fMet-Leu-Phe-dependent FPR1 phosphorylation is insensitive to the PMN environment, unless in cellular aggregates observed during PAF activation, or in inflammatory bowel disease abscesses.
Keywords: fMLF, FMLP, FPR1, inflammation, inflammatory bowel disease
Abstract
Bacterial/mitochondrial fMLF analogs bind FPR1, driving accumulation/activation of PMN at sites of infection/injury, while promoting wound healing in epithelia. We quantified levels of UFPR1 and TFPR1 in isolated PMN by use of phosphosensitive NFPRb and phosphorylation-independent NFPRa antibodies. UFPR1 and total TFPR were assessed inflamed mucosa, observed in human IBD. In isolated PMN after fMLF stimulation, UFPR1 declined 70% (fMLFEC50 = 11 ± 1 nM; t1/2 = 15 s) and was stable for up to 4 h, whereas TFPR1 changed only slightly. Antagonists (tBoc-FLFLF, CsH) and metabolic inhibitor NaF prevented the fMLF-dependent UFPR1 decrease. Annexin A1 fragment Ac2-26 also induced decreases in UFPR1 (Ac2-26EC50 ∼ 3 µM). Proinflammatory agents (TNF-α, LPS), phosphatase inhibitor (okadaic acid), and G-protein activator (MST) modestly increased fMLFEC50, 2- to 4-fold, whereas PTX, Ca2+ chelators (EGTA/BAPTA), H2O2, GM-CSF, ENA-78, IL-1RA, and LXA4 had no effect. Aggregation-inducing PAF, however, strongly inhibited fMLF-stimulated UFPR1 decreases. fMLF-driven PMN also demonstrated decreased UFPR1 after traversing monolayers of cultured intestinal epithelial cells, as did PMN in intestinal mucosal samples, demonstrating active inflammation from UC patients. Total TFPR remained high in PMN within inflamed crypts, migrating through crypt epithelium, and in the lamina propria-adjoining crypts, but UFPR1 was only observed at some peripheral sites on crypt aggregates. Loss of UFPR1 in PMN results from C-terminal S/T phosphorylation. Our results suggest G protein–insensitive, fMLF-dependent FPR1 phosphorylation in isolated suspension PMN, which may manifest in fMLF-driven transmigration and potentially, in actively inflamed tissues, except at minor discrete surface locations of PMN-containing crypt aggregates.
Introduction
Human neutrophils or PMN are the frontline host-defensive cells of the blood. In their “quiescent” state, carried by blood flow and shear-induced rolling, PMN sense inflammatory mediators along the endothelial luminal surface [1], acting as sentinels of infection and injury [2, 3]. They circulate until abnormal concentrations of stimuli or mediators of inflammation are presented by intact or injured endothelium. Such exposure activates their tight adherence and subsequent diapedesis through the endothelium, followed by their migration into underlying tissue, such as mucosal surfaces. This process is driven, in part, by N-formyl peptides, released by bacteria [4] or mitochondria [5] from injured host cells. Human PMN use 2 chemotactically active GPCRs in the FPR family, or FPR (FPR1 and FPR2/ALX) [6] to sense such activating agents. High-affinity binding of formyl peptides to FPR1 serves to guide PMN to the inflammatory stimulus, where they may become terminally activated by aggregated Igs and other location-specific stimuli [7]. Such activation results in the release of destructive proteases, oxidants, peroxidases, and agents to enhance and dampen the inflammatory response [8]. It is assumed that FPR1 is turned off by phosphorylation-dependent desensitization [9–11], contributing to a “tug of war” of sorts, for which the outcomes, resolution, or propagation of inflammation determine the health of the tissue involved [12]. Thus, examination of the phosphorylation state of PMN FPR1 on PMN under defined laboratory conditions and in situ is an important medically relevant goal.
In the intestinal milieu, where luminal concentrations of N-formyl peptides are at least in the micromolar range [13], the response to injury by PMN is of particular importance. Release of formyl peptides to tissues underlying the injured epithelium at such concentrations activate PMN FPR1 and may contribute to formation of intestinal crypt abscesses [14] in major acute and chronic inflammatory conditions of the intestine [15]. Although epithelial FPR1 activation also accelerates mucosal wound-healing [16, 17], the disposition of PMN FPR1 to a contributory or dampening role in inflammation may be mediated predominantly by the PMN environment.
Phosphorylation of GPCRs is a central mechanism of their regulation, controlling interactive affinity changes and the spectrum of regulatory partners with which they interact. Recently, we showed that 2 mAb generated in our laboratory [18] could be exploited to recognize and report on the phosphorylation state of FPRs in human PMN after activation by formyl peptides [19]. In heterologous systems, FPR1 phosphorylation has been shown to dampen the response to formyl peptides, reducing the effectiveness of fMLF-occupied FPR1 interaction with G proteins [20]. This receptor modification appears to mediate its sequestration by physical association with arrestins [21] and by internalization of the receptor [22]. However, the regulation of FPR1 by phosphorylation in PMN still remains relatively unstudied [23].
In the current work, we have indirectly characterized fMLF-induced phosphorylation of FPR1 by quantifying the levels of UFPR1 recognized by the phosphorylation-sensitive antibody, NFPRb [18, 19] under a variety of conditions that modulate PMN activation. We also measured the total PMN content of FPR1 (TFPR1), whether phosphorylated or not, by quantifying the 60K immunoblotting species recognized by the phosphorylation-insensitive antibody, NFPRa [18, 19]. This analysis showed that fMLF caused the loss of UFPR1, while leaving TFPR1 little changed in suspension PMN. Because in our previous work [18, 19], we showed that such a loss of UFPR1 results from the phosphorylation of at least 1 of 7 different S/T sites, in or near the epitope recognized by NFPRb, we were able to use this indirect assay of phosphorylation to conclude that fMLF-induced FPR1 phosphorylation: 1) is independent of G-protein activation but agonist specific, demonstrating correlation with agonist affinity and sensitivity to antagonists; 2) is relatively stable in suspension cells even after longer periods of fMLF exposure and a range of modulating cytokines, chemokines, and other agents, except for PAF and the metabolic inhibitor NaF, both of which nearly, completely inhibited fMLF-stimulated UFPR1 declines; 3) appears to also occur in PMN that have transmigrated across epithelial monolayers, consistent with reduction of UFPR1; and 4) appears to be maintained in PMN engaged in active intestinal inflammation in lamina propria, transmigrating through crypt epithelia, and in crypt abscesses, also consistent with the absence of UFPR1 but high TFPR labeling of tissue samples from patients with IBD. In light of inhibition of the reactivation of desensitized FPR1, reported by Forsman et al. [24], we speculate that inflammatory conditions may exist that dysregulate fMLF-stimulated FPR1 inactivation by phosphorylation, allowing for reactivation of intact FPR1 by dephosphorylation.
MATERIALS AND METHODS
Reagents
Octyl β-D-glucopyranoside, PMSF, and DTT and LXA4 were purchased from Calbiochem (EMD Millipore, Billerica, MA, USA) and DDM was from Anatrace (Affymetrix, Santa Clara, CA, USA). Goat anti-mouse IgG (H+L) DyLight 800–conjugated antibody was from Thermo Fischer Scientific (Waltham, MA, USA), and Odyssey infrared imaging blocking buffer was from LiCor Biosciences (Lincoln, NE, USA). The PMN activation modulator CsH was obtained from Enzo Life Sciences (Farmingdale, NY, USA); tBoc-FLFLF from Bachem (Torrance, CA, USA); TNF-α, ENA-78, IL-1RA, as well as GM-CSF from PeproTech (Rocky Hill, NJ, USA); and Annexin I peptide Ac2-26 from Tocris Biosciences (Bristol, United Kingdom). Baxter Healthcare injection-grade sterile water in Viaflex bags was obtained from Baxter Travenol (Deerfield, IL, USA). ProSieve color protein markers were from Lonza Rockland (Rockland, ME, USA). Dextran (average molecular weight = 500,000 as a 20% autoclaved solution), fMLF, and all other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies
NFPRa and NFPRb, formerly NFPR1 and NFPR2, are described in publications by Jesaitis and coworkers [18, 19]. Rabbit anti-human Lactoferrin was obtained from ICN/Valeant Pharmaceuticals (Laval, QC, Canada). Monoclonal mouse antiphosphotyrosine was obtained from Sigma-Aldrich. Antigen affinity-purified rabbit anti-selectin L (human CD62L, SELL) polyclonal antibody was obtained from Sino Biological (Beijing, China).
Buffers
Buffers used for cell resuspension and stimulation buffers
DPBS[+]: 136 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, Na2HPO4, supplemented with 1 mM MgCl2, 0.9 mM CaCl2, and 0.1% dextrose was used with the gelatin method of cell preparation.
RPMI (Sigma-Aldrich), without phenyl red, was made as directed by the manufacturer and used for the Dextran/Ficoll method of cell preparation.
RBC lysis buffer: 0.83% NH4Cl for gelatin method; dH2O for Dextran method.
PMN lysis buffer: 100 mM KCl, 10 mM NaCl, 10 mM Hepes, 2 mM MgCl2, 1 mM EDTA, 100 μM DTT, 10 μg/ml chymostatin, 1 mM PMSF (added just before use), 1:1000 diluted Sigma protease inhibitor cocktail P8340, 1:100 diluted phosphatase inhibitor cocktails 1 (P2850) and 2 (P5726), with or without the nonionic detergent, 1% DDM, pH 7.4.
Buffers for SDS-PAGE
SDS sample buffer: 0.2 M Tris-HCl, pH 8, 18 mM DTT, and 2% Na-dodecylsulfate.
TS buffer (2× nonreducing SDS-PAGE sample buffer): 4% SDS, 0.12 M Tris-HCl, pH 6.8, 20% glycerol, 0.01% bromophenol blue.
Special FPR electrotransfer buffer: 0.19 M Na-glycine, 20% methanol, 25 mM Tris base, 0.02% SDS, pH 8.5.
Buffers for immunoblotting
Blocking buffer: LiCor Odyssey blocking buffer undiluted.
Primary antibody incubation buffer (I°): blocking buffer plus 0.2% Tween-20.
Secondary antibody incubation buffer (II°): blocking buffer plus 0.2% Tween-20 and 0.01% SDS.
Postprimary and postsecondary antibody incubation wash buffer: PBS + 0.1% Tween.
PMN preparations
Human PMN were prepared by the gelatin method, as described by Henson and Oades [25], which involves discarding of the buffy coat, followed by 1 g sedimentation in porcine-derived gelatin (Sigma-Aldrich) at 37°C and red cell lysis in 0.83% NH4Cl, with intermediate washes and resuspension in DPBS(+). The Dextran method, as described by DeLeo et al. [26], with minor modifications, involves 1 g sedimentation of heparinized blood, mixed 1:1 at room temperature with 3% Dextran in 150 mM NaCl with pelleting and resuspension of the leukocyte-rich supernatant in 150 mM NaCl, followed by sedimentation in Ficoll-Hypaque, red cell lysis in dH2O (1 or 2 times), and resuspension in 150 mM NaCl. With the use of the latter method, all final cell-suspension buffers were made in Baxter Healthcare injection-grade sterile water, including RPMI without phenol red, and was confirmed as LPS free by use of a standard limulus assay (Sigma-Aldrich), showing <1 ng/ml LPS. The Dextran method was used mostly for cases in which modulators, known for affecting PMN priming, were examined (e.g., LPS and cytokines). Priming was confirmed by the sensitivity of the uncleaved form of CD62L to fMLF stimulation, as measured by immunoblot analysis by use of the polyclonal anti-CD62L. The gelatin method, long a standard method in our laboratory [27], purified fMLF-sensitive granulocyte populations, confirmed by measuring stimulation of superoxide production and degranulation [28]. It was used to examine the fMLF-induced FPR1 phosphorylation under the numerous other conditions described below and in Table 1.
TABLE 1.
