < 0.01).
Table 1.
Baseline characteristics of all patients included in the study and after stratification according to PTX-3 tertile variation categories in MIMICK subjectsa
| All patients (n = 188) | Stable lowa (n = 75) | Decreasea (n = 36) | Increasea (n = 34) | Stable higha (n = 43) | P-value | |
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Men, %b | 55 | 53 | 39 | 59 | 67 | ns |
| Age, yearsc | 66 (43–80) | 68 (43–81) | 64 (43–77) | 65 (41–78) | 67 (47–81) | ns |
| CVD, %b | 62 | 55 | 61 | 68 | 72 | ns |
| Dialysis vintage, monthsc | 29 (7–104) | 29 (7–94) | 33 (7–109) | 24 (4–53) | 36 (6–143) | ns |
| SGA > 1, %b | 46 | 35 | 50 | 55 | 46 | |
| Low/medium/high Davies score, %b | 39/105/44 | 30/55/15 | 20/58/22 | 15/59/26 | 9/54/37 | <0.05 |
| BMI, kg/m2c | 24 (18–31) | 25 (19–33) | 23 (18–32) | 26 (20–32) | 22 (17–27) | <0.01 |
| LBMI, kg/m2c | 16 (13–20) | 17 (13–21) | 16 (13–20) | 17 (14–21) | 16 (13–18) | <0.01 |
| FBMI, kg/m2c | 8.0 (4.4–13) | 8.3 (5.2–14) | 8.2 (4.2–14) | 8.8 (4.5–11) | 6.7 (3.5–9.4) | <0.01 |
| CRP, mg/Lc | 6.3 (0.9–45) | 5 (0.8–24) | 6 (1.4–63) | 7 (0.5–31) | 9 (1.1–103) | ns |
| Hcy, µmol/Lc | 32 (14–60) | 33 (16–60) | 27 (16–54) | 37 (14–66) | 25 (10–52) | ns |
| S-Albumin, g/Lc | 35 (30–41) | 37 (32–41) | 37 (28–42) | 35 (30–40) | 33 (26–39) | <0.01 |
| Characteristics during follow-up | ||||||
| Number of deaths, n (%)b | 88 (47) | 28 (37) | 14 (39) | 17 (50) | 29 (67) | <0.05 |
| Time to death, months | 22 (7–40) | 22 (11–42) | 24 (6–43) | 26 (10–39) | 20 (5–39) | ns |
ns, not significant.
aPTX-3 variation groups were constructed according to changes in tertiles of the distribution at baseline and 3-month measurement (see Materials and methods).
bDifferences between groups in categorical data were tested by means of a χ2 test.
cData are expressed as median (10–90 percentiles). Differences between groups were tested by means of Kruskal–Wallis tests.
Longitudinally, ΔPTX-3 was significantly associated to ΔCRP and inversely associated to Δalbumin but not to ΔHcy. There was a significant inverse association between ΔCRP and Δalbumin, but there was no association between ΔCRP and ΔHcy (Table 2).
Table 2.
Correlation matrix of changes in inflammatory markers
| ΔPTX3 | ΔHcy | ΔAlbumin | |
|---|---|---|---|
| ΔHcy | −0.12 | ||
| ΔAlbumin | −0.17a | 0.10 | |
| ΔCRP | 0.32a | −0.10 | −0.43b |
Correlations (rho) were calculated by Spearman's rank tests.
aP < 0.05.
bP < 0.01.
Variability of PTX-3, CRP, Hcy and albumin
From the variation of PTX-3, CRP, albumin and Hcy, the ICC was calculated and the within-subject variation was most pronounced for PTX-3 having the lowest ICC (44%). Hcy had the highest ICC (81%), indicating that most of the observed variability in a given assay is explained by between-subject variation, implying greater stability within individual patients upon repeated measurements over time. In contrast, the low ICC of PTX-3 implies that the within-subject variation is high and that repeated measurements in the same individuals over time will yield more disparate results (Figure 1).