PMN modulator incubation conditions
| Name | Abbreviation |
Preincubation |
|
|---|---|---|---|
| Concentration | Duration | ||
| Lipopolysaccharide | LPS | 100 ng/ml | 1 h |
| Tumor necrosis factor alpha | TNF-α | 25 ng/ml | 10 min |
| Human PMN-activating peptide (CXCL5) | ENA-78 | 100 ng/ml | 10 min |
| Interleukin 1 receptor antagonist | IL-1RA | 100 nM | 10 min |
| Granulocyte macrophage colony-stimulating factor | GM-CSF | 10 ng/ml | 10 min |
| Lipoxin A4 | LXA4 | 2 µM | 10 min |
| Okadaic acid | OKA | 2 µM | 30 min |
| Mastoparan-7 | MST | 10 µM | 10 min |
| Sodium fluoride | NaF | 40 mM | 15 min |
| Pertussis toxin | PTX | 250, 500 ng/ml | 2–4 h |
| Cyclosporin H | CsH | 3 µM | 10 min |
| tBoc-Phe-Leu-Phe-Leu-Phe | tBoc-FLFLF | 1 µM | 10 min |
| bis Aminophenoxyethane-tetraacetic acid | BAPTA | 10 µM | 30 min |
| Ethylene glycol tetra-acetic acid | EGTA | 1.5 mM (1 mM in excess of [Ca2+]) | 1 min |
| Hydrogen peroxide | H2O2 | 10, 100, 1000 µM | 10 min |
| Annexin A1 mimetic peptide | Ac2-26 | 0.3 nM–10 µM | 10 min |
| Platelet-activating factor | PAF | 100 nM | 10 min |
PMN were preincubated with the above agents for the times and concentrations indicated at 37°C in RPMI, except for NaF, which was substituted for 40 mM NaCl in DPBS. The incubations were then followed by a 10 min exposure to vehicle or 0.3 nM–10 µM fMLF or formyl-norLeu-Leu-Phe (for incubation with H2O2) before harvesting the cells for analysis. All agents were checked for activity, as described in Materials and Methods.
Assay of UFPR1, TFPR1, and TFPR2 by ratio immunoblotting
Cell treatment and disruption
To determine the effect of fMLF stimulation of PMN on the level of UFPR1, TFPR1, or TFPR2, PMN, prepared as above, were resuspended in 500 µL RPMI in polyethylene centrifugation vials at a cell density of 5 × 106/ml, equilibrated in buffer for 10 min at 37°C, followed by exposure with test agents (i.e., inhibitors, cytokines, or other compounds that were candidates for perturbing FPR1 phosphorylation; see Table 1 for conditions). Subsequent to this incubation, fMLF was added by a 1 in 10 dilution of 10-fold-concentrated stock solution in the cell resuspension buffer. After the appropriate exposure, the stimulation was stopped by addition of 1 ml ice-cold cell resuspension buffer, followed by centrifugation in a table-top centrifuge cooled to 4°C at 6000 g for 2 min. The supernatant buffer was then removed by aspiration and pellets resuspended in 300 μl DDM-containing PMN lysis buffer. Pellets were solubilized in the lysis buffer by vortexing, followed by end-over-end tumbling of suspensions in capped tubes at 4°C for 45 min. The resultant lysate was centrifuged at 13,000 g for 30 min at 4°C and 200 µl supernatant used for assay by quantitative immunoblotting as described below. Control analyses confirmed that PMN agonists, inhibitors, and modulating agents used in assays were active by examining priming-associated cleavage of CD62L [29], released lactoferrin [30, 31], or activated tyrosine kinases [32], respectively (not shown).
SDS-PAGE
Samples (200 μl) from the DDM extraction were diluted 1:1 in TS 2× SDS-PAGE sample buffer containing 18 mM DTT, agitated, and then heated to 60°C for 5 min. The samples were then cooled for 5 min at room temperature, alkylated by adding 100 μl of 300 mM N-ethylmaleimide to give a final concentration of 60 mM, and allowed to react for 5 min at room temperature. The reduced and alkylated samples were then diluted 1:1 with TS sample buffer (1×), mixed, and stored frozen at −20°C.
Immunoblotting
Between 10 and 50 μl sample, prepared as above, was loaded into the stacking wells of 9% polyacrylamide gels, prepared and run, and then electroeluted onto Immobilon-FL (EMD Millipore) 0.45 µm PVDF membranes in special FPR electrotransfer buffer, as described previously [19]. PVDF blots were rinsed briefly in dH2O, followed by fixation for 1 min in 100% methanol, rinsed for 1 min in dH2O, and then dried between 2 sheets of Whatman 3MM Chr filter paper overnight. The dried PVDF was then rewet in 100% MeOH for 15 s, rinsed in dH2O, rinsed again in PBS for 2 min, and then blocked at 4°C in undiluted LiCor Odyssey blocking buffer for 1 h at ambient temperature with gentle rocking. After blocking, the blots were transferred directly into LiCor Odyssey blocking buffer, supplemented with 0.2% Tween-20 plus 1 µg/ml NFPRa or 2 µg/ml NFPRb for 1 h at 37°C with gentle rocking. The blots were washed 5 times for 10 min in PBS + 0.1% Tween-20 at room temperature with rocking, after which, they were placed in the buffer from the original blocking step, saved, and supplemented with 0.2% Tween-20, 0.01% SDS, and a 1:10,000 dilution of commercially conjugated DyLight 800 goat anti-mouse IgG secondary antibody. The blots were incubated with the secondary mAb at room temperature for 1 h in the dark, followed by 3 × 10 min washes in PBS containing 0.1% Tween-20 and 2 × 10 min washes in PBS without Tween-20, and then placed in PBS for scanning quantitation. The blots were then scanned with a LiCor Odyssey infrared digital scanner at the 780 nm excitation while wet. It is important to note that this protocol was optimized specifically to reduce nonspecific binding of antibodies to highly abundant proteins in the molecular weight range of the FPRs and to maximize the transfer and retention of FPRs to the PVDF.
Scans were quantified 2 ways. For some early kinetic experiments (not shown), immunoblots were quantified by a routine chemiluminescence method, development of film, followed by densitometry, as described in Riesselman et al. [18]. For the remainder of the experiments, 8-bit tif images from Odyssey scans of wet transfers were saved to files and analyzed with ImageQuant (GE Life Sciences, Pittsburgh, PA, USA) by inverting images, rotating 90°, and analyzing individual bands (see Fig. 1) at Mr = 60 K, 39 K, and 30 K (representing relative molecular masses in K) in a 28 lane gel as a single virtual lane in ImageQuant, allowing up and down quantitation for any molecular weight range for all conditions of the experiment. FPR1 (50–65 K on NFPRb and NFPRa blots) and FPR2/ALX (30–40 K) on NFPRa blots were so obtained. Occasionally, to confirm lane loads, the 30 K band corresponding to an unknown cytosolic molecular species [19], cross-reactive with NFPRa only when denatured, was also measured. The integrated signals minus background and corresponding ratios of background subtracted signals for FPR1 measured with NFPRb and NFPRa were calculated and plotted after normalizing for maximum range or against the control as indicated.
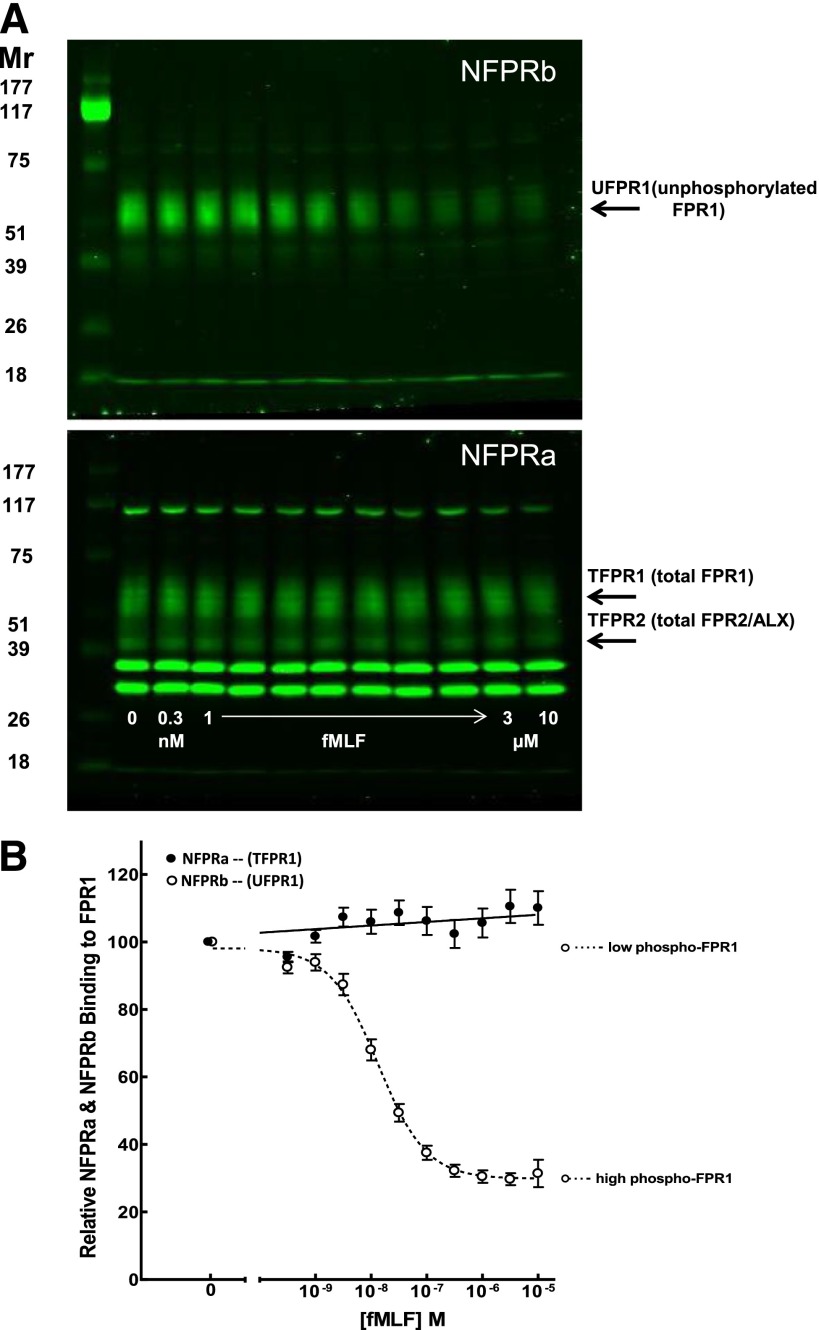
Figure 1. Binding of mAb NFPRa and NFPRb to DDM extracts of PMN pre-exposed to a range of fMLF concentrations.
Suspensions of purified human PMN, prepared by the Dextran/Ficoll method, as described in Materials and Methods, were incubated with logarithmically increasing (A, lower, arrow) concentrations of fMLF at 0, 3 × 10−10, 1 × 10−9, 3 × 10−9, 1 × 10−8, 3 × 10−8, 1 × 10−7, 3 × 10−7, 1 × 10−6, 3 × 10−6, and 1 × 10−5 M for 10 min at 37°C. Aliquots were diluted in ice-cold buffer and centrifuged at low speed for 2 min and the pellets solubilized in 1% DDM, cleared by centrifugation, denatured in SDS-PAGE sample buffer, reduced and alkylated, and immunoblotted at 1.5 × 105 cell equivalents/lane. Blotting was carried out with NFPRa primary antibody that recognizes phosphorylated (phospho) and nonphosphorylated juxtamembrane C-terminal tail regions of FPR1 (Mr ∼ 60 K) and FPR2/ALX (Mr ∼ 40 K; A, lower). As these forms are insensitive to fMLF treatment of PMN and contain no S/T phosphorylation sites, they represent TFPR1 and TFPR2, respectively. Immunoblotting was also performed by use of NFPRb (specific for the nonphosphorylated C-terminal 10 aa residue region of FPR1 only) as the primary antibody shown (A, upper). This form is sensitive to fMLF treatment, and its epitope has S/T phosphorylation sites and represents a measure of UFPR1. Mr indicates relative molecular masses of standards shown in the left-most lanes in K). LiCor DyLight 800 infrared fluorescing goat anti-mouse IgG was used as a secondary antibody. The blots were visualized by use of a LiCor infrared fluorescence imager, as shown in A. The intensity of each FPR1 (60 K) band of interest was quantified by use of ImageQuant and normalized to the [fMLF] = 0 control condition. The relative normalized NFPRa (●) and NFPRb (○) intensities were plotted against a logarithmic scale of fMLF concentration (B).
We found minimal correlation of NFPRa signals with fMLF stimulation over multiple runs under any condition examined in this study. The increase of ∼10% (see Fig. 1) was not significant by linear regression analysis. We believe this small, systematic difference may have more to do with cell recovery or other undetermined influences in the assay. We observed virtually no visual differences or correlations in NFPRa binding attributable to anything but random occurrences for any condition for all bands quantitated, including the FPR2 39 K band and cytosolic 25 K and 30 K bands (see Fig. 1). Thus, because of run-to-run variations in SDS-PAGE signal levels and lane-to-lane load variations, errors were minimized, and multiple repeat runs were normalized for averaging by use of the ratio of NFPRb:NFPRa integrated signals for plotting the dependencies of NFPRb on fMLF for all of the figures shown (except see Figs. 1 and 2) in this study. This normalized data were analyzed and plotted by use of GraphPad Prism (GraphPad Software, La Jolla, CA, USA), and where appropriate, a 1- or 2-site competition-binding curve, fit by nonlinear regression, was plotted as sigmoidal inhibition curves on a logarithmic scale. In most cases, modulators had little or no effect on the amount of control UFPR1 but demonstrated minor variations (∼10–20%) in levels of UFPR1 under maximal fMLF stimulation of PMN. To allow better graphical comparison of fMLFED50 of the modulators, the UFPR1 signal changes were normalized, where appropriate, by the maximal full-range change in NFPRb binding, allowing visualization and comparison of the shifts in fMLFED50 fMLF-induced UFPR1 changes on a 0–100% full-scale range.
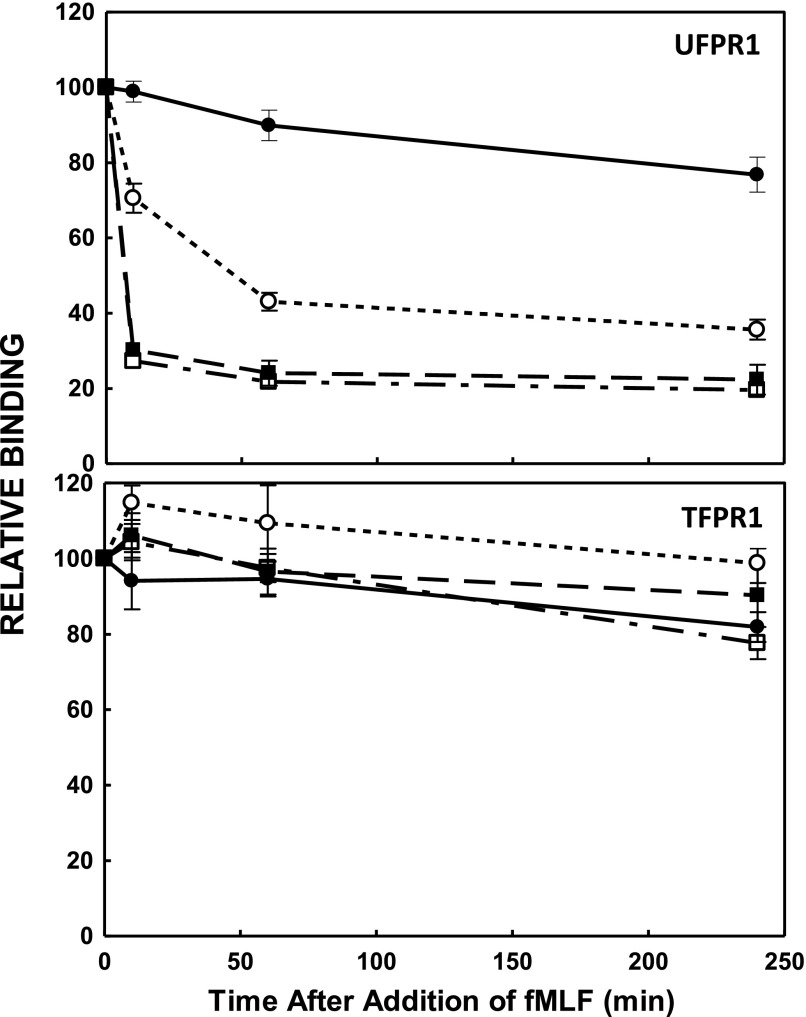
Figure 2. Kinetics of fMLF-induced sensitivity of mAb NFPRa and NFPRb binding to FPR1 in immunoblots.
PMN, prepared as in Fig. 1, were exposed to 1 µM fMLF for the indicated time periods and lysed as described in Materials and Methods. Cell equivalents (1.5 × 105) were solubilized and separated by SDS-PAGE, followed by immunoblotting, as described in Fig. 1. The binding of mAb NFPRa and mAb NFPRb to FPR1 (TFPR1 and UFPR1, respectively; relative to the t = 0, and [fMLF] = 0 value) was calculated from the signal intensities determined by LiCor infrared fluorescence and image analysis. These values are plotted as a function of the time of incubation of the cells with fMLF or vehicle at 37°C. The relative NFPRb binding to FPR1 (UFPR1), after 0, 10, 60, and 240 min stimulation with fMLF, is plotted (upper) for exposures to 0 (●), 10−8 M (○), 10−7 (▪), and 10−6 (□) M fMLF. (Lower) The same samples probed with NFPRa (TFPR1).
Assessment of PMN UFPR1 after transepithelial migration
PMN (1 × 106/well) were introduced into the upper chamber of Transwells containing confluent monolayers of polarized T84 cells [14] and were stimulated to transmigrate by fMLF gradients of 0–100 nM. Transmigrated PMN were collected from the bottom chambers of Transwells, permeabilized, and labeled with NFPRb antibodies. The phosphorylation state of FPR1 was examined by flow cytometry by comparing NFPRb-labeling intensity with PMN, introduced to the upper chamber of Transwells without fMLF stimulation. For immunofluorescence staining, collected cells were resuspended in cold FACS buffer (2% FBS in PBS + 0.1% Na azide) containing 5 µg/ml primary anti-NFPRb antibody, followed by incubation with 1 µg/ml secondary antibody conjugated with Alexa 488 and analyzed with a FACSCalibur (BD Biosciences, San Jose, CA, USA).
Immunofluorescence labeling and confocal microscopy
Colonic mucosal tissue was obtained from nondiseased areas in the colon cancer-resection specimens and diseased areas from colonic resection for active UC. Frozen-tissue sections were fixed in 100% vol/vol ethanol for 20 min at −20°C, washed 5 times with HBSS, and incubated in 3% wt/vol BSA for 1 h at room temperature, followed by primary antibody (NFPRa or NFPRb) overnight at 4°C in a humidity chamber. The following day, sections were washed 5 times with HBSS and subsequently incubated in the dark with fluorophore-labeled secondary antibodies for 1 h. Nuclei were visualized by staining with 1 μg/ml TO-PRO-3 (Life Technologies, Frederick, MD, USA) nuclear stain for 5 min at room temperature. Sections were mounted in p-phenylenediamine-glycerol. Images were captured by use of a Zeiss LSM 510 confocal microscope at the same day of preparation.
Human subjects
All work on human subjects was approved by the Montana State University and Emory University Internal Review Boards under guidelines approved by the National Institutes of Health of the U.S. Public Health Service.
RESULTS
Recently, we demonstrated that FPRs on human PMN become phosphorylated at any of 7 C-terminal tail S/T sites when suspensions of cells are stimulated with fMLF in the presence of cytochalasin B [19]. In an earlier study, we provided indirect evidence of fMLF-mediated FPR1 phosphorylation in Chinese hamster ovary cells expressing rFPR1 [18]. As heterologous systems do not necessarily reflect the functional milieu of various receptors that have been so expressed, we attempted to characterize the fMLF-induced FPR1 phosphorylation response by measuring changes in UFPR1 and TFPR1 in isolated human peripheral blood PMN and in PMN of normal and inflamed intestinal mucosal tissue. Secondary to measurement of these parameters, we also noted TFPR2 levels in some of the same samples.
UFPR1 sensitivity to fMLF is equivalent in 2 PMN preparations
In previous studies, cited above, we used PMN, prepared by a traditional method by use of plasma of buffy coat-depleted blood (by 1 g sedimentation) in porcine gelatin at 37°C. This method produces cells that are partially primed but demonstrate NFPRb sensitivity to FPR1 phosphorylation [18, 19]. As one of our original aims in this study was to characterize the fMLF-stimulated FPR1 phosphorylation response in PMN undergoing priming, we needed to establish a baseline against which we could compare PMN exposed to priming conditions for comparison with PMN in the unprimed state, as well as with our previous studies. We used the Dextran method of PMN isolation only to examine the effect of a limited range of priming agents on fMLF-induced loss of the UFPR1, TFPR1, and TFPR2 content, whereas gelatin-prepared PMN were used for the other experimental conditions to allow comparison with our previous studies [18, 19].
To show that the baseline responses of these 2 cell preparations were equivalent, we exposed suspensions of human PMN, prepared by either method, to a logarithmic range (0.3 nM–10 µM) and 0 control fMLF concentrations for 10 min at 37°C and examined the cellular content of these 2 receptors by quantitative immunoblotting (Fig. 1). These exposure conditions activate FPR1 and FPR2/ALX at the higher concentrations (1–10 µM) [33]. Figure 1A shows the immunoblotted molecular species bound by NFPRb (upper) and NFPRa (lower) in extracts of the fMLF-stimulated PMN prepared by the Dextran method. Immunoblots of gelatin-prepared cells are visually and graphically indistinguishable from those in Fig. 1 (not shown; also see ref. [19]). Each lane in this blot represents ∼2 × 105 PMN. We observed a clear, saturable decline in NFPRb binding (upper) to the FPR1 band (Mr range of ∼50–65 K) in the upper blot, which represents UFPR1 (see also below).
When quantitated (see Materials and Methods) and plotted as a function of the log of the fMLF concentration, as shown in Fig. 1B, it is clear that there is a sigmoidal dependence of the 60 K UFPR1 band on fMLF, suggesting a 70% decline in binding and representing a decline in the level of UFPR1 after maximal fMLF stimulation. As in prior studies [18], we have shown that the loss of binding can be reversed by exposure of FPR1-containing membranes to alkaline phosphatase and that FPR1 from fMLF-stimulated neutrophils is phosphorylated at or near its C-terminal epitope [19], we feel confident that this change is a result of phosphorylation of the receptor and have so suggested by indicating the high and low phosphorylated FPR1 levels in Fig. 1B and by use of the acronym UFPR1 for unphosphorylated FPR1.
The EC50 of the UFPR1 decline is 10.5 ± 0.2 nM (n = 18) for cells prepared by the Dextran method and 11.1 ± 0.7 nM (n = 17) for PMN prepared by the gelatin method (not shown) and suggests that in both preparations, UFPR1 responds to fMLF exposure equivalently. Thus, we used Dextran-prepared cells only for tests of priming agents (see below). The residual signal observed after exposure of the PMN to saturating concentrations of fMLF can be explained by the presence of at least 15–20% FPR1, sequestered in the specific granules [18], and is supported by our unpublished studies, where we compared the levels of UFPR1 in plasma membrane and specific granule fractions after PMN stimulation with fMLF [unpublished results]. Any residual FPR1 escaped phosphorylation, was dephosphorylated, or phosphorylated in 1 of 6 S/T outside of the identified epitope region of the FPR1 C terminus [19]. Thus, we feel safe to conclude that NFPRb recognizes UFPR1 most preferentially, if not exclusively, and has very little, if any, affinity for phosphorylated FPR1.
Characteristically, in the lower blot, the NFPRa-binding species included a prominent 60 K FPR1 band (between the 51 and 75 K Mr markers), as well a less-intense 40 K band or FPR2/ALX that was visualized between the 39 K and 51 K Mr markers [18]. All visible bands, including the 30 K and 35 K bright doublet, showed no significant dependence on fMLF concentration during the 10 min exposure. Quantitation of the FPR1 band revealed a small 10% linear and statistically significant (P = 0.03) and fMLF-dependent nonzero slope up to the maximal fMLF exposure of 10 µM. As in prior studies, we showed that the 110, 35, and 30 K bands were cytosolic in origin. Since recovery or extraction of TFPR1 is only slightly affected by the exposure of PMN to fMLF, we used the 60 K NFPRa band and, occasionally, the 30 K band as internal standards to correct for cell load variation for quantitation. The epitope for this antibody is identical in sequence on FPR1 and FPR2/ALX and does not contain any S/T sites that could be phosphorylated [18], and no phosphorylated forms of FPR2/ALX were found in our prior study [19] even after maximal stimulation with 1 µM fMLF in the presence of Cytochalasin B. Thus, we conclude that NFPRa, binding to FPR1 and FPR2/ALX, is not sensitive to the phosphorylation of either species in PMN and conclude that both bands provide a measure of TFPR1 and TFPR2 and together, represent TFPR.