Fig. 1.
Variance components for PTX-3, CRP, albumin and Hcy in HD patients. The ICC was calculated from estimates of between-subjects and within-subjects derived from mixed models.
Mortality risk showed as crude and adjusted HR
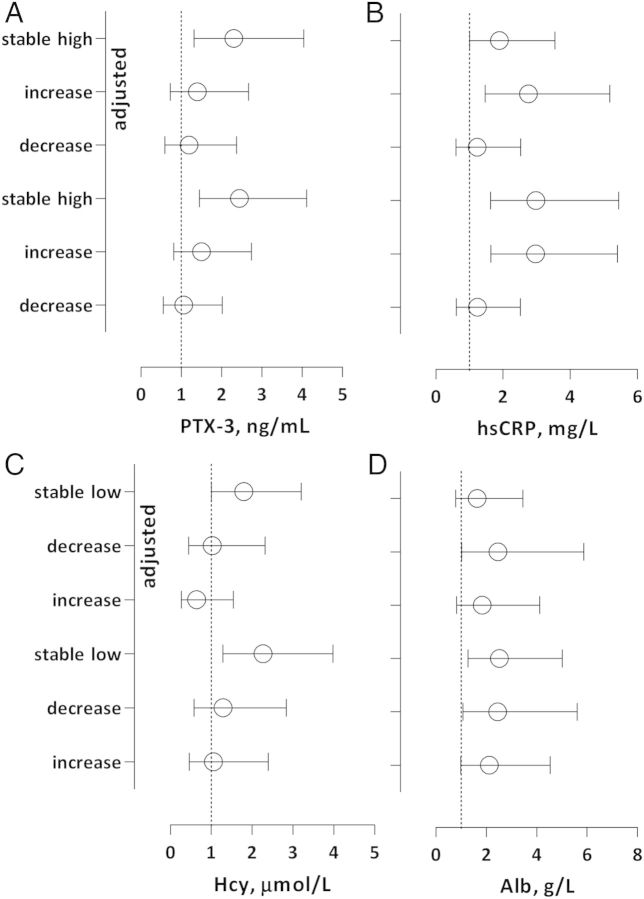
The mortality risk was assessed for groups of PTX-3, CRP, albumin and Hcy as crude and adjusted HRs. In the present study, 88 (47%) individuals died during the observation time of 41 (11–50) months and in unadjusted crude analysis the best survival was seen in the stable high-albumin group (Figure 2A–D). Patients in the stable high group of PTX-3 had a higher mortality risk than patients in the stable low-PTX-3 group [HR 2.45 (95% CI: 1.45–4.11), P < 0.01]. The risk remained significantly higher after adjustments for age, sex, CVD, DM, vintage and malnutrition [HR 2.31 (95% CI: 1.32–4.04), P < 0.01]. Patients in the increased and stable high-CRP groups had higher mortality risk than the stable low group, [HR 2.97 (95% CI: 1.63–5.40), P < 0.01] and [HR 2.98 (95% CI: 1.63–5.45), P < 0.01], respectively, and the risk remained significant after adjustments, [HR 2.76 (95% CI: 1.47–5.17), P < 0.01] and [HR 1.89 (95% CI: 1.01–3.55), P < 0.05], respectively. In contrast, patients in the stable low-Hcy group had a higher mortality risk compared with the stable high group before and after adjustments for age, sex and comorbidity, [HR 2.26 (95% CI: 1.29–3.98), P < 0.01] and [HR 1.80 (95% CI: 1.01–3.20), P < 0.05], respectively. Concerning albumin, patients in the decrease group had significant higher mortality risk [HR 2.46 (95% CI: 1.03–5.87), P < 0.05] than the stable high group after adjustments (Figure 3A–D).
Fig. 2.
Kaplan–Meier survival curves according to PTX-3 (A), CRP (B), albumin (C) and Hcy (D) variation groups.
Fig. 3.