Kinetics of fMLF-induced changes in UFPR1
To determine the time course of UFPR1 changes in response to fMLF, we examined the NFPRb binding to the 60 K species (FPR1) of PMN extracts, prepared as described in Fig. 1, after continuous exposure of PMN to 0, 10, 100, and 1000 nM fMLF for up to 4 h at 37°C. Figure 2 shows results of the average of 2 preparations measured in triplicate. The data suggest that the decline in UFPR1 after exposure of PMN to 1 µM fMLF is rapid and 90% complete before 10 min (single exponential t1/2 value for the decline is 15.4 s with a 95% confidence interval of 11–24 s; data not shown). After this initial decline, the amount of FPR1 recognized by NFPRb is relatively stable for up to 4 h. TFPR1, representing NFPRa binding to the 60 K species, increased by a small amount, at most 10–15%, during the same initial period but then shows a comparable decline of 10–15% during the subsequent period, whereas in unexposed control cells, TFPR1 levels decreased linearly and slowly, at most 15%, over 4 h, probably representing a relatively small loss of cells to the assay. By contrast, the 40 K TFPR2 levels declined by >50% over the same time period, independent of fMLF exposure (data not shown). We did not observe the accumulation of lower molecular weight fragments or a change in the size of the species identified as FPR1 or FPR2 during this time course. Together, these results suggest that the large UFPR1 changes are not a result of loss of receptors because of proteolysis or changes in their extractability.
Specificity of fMLF-stimulated UFPR1 modulation
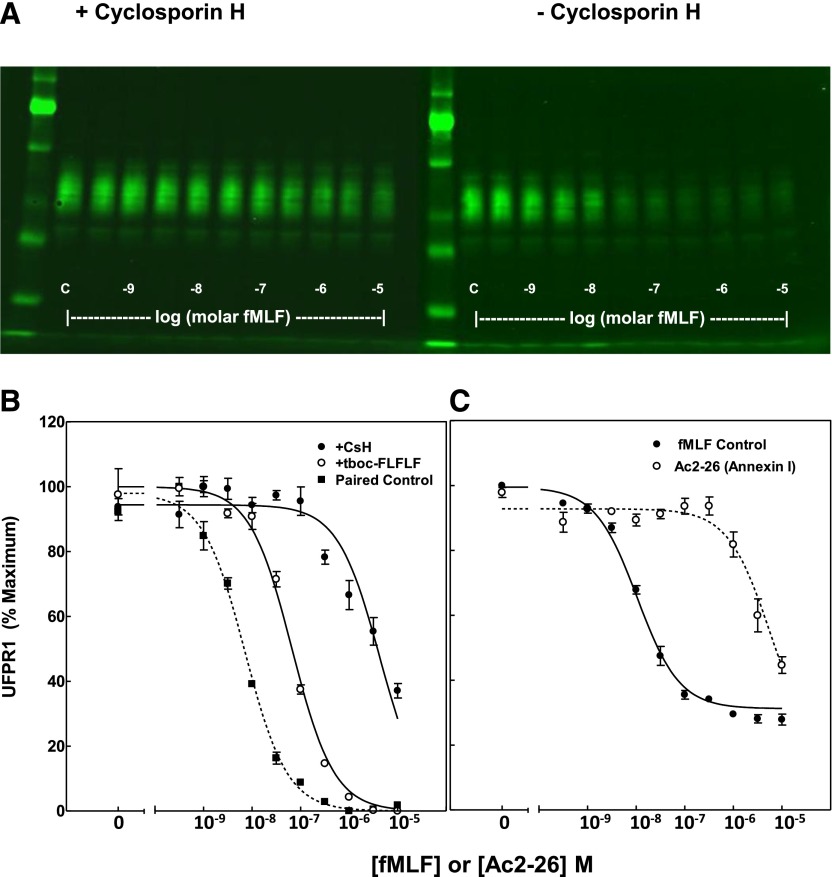
To show that fMLF-induced changes to UFPR1 levels are specific to FPR1 ligands, we examined the ability of 2 antagonists and 1 related agonist of FPR2/ALX to affect UFPR1 modulation after fMLF stimulation in PMN. For the antagonist case, we exposed PMN to 3 μM CsH, a competing antagonist of FPR1 [34], for 10 min at 37°C, followed by additional exposure to the same range of concentrations of fMLF as in Figs. 1 and 2. Figure 3A clearly shows that CsH inhibits the fMLF-induced UFPR1 declines compared with the vehicle control (right). This reduction in efficiency occurs with no change in NFPRa binding (not shown). With this exposure to 3 µM CsH, there is a shift in the fMLFEC50 to higher concentrations (4 × 10−6 M fMLF), shown in Fig. 3B, as would be expected by this competitive antagonist. Likewise, 1 μM tBoc-FLFLF, another competitive antagonist, also exerts an inhibitory effect on the fMLF-stimulated decrease in UFPR1 and is also shown graphically in Fig. 3B. The fMLfEC50 in this regime is shifted to 7 × 10−8 M in the presence of 1 μM tBoc-FLFLF. These results indicate that tBoc-FLFLF and CsH compete for the fMLF-binding site and act as competitive antagonists.
Figure 3. fMLF-stimulated FPR1 phosphorylation is specific and saturable.
Suspensions of human PMN were incubated with fMLF, as shown in Fig. 1, after a 10 min preincubation in the presence of 3 µM CsH, 1 µM tBoc-FLFLF, or vehicle. Extracts were prepared, as described in Fig. 1 and Materials and Methods. (A) A developed immunoblot, as visualized by a LiCor imager, shows the inhibitory effect of 3 µM CsH on the ability of different concentrations of fMLF to decrease the amount of UFPR1, as measured by the decrease of NFPRb binding to FPR1. (B) Concentration dependence of UFPR1 as a function of fMLF after a 10 min pre-exposure of PMN suspensions (before fMLF) at the indicated, above concentrations of CsH (●), tBoc-FLFLF (○), or vehicle (Paired Control; ▪). Quantitation was performed by use of ImageQuant software, as described in Materials and Methods. Values obtained from the image analysis were normalized for sample load by determining the ratio of the NFPRb:NFPRa intensities and then for experiment-to-experiment variation by determination of the percent of the maximum signal of controls before averaging. (C) Ac2-26 was used over the same range of concentration as fMLF to effect a reduction in UFPR1. The indicated concentrations of fMLF (●) or peptide Ac2-26 (○) induce FPR1 phosphorylation at EC50 = 10.6 ± 0.8 nM for fMLF or 5.9 ± 0.3 µM for Ac2-26. EC50 was calculated by a 1-site nonlinear regression analysis by use of GraphPad Prism software.
Annexin A1 and its N-terminal mimetic peptide AC2-26 have been identified as an agonist of FPR1 and FPR2/ALX [35, 36]. Release of Annexin A1 and its peptides is believed to be associated with cell injury, apoptosis, proresolution, and prorepair events [37]. To confirm the ligand specificity of induction of FPR1 phosphorylation, it was thus important also to examine the effects of Ac2-26 on the modulation of UFPR1 levels. Figure 3C shows that Ac2-26 resulted in a 50% decline in UFPR1 at 5.9 ± 0.3 µM, confirming that it is ∼1000-fold less potent than fMLF, consistent with it being a less-potent agonist of FPR1 [36]. This value is approximately 4-fold higher than the binding constant for FPR1 (EC50 = 1.4 µM) determined by homoligand competition for 125-I Ac2-26 on PMN [36].
UFPR1 loss does not involve G proteins
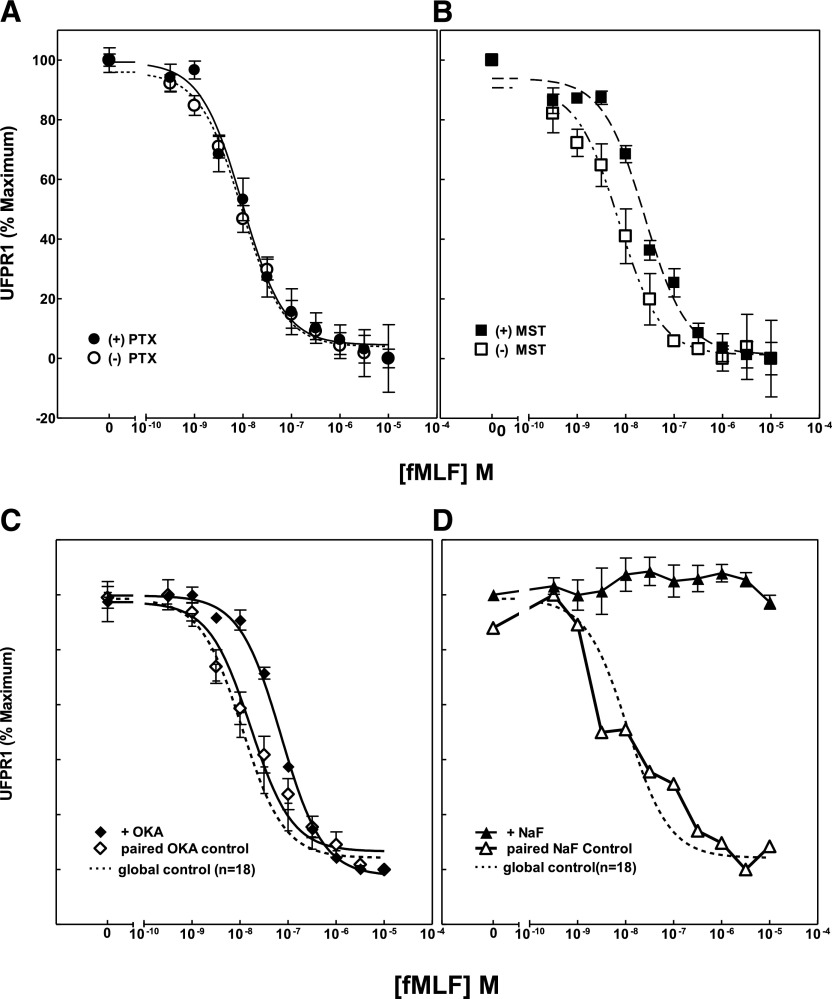
FPR1 is a GPCR that was suggested by Pitcher et al. [38] to promote the generation of Pleckstrin-homology domain membrane-binding sites for GRK2/3 and thus, might facilitate the interaction of the kinase with occupied receptors. To determine whether UFPR1 loss depends on G-protein activation, we used PTX and wasp venom, MST, to inhibit or activate, respectively, PMN G protein–mediated processes [39, 40]. The effect of both of these modulators of FPR1 phosphorylation is shown in Fig. 4A (PTX) and Fig. 4B (MST). In this experiment, the fMLF concentration dependence of FPR1 phosphorylation was examined in the presence and absence of inhibitory/stimulatory concentrations of PTX/MST, respectively. The expectation would be that PTX might inhibit and MST promote the activation of GRK2/3. If FPR1 phosphorylation were driven by an alternate G protein, such as Gq or G16, then MST might be expected to have an opposite effect on FPR1 phosphorylation compared with PTX. Alternatively, there could be a secondary effect on GRK efficiency as a result of its regulation by phosphatases/kinases that might skew the concentration dependence of fMLF-induced phosphorylation. Interestingly, the results of this experiment clearly show that PTX had no effect on the magnitude or fMLFEC50 of fMLF-stimulated UFPR1 changes, whereas MST induced a variable and, at most, 4-fold shift of the fMLFEC50 to higher concentrations. These results suggest that the kinase(s) that phosphorylate FPR1 are not downstream of fMLF activation of a G protein–coupled transduction pathway and that other kinases or phosphatases may control the activity of FPR1 kinases/phosphatases, thereby regulating the degree of FPR1 phosphorylation at any particular fMLF concentration.
Figure 4. Effects of modulators of G-protein activation and cellular phosphorylation on FPR1 phosphorylation.
(A) Suspensions of human PMN were incubated with 200 or 500 ng PTX for 4 h at 37°C (●), (B) with 10 µM MST for 10 min at 37°C (▪), or with vehicle for the corresponding times (○, □), respectively. Samples were then processed, as described in Fig. 1, and values for UFPR1 calculated. Preincubations were also carried out to inhibit cellular phosphatases with (C) 2 µM okadaic acid (OKA; ♦) for 10 min at 37°C or with (D) the metabolic inhibitor 40 mM NaF for 15 min (▴). Also shown are the paired controls for vehicle (◊, ∆). UFPR1 values were normalized to the full-scale maximum range of control values for averaging, display, and comparison.
Minimal UFPR1 changes after PMN exposure to modulators of phosphatase activity, cellular ATP, and calcium
Okadaic acid and NaF are well-known inhibitors of cellular metabolic processes. The former, a product of marine organisms, strongly inhibits protein S/T phosphatase 1, 2A, and 2B [41] and has been shown to prevent fMLF-induced NADPH oxidase desensitization [42]. The latter is not only a general phosphatase inhibitor [43] but also directly activates G proteins in PMN [44] and inhibits glycolysis [45], causing a general decline in ATP levels within the cell [46]. A third agent that inhibits phosphatases, H2O2 [47], is a byproduct of NADPH oxidase activation [48] and has been suggested to play an important role in regulating signaling [49]. Additionally, to examine PMN signaling function more generally, we examined the effect of intracellular and extracellular Ca2+ chelators BAPTA [50, 51] and EGTA, respectively.
The effect of the first 2 agents on the fMLF-sensitive UFPR1 modulation is shown in Fig. 4C. Okadaic acid treatment of PMN resulted in a counter-intuitive, enhanced level of nonphosphorylated FPR1, shifting the fMLFEC50 for UFPR1 decreases 3- to 4-fold to the right, i.e., to higher concentrations. However, exposure of PMN to 40 mM NaF for 15 min at 37°C, shown in Fig. 4D, completely blocked fMLF-induced UFPR1 decreases, maintaining the maximal binding of NFPRb to FPR1 compared with the paired controls. This treatment has been reported to cause occupied FPR1 dissociation from F-actin [52] induced by the decline in ATP levels [46] to 3% of their starting levels. We interpret the inability of FPR1 to become phosphorylated to be the result of substrate depletion of the FPR1 kinase GRK2/3 rather than inhibition of an FPR1 phosphatase or activation of G proteins. Lastly, a 10 min exposure of PMN suspensions to H2O2, followed by simultaneous formyl-norLeu-Leu-Phe stimulation over another 10 min, also showed no effect on the concentration dependence of induced phosphorylation of FPR1 (not shown), as was also observed for the intracellular and extracellular Ca2+ chelators, 10 µM BAPTA and 1.5 mM EGTA (not shown; see Table 1 for incubation conditions), respectively.
Modulation of UFPR1 by endogenous pro- and anti-inflammatory agents
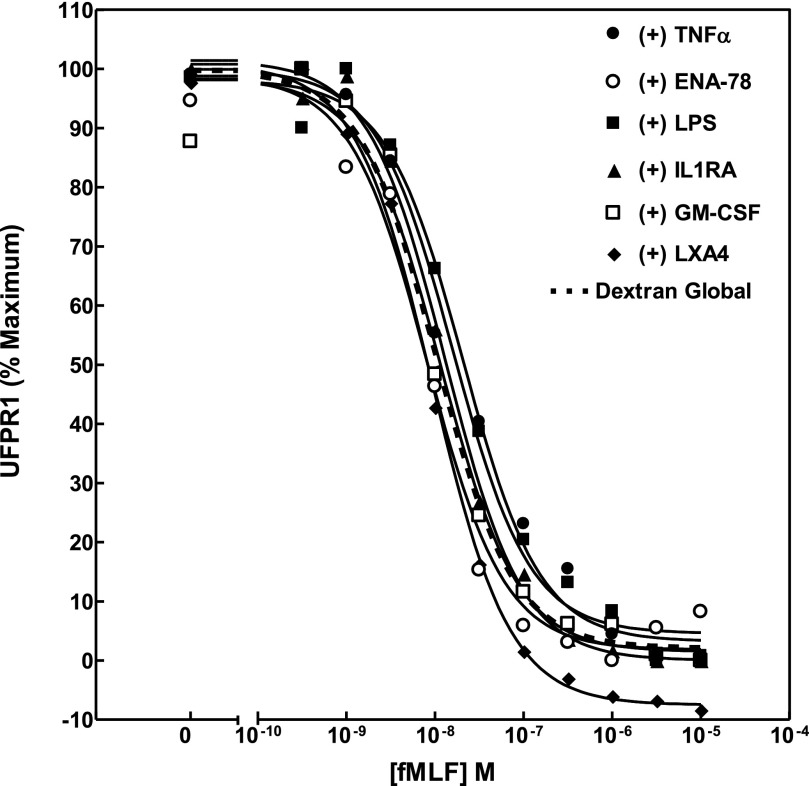
Various cytokines and lipids have been shown to be pro- or anti-inflammatory in their ability to enhance or abate PMN reactivity. One way that such activity could be manifested would be as a cytokine-dependent shift in concentration dependence of fMLF-induced phosphorylation of FPR1. To survey the effects of a variety of such agents, we compared the EC50 of fMLF-induced decline in NFPRb binding on cells that had been preincubated with TNF-α (25 ng/ml), ENA-78 (10 nM), IL-1RA (100 nM), GM-CSF (10 ng/ml), and LXA4 for 10 min at 37°C and LPS (100 ng/ml) for 1 h at 37°C. All of these agents had minimal effects and are summarized in Fig. 5 and Table 2.
Figure 5. Comparison screen of the effect of modulators of inflammation on the sensitivity of levels of UFPR1.
Unprimed human PMN were prepared by the Dextran/Ficoll method and exposed at 37°C to 2.5 ng/ml TNF for 10 min, 100 ng/ml ENA-78 for 10 min, 100 ng LPS for 1 h, 100 nM IL-1RA for 10 min, and 10 ng/ml GM-CSF or 1 µM LXA4 for 10 min before processing, as described in Fig. 1. The average UFPR1 values for each condition are plotted as a function of fMLF concentration, to which PMN were exposed. The dotted line represents the average of all of the control values for all paired experiments conducted on Dextran prepared cells. Table 2 presents the calculated EC50 for each experimental condition, the 95% confidence interval, as well as the calculated P values for comparison of the paired EC50 in each experimental vehicle control with the experimental test values for each cytokine exposure.
TABLE 2.
Effect of PMN modulators on UFPR1
| Modulator | EC50 (nM) | 95% | P value | |
|---|---|---|---|---|
| LPS | (+) | 20.8 | 14.3–30 | |
| (−) | 8.5 | 7.1–10.2 | 0.0013 | |
| TNF-α | (+) | 16.0 | 10.4–24.5 | |
| (−) | 8.5 | 7.1–10.2 | 0.001176 | |
| ENA-78 | (+) | 8.2 | 5.2–13.0 | |
| (−) | 12.2 | 8.8–17.0 | 0.007999 | |
| IL-1RA | (+) | 13.1 | 10.4–16.6 | |
| (−) | 13.3 | 11.5–15.3 | 0.696509 | |
| GM-CSF | (+) | 11.6 | 7.07–18.9 | |
| (−) | 9.2 | 7.4–11.3 | 0.730119 | |
| LXA4 | (+) | 10.2 | 8.7–12.0 | |
| (−) | n.s. | n.s. | n.s. | |
n.s., Not significant.
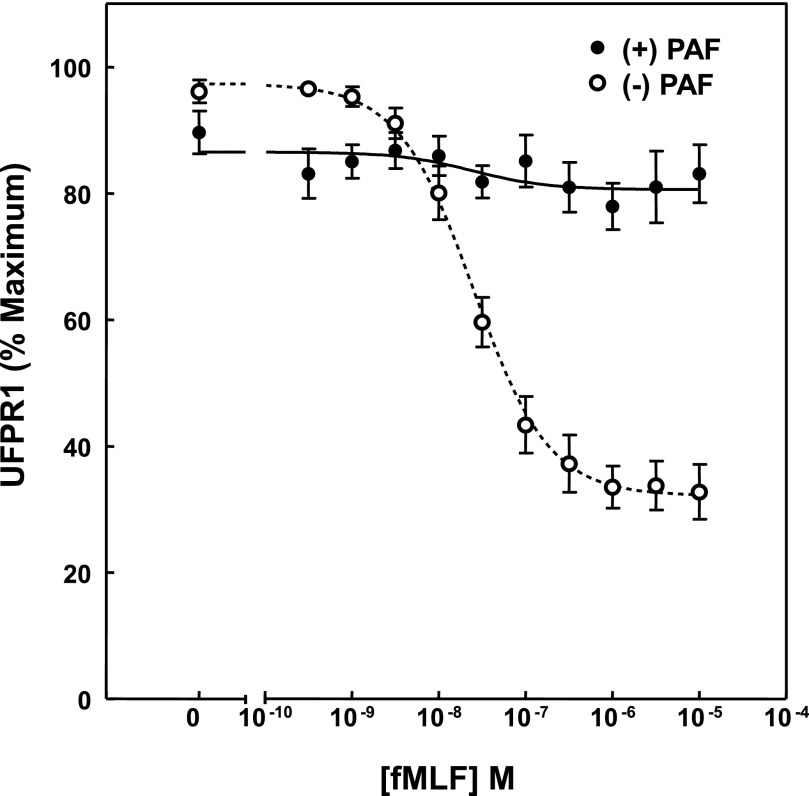
A notable exception to this pattern was, however, demonstrated by PAF, which is a choline phosphoglyceride activator of PMN and mediator of inflammation [53, 54], with high affinity and high potency and known to activate PMN chemotaxis, degranulation, adherence, aggregation, and superoxide generation [55, 56]. Recently Forsman and colleagues [24] showed that PAF also mediates an FPR1 ligand-dependent reactivation of desensitized, FPR1-stimulated superoxide production. As shown in Fig. 6, treatment with PAF resulted in almost complete elimination of the fMLF-dependent loss of UFPR1. Of interest is the observation that the recovery of TFPR1 and UFPR1 was reduced significantly by 30–40% after PAF and PMN formed visual aggregates, suggesting that PMN self-adherence may play a role in the inhibition and perhaps the reactivation noted above. It would be of interest, however, to examine the effect of PAF on previously adherent cells and after stimulation of PMN with fMLF to explore whether these process could be related to reactivation of phosphorylated FPR1.
Figure 6. PAF inhibits fMLF-stimulated UFPR1 decreases.
PMN, prepared by gelatin method, were in preincubated for 10 min at 37°C in cell buffer containing vehicle (○) or 100 nM PAF (●). The exposure was followed by 10 min of stimulation with the indicated concentration of fMLF. The average UFPR1 values for each condition were determined, as indicated in Fig. 1, and are plotted as a function of fMLF concentration, to which PMN were exposed. The dotted and solid lines represent the nonlinear regression curve fits determined by GraphPad Prism analysis to averages of all of the control values for all paired experiments conducted on Dextran-prepared cells.