(A–D) Crude and adjusted mortality risk for each variation category according to the 33rd and 67th percentiles of PTX-3, CRP, alb and Hcy. The groups with highest survival rate were set as reference groups; the stabile low group was the reference category for PTX-3 and CRP and the stable high group was the reference category for alb and Hcy. Adjustments were made for age, sex, CVD, DM, vintage and malnutrition (SGA > 1). Mortality risks were expressed as HRs and 95% CI.
Relative contributions of factors explaining the variation of clinical outcome
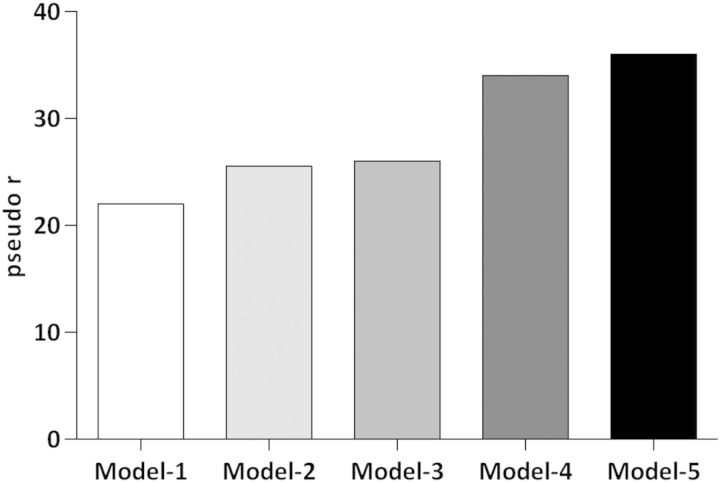
We studied the impact of various clinical and demographic factors on the mortality risk by logistic regression. In the first model, we included age, sex and Davies comorbidity score (pseudo r = 0.22). In Model 2, we added the effect of being in the Hcy decrease group, increase group and stable low group compared with the reference stable high group (pseudo r = 0.25). In Model 3, we added the effect of being in the albumin decrease group, increase group and stable low group and used stable high group as reference (pseudo r = 0.26). Similarly, in Model 4, we added the effect of being in the CRP decrease group, increase group and stable high group compared with the stable low group as reference (pseudo r = 0.34). In Model 5, we added the effect of being in the PTX-3 decrease group, increase group and stable high group compared with the stable low group as reference (pseudo r = 0.36) (Figure 4).
Fig. 4.
The impact of age, gender and Davies comorbidity score is shown as pseudo r for the clinical outcome (Model 1), Model 1 + Hcy (Model 2), Model 2 + albumin (Model 3), Model 3 + CRP (Model 4) and Model 4 + PTX-3 (Model 5).
Discussion
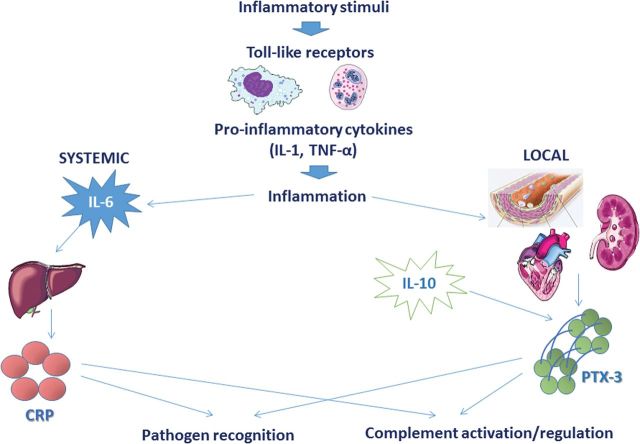
The long pentraxin PTX-3 has an important role in the innate immune system as outlined in Figure 5. Together with the short pentraxins, CRP and serum amyloid P, PTX-3 is a member of a family of ancient proteins whose structure is well-preserved throughout evolution [21]. CRP is synthesized in the liver in response to interleukin 6 (IL-6) and other inflammatory factors while PTX-3 is synthesized in the vasculature in response to inflammatory mediators such as lipopolysaccharides, interleukin 1β and tumour necrosis factor α but not to IL-6 [22]. As a tuner of the immune system, PTX-3 is involved in pathogen recognition, complement activation and regulation, which improves the defence against various infections, but also in angiogenesis and atherosclerotic lesion development. The role of PTX-3 in the atherosclerosis process is debated; on one hand studies show that PTX-3 has an active part in foam formation in plaques and takes part in activation of the classical complement cascade [23–25], and on the other hand another study shows cardioprotective effects of PTX-3 in healthy men [26].
Fig. 5.
Role of PTX-3 in the innate immune system. Toll-like receptors are activated by inflammation. While CRP secretion from the liver is stimulated by systemic IL-6, PTX-3 is released locally in the vasculature. IL-10 amplifies PTX-3 secretion. PTX-3 and CRP are involved in pathogen recognition, complement activation and regulation.
This is the first study to show repeated measurements of PTX-3 in a dialysis cohort. Our main findings are that PTX-3 is highly variable within individuals, even more so than CRP and that the trimestral variation of PTX-3 associate to mortality. According to the ICC analysis, a method to compare estimates of between-subjects and within-subject variances, PTX-3 had the highest intra-individual variation during the study followed by albumin, CRP and Hcy (Figure 1).
The inflammatory markers PTX-3 and CRP are positively associated while albumin and Hcy are negatively associated with a higher mortality risk. After adjustments for clinical factors, the PTX-3 stable high group and the CRP increase and stable high groups remained significantly associated with high mortality risk compared with stable low groups of these markers (Figure 3). Moreover, in multivariate logistic regression we found that stable high PTX-3 adds to the mortality risk, even after inclusion of clinical factors and the three other biomarkers (Figure 4).
Low serum albumin is associated with poor outcome in dialysis patients [27] and is widely used as a surrogate marker for nutritional status in CKD patients. However, albumin is a negative acute-phase reactant that has a much stronger inverse association to inflammation in CKD patients and should mainly be considered as an inflammatory marker [28, 29]. In the crude analysis, patients in the stable low and decrease albumin groups had higher mortality risk. In the adjusted model, only the decrease albumin group had a significantly higher mortality risk demonstrating that a decreasing albumin level is a clinical warning sign.
In the general population, high Hcy levels are associated with worse cardiovascular outcome. However, in CKD patients, there is an inverse association between Hcy levels and cardiovascular complications and mortality [30, 31]. We confirm a similar association in the crude analysis between low Hcy levels and a higher mortality risk. Patients in the stable low Hcy group had significantly increased mortality risk after adjustments for age, sex and comorbidity compared with stable high Hcy group. As discussed previously, the association between low Hcy levels and worse outcome may be explained by malnutrition in HD patients.
The variability of CRP (ICC = 0.59) in our study is in agreement with a recent study of healthy subjects which showed an ICC level of CRP (ICC = 0.62) [32]. In contrast, ICC for PTX-3 was 0.44 and thus had much higher intra-individual variation which may reflect more rapid changes than for CRP due to local production of PTX-3 while CRP is produced in the liver.
Local ischaemic and inflammatory events quickly stimulate production of PTX-3 in the vasculature and PTX-3 is rapidly detectable in the systemic circulation. In patients with coronary disease, PTX-3 plasma levels peak much earlier after a myocardial infarction (MI) than CRP levels (7.5 versus 50 h) [33]. Although constantly increased levels in HD patients, the plasma PTX-3 levels increase significantly in the first hour of a HD treatment while CRP does not change [34]. This may be a consequence of local synthesis of PTX-3 following the activation of both immune and inflammatory responses. Furthermore, changes in PTX-3 and CRP levels over a 3-month study period show a significant association while there is an inverse association between changes in PTX-3 and albumin levels as expected (Table 2).