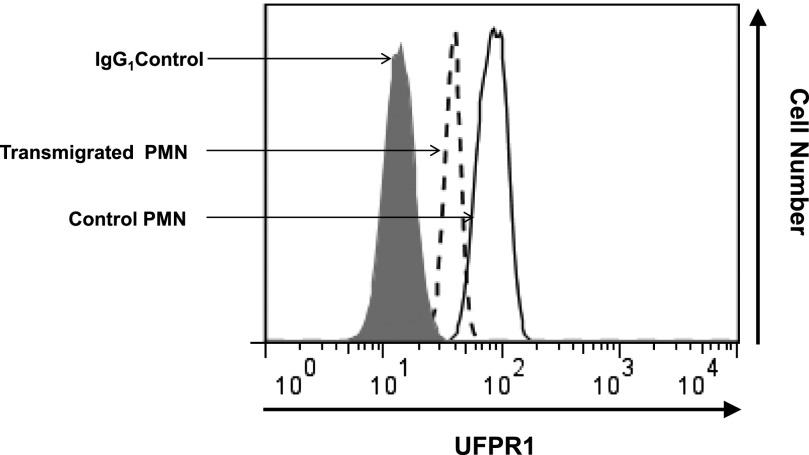
UFPR1 is reduced after fMLF-induced transmigration
PMN, normally engaged in chemotactic behavior, are adherent, first to the endothelium and then to migrate through a 3-dimensional matrix during and after diapedesis, exhibiting a bell-shaped curve of sensitivity to fMLF [57]. To approximate such activity, we examined PMN, before and after transmigration, across model epithelial monolayers of the T84 cell line [58], a process that normally peaks at ∼1 µM fMLF. Epithelial cells were cultured on permeable supports, and PMN migration from the basolateral to the apical compartment was induced by a 0 to 100 nM gradient of fMLF. After transmigrating across the epithelium, PMN were harvested and analyzed for UFPR1 content and compared with PMN that did not migrate across the epithelium and were not exposed to fMLF. UFPR1 levels were judged by intensities of NFPRb binding to permeabilized cells in each population by flow cytometry. As shown in Fig. 7, PMN that had transmigrated across epithelial monolayers in response to a 100 nM fMLF gradient had a 2-fold reduction in NFPRb labeling, consistent with decreased UFPR1.
Figure 7. FPR1 is phosphorylated after transepithelial migration.
Human PMN (1 × 106/well) were introduced to the apical surface of confluent intestinal epithelial T84 cells and stimulated to migrate across to the basolateral surface by fMLF gradient (100 nM). Transmigrated PMN (from the bottom chambers of Transwells) and PMN before transmigration (from upper compartments of Transwells) were harvested, permeabilized, and fluorescently labeled with 5 µg/ml primary mAb NFPRb to reveal levels of UFPR1, followed by 1 µg/ml secondary antibody conjugated with Alexa 488. Fluorescence as an index of FPR1 phosphorylation was analyzed by use of FACSCalibur.
FPR in active UC
During an inflammatory response, PMN arrive at a site of injury or infection, where they sample the highest concentrations of chemoattractants, such as fMLF, and may respond, while they are also actively being turned off by receptor phosphorylation and its sequelae among other processes. Active mucosal inflammation in UC is characterized by an influx of PMN into the mucosa [59]. Thus, after the migration across the vascular endothelium, PMN traverse the lamina propria and migrate across the gastrointestinal epithelial layer into the intestinal crypt lumen. Under these conditions, PMN are exposed to micromolar concentrations of formyl peptides, derived mainly from gut bacteria [13].
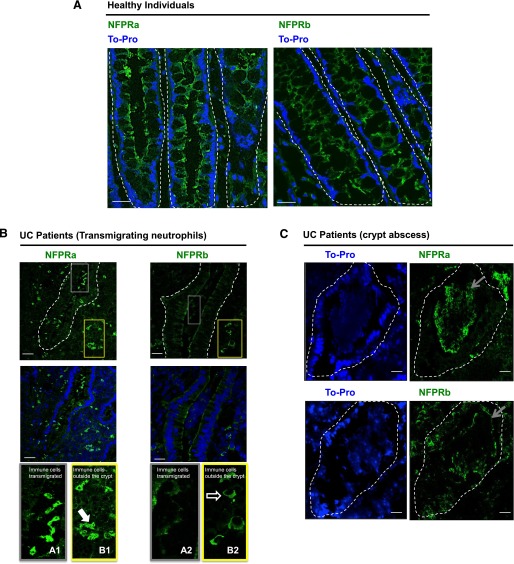
Our transmigration results suggest that UFPR1 modulation is detectable during transmigration across epithelial monolayers (see above). To corroborate these in vitro findings further, we examined the levels of UFPR1 and TFPR during intestinal mucosal inflammation. Frozen sections of intestinal mucosa from individuals with active UC were labeled with NFPRa to detect TFPR and NFPRb to detect UFPR1, and the results were compared with sections prepared from healthy colonic mucosa. Confocal microscopy of immunolabeled, healthy tissues revealed NFPRa and NFPRb labeling in the apical and subapical membrane of epithelial cells, with some additional labeling in the basolateral membrane (Fig. 8A), as has been reported previously [60]. However, NFPRa labeling of inflamed colonic mucosa revealed PMN-like cells with characteristic polymorphonuclear morphology (Fig. 8B) in the lamina propria, as well as in cells that had transmigrated across the intestinal epithelium into the crypt lumen. Whereas NFPRa and NFPRb labeling was decreased in the epithelium of inflamed colonic mucosa compared with healthy controls [60], NFPRa staining was consistent with significantly higher levels of TFPR in PMN-like cells, relative to epithelial cells in the lamina propria, as well as in those cells that had transmigrated across the epithelium into the crypt lumen (Fig. 8 B). Although Fig. 1 shows that NFPRa binds to other molecular species, as well as the 60 K FPR1 and 40 K FPR2/ALX, on immunoblots, we have shown previously that the native forms of these proteins, as well as their alcohol-fixed forms, are not recognized by NFPRa [18, 19], thus suggesting that the signals observed comprise the TFPR or their epitope-bearing C-terminal fragments.
Figure 8. FPR1 localization in colonic mucosa from individuals with UC.
Representative images showing TFPR immunolocalization by use of NFPRa and UFPR1 immunolocalization by use of NFPRb primary antibodies in healthy individuals (A, green) and in the inflamed intestinal mucosa of UC patients (B and C, green). In (B) gray and yellow outline boxes (top) identify crypt (gray) and lamina propria (yellow) regions, respectively and are shown at higher magnification (bottom) from NFPRa-stained sections, labeled as A1 and B1, respectively. Likewise, from NFPRb-labeled sections, crypt (grey) and lamina propria (yellow) regions are labeled as A2 and B2, respectively. TOPRO-3 (blue) was used as a nuclear marker. The solid arrow in B1 points to PMN in the lamina propria identified by their polymorphonuclear morphology. The open arrow in B2 shows NFPRb-staining of cells with monocytic morphology in the lamina propria. In crypt abscesses of UC patients (C), TO-PRO staining shows nuclear localization in tissues and degraded nuclei in PMN aggregates within crypts. Gray arrows point to PMN within crypt aggregates stained with NFPRa (upper) and NFPRb (lower). Original scale bars, 50 μm.
NFPRb labeling of PMN-like cells, signifying UFPR1, was faint and equivalent in intensity to that observed in the epithelium (where it is much less abundant), suggesting that indeed, UFPR1 was reduced in these cells. Other cells in the subepithelial lamina propria with monocytic morphology were also faintly labeled with NFPRb. Crypt abscesses of the same samples, both staining positively with NFPRa and NFPRb, are shown in Fig. 8C. These views show large aggregates of PMN staining positively with NFPRa and more weakly and patchy with NFPRb, surrounded by somewhat degraded crypt epithelia. NFPRa labeling was especially strong in the aggregated, PMN-like cells in the crypt lumen that were in close proximity to the epithelium with decreasing intensity in damaged cells in the center of crypt abscess cellular aggregates (Fig. 8C). A similar staining but sharper intensity gradient was observed for NFPRb in the periphery compared with the center of crypt abscess cell aggregate.
DISCUSSION
The phosphorylation of GPCRs after activation has been examined as a mechanism that regulates signal transduction for >40 years [61]. After numerous studies on the phosphorylation of hundreds of different types of GPCRs, primarily in recombinant systems, the role of GRKs and arrestins in this process has been firmly established as a way of inactivating receptor interaction with G proteins [62]. Now, investigations center on the understanding of the state of GPCR in the context of their native cellular and tissue environments [63]. Through understanding of the sequential steps and kinetics of the phosphorylation of the FPRs in leukocytes, the consequences of inappropriate FPR regulation and its relationship to disease may be revealed.
FPRs are considered to be part of a class of GPCR that recognize pathogen-associated molecular patterns(or PAMPs), stemming from the fact that bacteria initiate protein synthesis with formyl methionines instead of methionines as in eukaryotes. As mitochondria and chloroplasts retain bacterial protein synthetic capacity, they too have proteins that have N-formylated methionyl N termini. Damage to these organelles results in release of N-formylated peptides and thus, represents a danger or damage-associated molecular patterns (or DAMPs) [6]. Hence, invasion of tissues by bacteria or the destruction of cells at the organelle level would be expected to produce local concentrations of N-formyl peptides that may not only activate wound healing in epithelia [60] but also may attract and activate PMN as part of the inflammatory response. As the regulation of PMN responses is of critical importance to the host, and some of this regulation is mediated through FPR, understanding of a PMN GPCR prototype, such as FPR1, is of basic and medical interest.
Tight control of PMN function is particularly important at epithelial barriers, such as in the intestine, which serves to prevent invasion of enormous numbers of luminal bacteria that constitute the gut microbiome [64]. Direct measurement of fMLF concentrations in the colonic lumen suggests that the concentration of this peptide alone is ∼1 μM [13]. In cultured human colonic samples, Chadwick and colleagues [65] showed that growth media of bacterial species, isolated from the human gut, produced peptide fragments at concentrations equivalent to 1 μM fMLF and that a number of related peptide species are present in normal and diseased colonic lumens [13]. If this represents only 10% of all similar peptides from all species resident in the gut, then the concentration of N-formylated bacterial peptide activators in the gut lumen could be manyfold greater than that produced by Escherichia coli alone. Additionally, it is clear that during an inflammatory insult to this tissue, such as IBD, neutrophils are bathed in a complex mixture of chemokines, cytokines, resolvins, and other influences that determine their state of activation and progression of disease [66, 67]. Consequently, regulation of the PMN response that is capable of detecting bacterial peptide degradation products at infinitesimal concentrations with FPR [68] is paramount for controlling PMN invasion into the gastrointestinal lumen . One protective function of such a process might be to drive huge numbers of PMN into dysbiotic colons to encase and isolate pathogens from contact with colonic epithelia [69]. The purpose of our study was to examine some of the mechanisms that may regulate this process in human cells and tissues.
We used two epitope-mapped mAb, produced in our laboratory [18], to quantify FPR1 phosphorylation. One mAb NFPRa (NFPR1) recognizes the juxtamembrane N-terminal region of the FPR1 (and FPR2/ALX) C-terminal tail, encompassing 306-QDFRERLI-313 and the corresponding exact sequence in FPR2/ALX. The other mAb, NFPRb (aka NFPR2), recognizes the C terminus containing 338-STLPSAEVALQAK-350 of only FPR1. The latter epitope has a S/T amino acid residue T339 that can be phosphorylated and is spatially near 6 other potential sites (T325, S328, T329, T331, S332, and T334). Maaty et al. [19] identified 2 contiguous C-terminal-tail peptides from FPR1 and FPR2/ALX by mass analysis mixtures of FPR1 and FPR2/ALX, immunopurified from fMLF-stimulated and unstimulated PMN on NFPRa and NFPRb affinity matrices. They found no phosphorylated amino acid residues in unstimulated PMN, prepared by the gelatin method. However, after stimulation by 1 µM fMLF at 37°C, in the presence of cytochalasin B, they found direct evidence that all of the above sites of FPR1 become uniquely and individually phosphorylated. Interestingly, no FPR2/ALX-phosphorylated peptides were found, even though the concentrations used have been shown to activate neutrophils through this GPCR [35, 37]. The epitope recognized by the NFPRa mAb does not have any S/T sites. Therefore, we interpret the loss of NFPRb binding after fMLF stimulation of PMN without any significant change in the amount or the SDS-PAGE molecular weight of FPR1 or FPR2/ALX as evidence that the levels of UFPR1 decline as a result of FPR1 phosphorylation. Unfortunately, we cannot infer how much of the total FPR1 becomes phosphorylated, what the percent of the total phosphorylation sites is phosphorylated, or what the phosphorylation state of S/T residues might be outside of the region influencing the epitope. Our current study thus characterizes the decline in UFPR1 as a function of prior exposure of PMN to fMLF, from 0.3 nM to 10 µM, under various cell treatments. As this relative change might also be observable in tissue, we examined FPR1 phosphorylation on PMN that had transmigrated across cultured epithelial monolayers and in healthy and inflamed colonic mucosa.
Our studies, characterizing the modulation of UFPR1 levels by fMLF exposure of PMN, demonstrated that it is specific, saturable, and consistent with FPR1 phosphorylation. We interpreted the kinetics of UFPR1 changes, as revealed by NFPRb binding, shown in Fig. 2, as evidence that FPR1 gets phosphorylated at a rate consistent with a role in the termination of responses, such as fMLF-stimulated superoxide generation, and appears, at least in steady state, not to be degraded. The measurement demonstrated high specificity with 2 well-known antagonists of FPR1, CsH and tBoc-FLFLF [34], that inhibited the conversion of FPR1 to its phosphorylated form. An endogenous ligand of FPR1, Annexin A1–derived peptide Ac2-26, was also shown to stimulate FPR1 phosphorylation at concentrations shown previously to activate FPR1-mediated responses in these cells [37] but 4-fold-less potent than the reported binding EC50 of 1.4 µM [36] to neutrophils, suggesting that it may reflect some receptor in a G protein–uncoupled state. In contrast, ENA-78 (or CXCL5), which binds the GPCR CXCR2 on neutrophils, had no effect on fMLF-induced FPR1 phosphorylation, confirming the lack of cross-desensitizing phosphorylation [11] and specificity of this process.
In gelatin-prepared cells, a maximal rate of superoxide production is achieved by 30 s of fMLF exposure, followed by a decline to 0 by 1 min [70]. The t1/2 for UFPR1 decline was calculated to be ∼15 s. Additionally, from the examination of the levels of TFPR1 from NFPRa binding to FPR1, we infer that FPR1 is not significantly degraded for up to 4 h of continuous exposure of PMN to fMLF. Given that GPCR phosphorylation has been invoked as a mechanism of desensitization [62], required for sequestration/internalization and ultimate degradation of GPCR, these times are entirely consistent with such a role in termination of superoxide production but suggest that receptor degradation is not involved.
It was important to measure the phosphorylation response in a preparation whose response and kinetics has been well characterized [70], and thus, these initial characterizations were performed in gelatin-prepared cells. However, priming, an important component of PMN physiology, is bypassed by use of the gelatin method, and thus, we also compared the concentration dependence of fMLF-induced UFPR1 in these two populations of cells and found them indistinguishable, showing equivalent fMLFEC50 for UFPR1 modulation. This permitted several cytokines and modulators of the primed state to be evaluated on Dextran-prepared, unprimed cells, with some understanding of the relationship of the 2 preparations.
In these studies, examination of UFPR1 modulation by fMLF in PMN under the influence of these modulators revealed some potentially important results, suggesting that the PMN FPR1 kinases/phosphatases are relatively insensitive to at least a limited spectrum of activation conditions. For example, conditions that foster activation of protein kinases, such as stimulating PMN with fMLF in the presence of cytochalasin B [19], or conditions that involve inhibition of phosphatases with okadaic acid [71] or H2O2 had only a modest inhibitory or no effect, respectively. In no cases examined were there shifts in the sensitivity to lower concentrations of fMLF. With the comparison of the preincubation of PMN with the pro- and anti-inflammatory agents, GM-CSF, IL-1RA, ENA-78, and LXA4, only LPS and TNF-α had a modest effect on fMLF-induced FPR1 phosphorylation, again shifting the fMLFEC50 only 2-fold to higher concentrations compared with untreated PMN. Even MST, which is known to promote the exchange of GDP for GTP in the α subunits of G proteins [72, 73], did not stimulate the kinases sufficiently to appreciably increase receptor phosphorylation. Instead, it appears that MST modestly stimulated the activities that promote receptor phosphatases or inhibited receptor kinases. When the MST result is examined, in light of the observation that PTX had virtually no effect on a fMLF-induced reduction of UFPR1 in PMN, one may conclude that the involvement of G proteins in these processes is minimal and that the activation of Gi/Go does not have any significant effect on activating GRK2/3-mediated FPR1 phosphorylation.
NaF exposure, under conditions that deplete ATP within PMN and PAF under conditions that cause the PMN to aggregate, were the only agents we tried, aside from competitive antagonists of FPR1 that could completely inhibit fMLF-induced UFPR1 decreases. This finding suggests that GRK2/3-mediated phosphorylation of PMN FPR1, as measured by UFPR1 levels, depends directly on the availability of the fMLF-bound conformation of FPR1 in energy-competent cells and that these are minimally perturbed by many activation states that the PMN acquire. Together, this analysis of PMN regulation of FPR1 phosphorylation suggests that the receptor becomes saturably phosphorylated at 100–1000 nM fMLF and is probably deactivated to formyl peptides under conditions when regulatory pro- and/or anti-inflammatory cytokines are present, such as in inflamed tissue. Our results also suggest that there might exist conditions, such as in adherent or aggregated PMN, where phosphorylation of FPR1 is prevented or reversed.
Our studies also suggest that the agonist-dependent phosphorylation of FPR1 may have more important implications in PMN recruitment and migration into tissues during inflammation than previously envisioned. As we have shown in vitro that NFPRb binding to human PMN, induced to transmigrate across monolayers of epithelial cells, appears to be diminished by an exposure to a 0 to 100 nM fMLF gradient, we would expect relatively reduced staining by NFPRb in inflamed human colonic mucosa. Indeed, in tissues obtained from patients with UC, we observed bright staining of PMN with NFPRa, supporting the presence of a receptor. In this scenario, we observed decreased labeling of PMN as identified by their characteristic nuclear morphology, with NFPRb. Epithelia also express FPR1 [60], as do certain monocytic cells, although at much lower levels [74, 75], also demonstrated minimal staining with NFPRb . Significantly, we found that in PMN-rich aggregates in intestinal crypts, where, as a rule, monocytic cells are not found, the labeling with NFPRb was also minimal, showing equivalent staining intensity to that found in crypt epithelial cells, whereas NFPRa labeling was much stronger. However, in a small percentage of cells at the very periphery of PMN-rich aggregates in crypts, more prominent NFPRb labeling was observed. One speculative interpretation of these differences in NFPRb labeling is that most FPR1 is phosphorylated (i.e., reduced UFPR1) and thus, desensitized to fMLF, unless exposed to specific agents with actions similar to PAF. Such agents might promote FPR1 dephosphorylation and possibly reactivate FPR1 in a process similar to that described by Forsman and colleagues [24]. The 2-fold difference in intensity of staining observed in the in vitro transmigration assay suggests that such differences might be difficult to quantitate in tissue sections. However, it is clear from Fig. 8C that TFPR or their fragments are detectable in crypt PMN. Thus, it would be of interest to examine such receptor modulation in situ by use of fluorescently labeled, affinity-purified primary antibodies as multiplex antibody probes that would allow ratio imaging of the two signals. A microbiochemical analysis to determine whether the molecular size of the detected receptors remained unchanged would suggest FPR reactivation.
In summary, our results highlight stimulus and context-specific differences in phosphorylation of FPR1 in human PMN. They suggest that FPR1 phosphorylation is a highly stable process in suspension PMN that is consistent with GRK2/3 function, requiring energy-competent cells and an agonist-occupied receptor under modest control by cellular activation state, independent of the Gi/Go signaling pathway. By assessing FPR1 phosphorylation in histologic sections of inflamed mucosa, we speculate that FPR1 phosphorylation might be reversed at the surfaces of cellular aggregates within colonic crypt abscesses, similar to those found by Molloy et al. [69]. Such “reactivation” of FPR1 might thus be capable of perpetuating inflammatory processes in such pathologic states.
ACKNOWLEDGMENTS
Certain foundational aspects of the work were carried out under U. S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases Grant R01-AI22735 and Crohn's and Colitis Foundation of America Senior Research Award 2785 supported by the Helmsley Charitable Trust to A.J.J. This work was also supported by the NIH Grants R01DK089763 and R01DK055679 to A.N. and DK061379, DK072564, and DK079392 to C.A.P. The authors thank The Crohn's and Colitis Foundation for the Helmsley Scholar award to A.J.J. and a Career Development Award to R.S.
Glossary
- Ac2-26
N-terminal Annexin I mimetic peptide
- ALX
lipoxin A4 receptor
- CD62L
cluster of differentiation 62 ligand
- CsH
cyclosporin H
- DDM
dodecyl β-D-maltoside
- DPBS[+]
Dulbecco’s PBS plus dextrose
- ENA-78
epithelial cell-derived neutrophil-activating peptide 78
- fMLF
fMet-Leu-Phe
- FPR1
formyl peptide receptor 1
- FPR2/ALX
formyl peptide receptor 2/lipoxin A4 receptor
- GPCR
G protein–coupled receptor
- GRK2/3
G protein-coupled receptor kinase 2/3
- IBD
inflammatory bowel disease
- IL-1RA
IL-1R antagonist
- K
kilodalton(s)
- LXA4
lipoxin A4
- MST
mastoparan
- NFPRa
anti-total formyl peptide receptor mAb
- NFPRb
anti-unphosphorylated formyl peptide receptor 1
- PAF
platelet-activating factor
- PMN
polymorphonuclear leukocyte(s), neutrophil
- PTX
pertussis toxin
- PVDF
polyvinylidene difluoride
- S/T
serine/threonine
- tBoc-FLFLF
tBoc-Phe-Leu-Phe-Leu-Phe
- TFPR
total formyl peptide receptor 1 + total formyl peptide receptor 2
- TFPR1
total formyl peptide receptor 1, unphosphorylated formyl peptide receptor 1 + phosphorylated formyl peptide receptor 1
- TFPR2
total formyl peptide receptor 2/lipoxin A4 receptor
- UC
ulcerative colitis
- UFPR1
unphosphorylated formyl peptide receptor 1
AUTHORSHIP
All authors have made substantial contributions to the study's conception, design, and/or performance of experiments.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Sundd P., Pospieszalska M. K., Ley K. (2013) Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings. Mol. Immunol. 55, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams M. R., Azcutia V., Newton G., Alcaide P., Luscinskas F. W. (2011) Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 32, 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixit N., Simon S. I. (2012) Chemokines, selectins and intracellular calcium flux: temporal and spatial cues for leukocyte arrest. Front. Immunol. 3, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. (1984) Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 259, 5430–5439. [PubMed] [Google Scholar]
- 5.Carp H. (1982) Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J. Exp. Med. 155, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., Serhan C. N., Murphy P. M. (2009) International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatham W. W., Turkiewicz A., Blackburn W. D. Jr (1994) Determinants of neutrophil HOCl generation: ligand-dependent responses and the role of surface adhesion. J. Leukoc. Biol. 56, 654–660. [DOI] [PubMed] [Google Scholar]
- 8.Nauseef W. M. (2007) How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 219, 88–102. [DOI] [PubMed] [Google Scholar]
- 9.Luttrell L. M., Lefkowitz R. J. (2002) The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 115, 455–465. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson J., Bylund J., Movitz C., Björkman L., Forsman H., Dahlgren C. (2010) A methodological approach to studies of desensitization of the formyl peptide receptor: role of the read out system, reactive oxygen species and the specific agonist used to trigger neutrophils. J. Immunol. Methods 352, 45–53. [DOI] [PubMed] [Google Scholar]
- 11.Ali H., Richardson R. M., Haribabu B., Snyderman R. (1999) Chemoattractant receptor cross-desensitization. J. Biol. Chem. 274, 6027–6030. [DOI] [PubMed] [Google Scholar]