PTX-3 also reflects ischaemic and inflammatory events in the vasculature and is produced both by endothelial cells, neutrophils and macrophages. In CKD patients, high concentrations of PTX-3 predict all-cause mortality and are independently associated with endothelial dysfunction indicating that PTX-3 is a biomarker of peripheral vascular damage [9]. In diabetic patients CKD Stage 1, serum PTX-3 levels show association with endothelial dysfunction independent of CRP [35] which is conformed in a recent study where elevated levels of PTX-3 in CKD patients reflect endothelial dysfunction [14].
From our data, we report that there are significant associations between PTX-3 and BMI, LBMI and FBMI. We also found that PTX-3 stable high group shows significant lower BMI, LBMI and FBMI compared with PTX-3 stable low group. Our data conform with a recent study of Witasp et al. [36], showing that loss of BMI causes increased PTX-3 which indicate that high PTX-3 reflects wasting.
Although many studies during the last decade have found that PTX-3 is important in the context of CVD, its role is debated. Is PTX-3 just a biomarker or has it a causative or preservative role in the development of atherosclerosis? In an experimental model of MI greater lesions were found after induced MI in PTX-3 knock-out mice than in animals with normal PTX-3, suggesting a protective role of PTX-3 after an ischaemic insult [37]. Therefore, the PTX-3 level may rather be a marker of the magnitude of the defence activation, and thus a marker for the size of the injury. However, other studies suggest that PTX-3 is a biomarker of or part of the atherogenesis; immunohistochemical staining of atherosclerotic plaque in humans reveal a strong expression of PTX-3, and large amount of PTX-3 is found in human thrombi after an acute MI and increased levels of PTX-3 correlate with worse outcome in patients with heart failure [38, 39]. Further studies are needed to understand the role of PTX-3.
Several limitations should be considered when evaluating the relevance of our study: first, this is an observational study with a limited number of conventional HD patients, the PTX-3 variation may differ in patients treated with other dialysis modalities. Secondly, blood tests are drawn before an HD treatment and the patients were non-fasting. Third, the variation is assessed only by two measurements with 3 months in between and finally, the pathophysiologic mechanisms of PTX-3 in relation to the process of vascular damage in CKD patients have not been investigated.
When analysing the trimestral variations of biomarkers in this cohort of 188 HD patients, we found that the PTX-3 stable high group and the CRP increase group remained significantly associated with high mortality risk compared with stable low groups of these markers after adjustments for clinical factors. This is in concordance with the hypothesis that high PTX-3 levels as well as decreasing albumin levels reflect PEW in HD patients. Interestingly, PTX-3 showed higher variation within patients than CRP, albumin and Hcy according to the ICC analysis, which may be a reflection of the more rapid changes in PTX-3 levels compared with other biomarkers. These findings show that repeated measurements help in predicting mortality risk in HD patients and PTX-3 add to the mortality risk evaluation, even after inclusion of clinical factors and the three other biomarkers. Further studies are needed to investigate the underlying mechanisms linking PTX-3 to endothelial dysfunction and CVD.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the writing of this paper.
Acknowledgements
We thank the HD patients in the MIMICK study. We are indebted to the personnel at the Clinical Research Centre (KFC) and to our research staff at the Clinical Treatment Unit (KBC). Thanks to CLINTEC, Baxter Novum and Karolinska Institutet.
References
- 1.Zoccali C, Tripepi G, Mallamaci F. Dissecting inflammation in ESRD: do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol. 2006;17:S169–S173. doi: 10.1681/ASN.2006080910. [DOI] [PubMed] [Google Scholar]
- 2.Stenvinkel P, Carrero JJ, Axelsson J, et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Elzen WP, van Manen JG, Boeschoten EW, et al. The effect of single and repeatedly high concentrations of C-reactive protein on cardiovascular and non-cardiovascular mortality in patients starting with dialysis. Nephrol Dial Transplant. 2006;21:1588–1595. doi: 10.1093/ndt/gfk092. [DOI] [PubMed] [Google Scholar]
- 4.Nascimento MM, Pecoits-Filho R, Qureshi AR, et al. The prognostic impact of fluctuating levels of C-reactive protein in Brazilian haemodialysis patients: a prospective study. Nephrol Dial Transplant. 2004;19:2803–2809. doi: 10.1093/ndt/gfh493. [DOI] [PubMed] [Google Scholar]
- 5.Snaedal S, Heimbürger O, Qureshi A, et al. Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: implications for patient survival. Am J Kidney Dis. 2009;53:1024–1033. doi: 10.1053/j.ajkd.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AR, Alvestrand A, Divino-Filho JC, et al. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol. 2002;13(Suppl 1):S28–S36. [PubMed] [Google Scholar]
- 7.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 8.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 9.Tong M, Carrero J, Qureshi A, et al. Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol. 2007;2:889–897. doi: 10.2215/CJN.00870207. [DOI] [PubMed] [Google Scholar]
- 10.Stenvinkel P, Lindholm B. C-reactive protein in end-stage renal disease: are there reasons to measure it? Blood Purif. 2005;23:72–78. doi: 10.1159/000082014. [DOI] [PubMed] [Google Scholar]
- 11.Racki S, Zaputovic L, Mavric Z, et al. C-reactive protein is a strong predictor of mortality in hemodialysis patients. Ren Fail. 2006;28:427–433. doi: 10.1080/08860220600683581. [DOI] [PubMed] [Google Scholar]
- 12.Meuwese CL, Snaedal S, Halbesma N, et al. Trimestral variations of C-reactive protein, interleukin-6 and tumour necrosis factor-alpha are similarly associated with survival in haemodialysis patients. Nephrol Dial Transplant. 2011;26:1313–1318. doi: 10.1093/ndt/gfq557. [DOI] [PubMed] [Google Scholar]
- 13.Jaillon S, Peri G, Delneste Y, et al. The humoral pattern recognition receptor ptx3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witasp A, Ryden M, Carrero JJ, et al. Elevated circulating levels and tissue expression of pentraxin 3 in uremia: a reflection of endothelial dysfunction. PLoS ONE. 2013;8:e63493. doi: 10.1371/journal.pone.0063493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies SJ, Phillips L, Naish PF, et al. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17:1085–1092. doi: 10.1093/ndt/17.6.1085. [DOI] [PubMed] [Google Scholar]
- 16.Kyle UG, Schutz Y, Dupertuis YM, et al. Body composition interpretation. Contributions of the fat-free mass index and the body fat mass index. Nutrition. 2003;19:597–604. doi: 10.1016/s0899-9007(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi A, Alvestrand A, Danielsson A, et al. Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int. 1998;53:773–782. doi: 10.1046/j.1523-1755.1998.00812.x. [DOI] [PubMed] [Google Scholar]
- 18.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 19.Suliman M, Qureshi A, Bárány P, et al. Hyperhomocysteinemia, nutritional status, and cardiovascular disease in hemodialysis patients. Kidney Int. 2000;57:1727–1735. doi: 10.1046/j.1523-1755.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 20.Ockene IS, Matthews CE, Rifai N, et al. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem. 2001;47:444–450. [PubMed] [Google Scholar]
- 21.Bottazzi B, Bastone A, Doni A, et al. The long pentraxin ptx3 as a link among innate immunity, inflammation, and female fertility. J Leukoc Biol. 2006;79:909–912. doi: 10.1189/jlb.1005557. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Garlanda C, Bottazzi B, et al. The long pentraxin ptx3 in vascular pathology. Vascul Pharmacol. 2006;45:326–330. doi: 10.1016/j.vph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Klouche M, Peri G, Knabbe C, et al. Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis. 2004;175:221–228. doi: 10.1016/j.atherosclerosis.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Nauta A, de Haij S, Bottazzi B, et al. Human renal epithelial cells produce the long pentraxin ptx3. Kidney Int. 2005;67:543–553. doi: 10.1111/j.1523-1755.2005.67111.x. [DOI] [PubMed] [Google Scholar]
- 25.Taskinen S, Hyvonen M, Kovanen PT, et al. C-reactive protein binds to the 3beta-oh group of cholesterol in LDL particles. Biochem Biophys Res Commun. 2005;329:1208–1216. doi: 10.1016/j.bbrc.2005.02.091. [DOI] [PubMed] [Google Scholar]
- 26.Miyaki A, Maeda S, Otsuki T, et al. Plasma pentraxin 3 concentration increases in endurance-trained men. Med Sci Sports Exerc. 2011;43:12–17. doi: 10.1249/MSS.0b013e3181e84bce. [DOI] [PubMed] [Google Scholar]
- 27.Kaysen GA, Johansen KL, Cheng SC, et al. Trends and outcomes associated with serum albumin concentration among incident dialysis patients in the United States. J Ren Nutr. 2008;18:323–331. doi: 10.1053/j.jrn.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaysen GA, Dubin JA, Muller HG, et al. Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int. 2004;65:1408–1415. doi: 10.1111/j.1523-1755.2004.00520.x. [DOI] [PubMed] [Google Scholar]
- 29.Gama-Axelsson T, Heimburger O, Stenvinkel P, et al. Serum albumin as predictor of nutritional status in patients with ESRD. Clin J Am Soc Nephrol. 2012;7:1446–1453. doi: 10.2215/CJN.10251011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suliman M, Stenvinkel P, Qureshi AR, et al. The reverse epidemiology of plasma total homocysteine as a mortality risk factor is related to the impact of wasting and inflammation. Nephrol Dial Transplant. 2007;22:209–217. doi: 10.1093/ndt/gfl510. [DOI] [PubMed] [Google Scholar]
- 31.Ducloux D, Klein A, Kazory A, et al. Impact of malnutrition-inflammation on the association between homocysteine and mortality. Kidney Int. 2006;69:331–335. doi: 10.1038/sj.ki.5000096. [DOI] [PubMed] [Google Scholar]
- 32.DeGoma EM, French B, Dunbar RL, et al. Intraindividual variability of C-reactive protein: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2012;224:274–279. doi: 10.1016/j.atherosclerosis.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peri G, Introna M, Corradi D, et al. Ptx3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 34.Sjoberg B, Qureshi A, Anderstam B, et al. Pentraxin 3, a sensitive early marker of hemodialysis-induced inflammation. Blood Purif, 2012;34:290––297. doi: 10.1159/000342630. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz M, Axelsson J, Sonmez A, et al. Effect of renin angiotensin system blockade on pentraxin 3 levels in type-2 diabetic patients with proteinuria. Clin J Am Soc Nephrol. 2009;4:535–541. doi: 10.2215/CJN.04330808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witasp A, Carrero JJ, Michaelsson K, et al. Inflammatory biomarker pentraxin 3 (ptx3) in relation to obesity, body fat depots, and weight loss. Obesity. 2014;22:1373–1379. doi: 10.1002/oby.20695. [DOI] [PubMed] [Google Scholar]
- 37.Salio M, Chimenti S, De Angelis N, et al. Cardioprotective function of the long pentraxin ptx3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 38.Rolph MS, Zimmer S, Bottazzi B, et al. Production of the long pentraxin ptx3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–e14. doi: 10.1161/01.atv.0000015595.95497.2f. [DOI] [PubMed] [Google Scholar]
- 39.Imamura M, Kawasaki T, Savchenko AS, et al. Lipopolysaccharide induced expression of pentraxin 3 in human neutrophils and monocyte-derived macrophages. Cell Immunol. 2007;248:86–94. doi: 10.1016/j.cellimm.2007.09.003. [DOI] [PubMed] [Google Scholar]