- 12.Fullerton J. N., O’Brien A. J., Gilroy D. W. (2013) Pathways mediating resolution of inflammation: when enough is too much. J. Pathol. 231, 8–20. [DOI] [PubMed] [Google Scholar]
- 13.Roberts E. C., Hobson C. H., Anderson R. P., Chadwick V. S. (1990) Radio-immunoassay for formyl methionyl leucyl phenylalanine. II. Demonstration of an enterohepatic circulation of immunoreactive bacterial chemotactic peptides in man. J. Gastroenterol. Hepatol. 5, 38–43. [DOI] [PubMed] [Google Scholar]
- 14.Nash S., Parkos C., Nusrat A., Delp C., Madara J. L. (1991) In vitro model of intestinal crypt abscess. A novel neutrophil-derived secretagogue activity. J. Clin. Invest. 87, 1474–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chester J. F., Ross J. S., Malt R. A., Weitzman S. A. (1985) Acute colitis produced by chemotactic peptides in rats and mice. Am. J. Pathol. 121, 284–290. [PMC free article] [PubMed] [Google Scholar]
- 16.Leoni G., Alam A., Neumann P. A., Lambeth J. D., Cheng G., McCoy J., Hilgarth R. S., Kundu K., Murthy N., Kusters D., Reutelingsperger C., Perretti M., Parkos C. A., Neish A. S., Nusrat A. (2013) Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest. 123, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam A., Leoni G., Wentworth C. C., Kwal J. M., Wu H., Ardita C. S., Swanson P. A., Lambeth J. D., Jones R. M., Nusrat A., Neish A. S. (2013) Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 7, 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riesselman M., Miettinen H. M., Gripentrog J. M., Lord C. I., Mumey B., Dratz E. A., Stie J., Taylor R. M., Jesaitis A. J. (2007) C-Terminal tail phosphorylation of N-formyl peptide receptor: differential recognition of two neutrophil chemoattractant receptors by monoclonal antibodies NFPR1 and NFPR2. J. Immunol. 179, 2520–2531. [DOI] [PubMed] [Google Scholar]
- 19.Maaty W. S., Lord C. I., Gripentrog J. M., Riesselman M., Keren-Aviram G., Liu T., Dratz E. A., Bothner B., Jesaitis A. J. (2013) Identification of C-terminal phosphorylation sites of N-formyl peptide receptor-1 (FPR1) in human blood neutrophils. J. Biol. Chem. 288, 27042–27058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Key T. A., Bennett T. A., Foutz T. D., Gurevich V. V., Sklar L. A., Prossnitz E. R. (2001) Regulation of formyl peptide receptor agonist affinity by reconstitution with arrestins and heterotrimeric G proteins. J. Biol. Chem. 276, 49204–49212. [DOI] [PubMed] [Google Scholar]
- 21.Key T. A., Foutz T. D., Gurevich V. V., Sklar L. A., Prossnitz E. R. (2003) N-Formyl peptide receptor phosphorylation domains differentially regulate arrestin and agonist affinity. J. Biol. Chem. 278, 4041–4047. [DOI] [PubMed] [Google Scholar]
- 22.Key T. A., Vines C. M., Wagener B. M., Gurevich V. V., Sklar L. A., Prossnitz E. R. (2005) Inhibition of chemoattractant N-formyl peptide receptor trafficking by active arrestins. Traffic 6, 87–99. [DOI] [PubMed] [Google Scholar]
- 23.Haribabu B., Richardson R. M., Verghese M. W., Barr A. J., Zhelev D. V., Snyderman R. (2000) Function and regulation of chemoattractant receptors. Immunol. Res. 22, 271–279. [DOI] [PubMed] [Google Scholar]
- 24.Forsman H., Önnheim K., Andréasson E., Christenson K., Karlsson A., Bylund J., Dahlgren C. (2013) Reactivation of desensitized formyl peptide receptors by platelet activating factor: a novel receptor cross talk mechanism regulating neutrophil superoxide anion production. PLoS ONE 8, e60169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henson P. M., Oades Z. G. (1975) Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J. Clin. Invest. 56, 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLeo F. R., Renee J., McCormick S., Nakamura M., Apicella M., Weiss J. P., Nauseef W. M. (1998) Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Invest. 101, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jesaitis A. J., Naemura J. R., Painter R. G., Schmitt M., Sklar L. A., Cochrane C. G. (1982) The fate of the N-formyl-chemotactic peptide receptor in stimulated human granulocytes: subcellular fractionation studies. J. Cell. Biochem. 20, 177–191. [DOI] [PubMed] [Google Scholar]
- 28.Jesaitis A. J., Tolley J. O., Painter R. G., Sklar L. A., Cochrane C. G. (1985) Membrane-cytoskeleton interactions and the regulation of chemotactic peptide-induced activation of human granulocytes: the effects of dihydrocytochalasin B. J. Cell. Biochem. 27, 241–253. [DOI] [PubMed] [Google Scholar]
- 29.Kahn J., Ingraham R. H., Shirley F., Migaki G. I., Kishimoto T. K. (1994) Membrane proximal cleavage of L-selectin: identification of the cleavage site and a 6-kD transmembrane peptide fragment of L-selectin. J. Cell Biol. 125, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkos C. A., Cochrane C. G., Schmitt M., Jesaitis A. J. (1985) Regulation of the oxidative response of human granulocytes to chemoattractants. No evidence for stimulated traffic of redox enzymes between endo and plasma membranes. J. Biol. Chem. 260, 6541–6547. [PubMed] [Google Scholar]
- 31.Perez H. D., Elfman F., Marder S., Lobo E., Ives H. E. (1989) Formyl peptide-induced chemotaxis of human polymorphonuclear leukocytes does not require either marked changes in cytosolic calcium or specific granule discharge. Role of formyl peptide receptor reexpression (or recycling). J. Clin. Invest. 83, 1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagisawa M., Saeki K., Okuma E., Kitamura T., Kitagawa S., Hirai H., Yazaki Y., Takaku F., Yuo A. (1999) Signal transduction pathways in normal human monocytes stimulated by cytokines and mediators: comparative study with normal human neutrophils or transformed cells and the putative roles in functionality and cell biology. Exp. Hematol. 27, 1063–1076. [DOI] [PubMed] [Google Scholar]
- 33.Perretti M. (2003) The annexin 1 receptor(s): is the plot unravelling? Trends Pharmacol. Sci. 24, 574–579. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel-Seifert K., Seifert R. (1993) Cyclosporin H is a potent and selective formyl peptide receptor antagonist. Comparison with N-t-butoxycarbonyl-L-phenylalanyl-L-leucyl-L-phenylalanyl-L-leucyl-L-phenylalanine and cyclosporins A, B, C, D, and E. J. Immunol. 150, 4591–4599. [PubMed] [Google Scholar]
- 35.Le Y., Ye R. D., Gong W., Li J., Iribarren P., Wang J. M. (2005) Identification of functional domains in the formyl peptide receptor-like 1 for agonist-induced cell chemotaxis. FEBS J. 272, 769–778. [DOI] [PubMed] [Google Scholar]
- 36.Hayhoe R. P., Kamal A. M., Solito E., Flower R. J., Cooper D., Perretti M. (2006) Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood 107, 2123–2130. [DOI] [PubMed] [Google Scholar]
- 37.Dalli J., Montero-Melendez T., McArthur S., Perretti M. (2012) Annexin A1 N-terminal derived peptide ac2-26 exerts chemokinetic effects on human neutrophils. Front. Pharmacol. 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitcher J. A., Touhara K., Payne E. S., Lefkowitz R. J. (1995) Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. J. Biol. Chem. 270, 11707–11710. [DOI] [PubMed] [Google Scholar]
- 39.Okajima F., Ui M. (1984) ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J. Biol. Chem. 259, 13863–13871. [PubMed] [Google Scholar]
- 40.Hirai Y., Yasuhara T., Yoshida H., Nakajima T., Fujino M., Kitada C. (1979) A new mast cell degranulating peptide “mastoparan” in the venom of Vespula lewisii. Chem. Pharm. Bull. (Tokyo) 27, 1942–1944. [DOI] [PubMed] [Google Scholar]
- 41.Vale C., Botana L. M. (2008) Marine toxins and the cytoskeleton: okadaic acid and dinophysistoxins. FEBS J. 275, 6060–6066. [DOI] [PubMed] [Google Scholar]
- 42.Harbecke O., Liu L., Karlsson A., Dahlgren C. (1997) Desensitization of the fMLP-induced NADPH-oxidase response in human neutrophils is lacking in okadaic acid-treated cells. J. Leukoc. Biol. 61, 753–758. [DOI] [PubMed] [Google Scholar]
- 43.Matula M., Mitchell M., Elbein A. D. (1971) Partial purification and properties of a highly specific trehalose phosphate phosphatase from Mycobacterium smegmatis. J. Bacteriol. 107, 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brom C., Brom J., König W. (1991) G Protein activation and mediator release from human neutrophils and platelets after stimulation with sodium fluoride and receptor-mediated stimuli. Immunology 73, 287–292. [PMC free article] [PubMed] [Google Scholar]
- 45.Gumińska M., Sterkowicz J. (1976) Effect of sodium fluoride on glycolysis in human erythrocytes and Ehrlich ascites tumour cells in vitro. Acta Biochim. Pol. 23, 285–291. [PubMed] [Google Scholar]
- 46.Sklar L. A., Mueller H., Omann G., Oades Z. (1989) Three states for the formyl peptide receptor on intact cells. J. Biol. Chem. 264, 8483–8486. [PubMed] [Google Scholar]
- 47.Thannickal V. J., Fanburg B. L. (2000) Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L1005–L1028. [DOI] [PubMed] [Google Scholar]
- 48.Follin P., Johansson A., Dahlgren C. (1991) Intracellular production of reactive oxygen species in human neutrophils following activation by the soluble stimuli FMLP, dioctanoylglycerol and ionomycin. Cell Biochem. Funct. 9, 29–37. [DOI] [PubMed] [Google Scholar]
- 49.Wright V. P., Reiser P. J., Clanton T. L. (2009) Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. J. Physiol. 587, 5767–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricci A. J., Wu Y. C., Fettiplace R. (1998) The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J. Neurosci. 18, 8261–8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naccache P. H., Therrien S., Caon A. C., Liao N., Gilbert C., McColl S. R. (1989) Chemoattractant-induced cytoplasmic pH changes and cytoskeletal reorganization in human neutrophils. Relationship to the stimulated calcium transients and oxidative burst. J. Immunol. 142, 2438–2444. [PubMed] [Google Scholar]
- 52.Klotz K. N., Jesaitis A. J. (1994) The interaction of N-formyl peptide chemoattractant receptors with the membrane skeleton is energy-dependent. Cell. Signal. 6, 943–947. [DOI] [PubMed] [Google Scholar]
- 53.Stafforini D. M., McIntyre T. M., Zimmerman G. A., Prescott S. M. (2003) Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit. Rev. Clin. Lab. Sci. 40, 643–672. [DOI] [PubMed] [Google Scholar]
- 54.Prescott S. M., McIntyre T. M., Zimmerman G. A., Stafforini D. M. (2002) Sol Sherry lecture in thrombosis: molecular events in acute inflammation. Arterioscler. Thromb. Vasc. Biol. 22, 727–733. [DOI] [PubMed] [Google Scholar]
- 55.Lad P. M., Olson C. V., Grewal I. S. (1985) Platelet-activating factor mediated effects on human neutrophil function are inhibited by pertussis toxin. Biochem. Biophys. Res. Commun. 129, 632–638. [DOI] [PubMed] [Google Scholar]
- 56.O’Flaherty J. T., Wykle R. L., Miller C. H., Lewis J. C., Waite M., Bass D. A., McCall C. E., DeChatelet L. R. (1981) 1-O-Alkyl-sn-glyceryl-3-phosphorylcholines: a novel class of neutrophil stimulants. Am. J. Pathol. 103, 70–78. [PMC free article] [PubMed] [Google Scholar]
- 57.Russo R. G., Liotta L. A., Thorgeirsson U., Brundage R., Schiffmann E. (1981) Polymorphonuclear leukocyte migration through human amnion membrane. J. Cell Biol. 91, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parkos C. A., Colgan S. P., Delp C., Arnaout M. A., Madara J. L. (1992) Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J. Cell Biol. 117, 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campbell E. L., Bruyninckx W. J., Kelly C. J., Glover L. E., McNamee E. N., Bowers B. E., Bayless A. J., Scully M., Saeedi B. J., Golden-Mason L., Ehrentraut S. F., Curtis V. F., Burgess A., Garvey J. F., Sorensen A., Nemenoff R., Jedlicka P., Taylor C. T., Kominsky D. J., Colgan S. P. (2014) Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Babbin B. A., Jesaitis A. J., Ivanov A. I., Kelly D., Laukoetter M., Nava P., Parkos C. A., Nusrat A. (2007) Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J. Immunol. 179, 8112–8121. [DOI] [PubMed] [Google Scholar]
- 61.Kühn H., Dreyer W. J. (1972) Light dependent phosphorylation of rhodopsin by ATP. FEBS Lett. 20, 1–6. [DOI] [PubMed] [Google Scholar]
- 62.Reiter E., Lefkowitz R. J. (2006) GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 17, 159–165. [DOI] [PubMed] [Google Scholar]
- 63.Butcher A. J., Kong K. C., Prihandoko R., Tobin A. B. (2012) Physiological role of G-protein coupled receptor phosphorylation. Handb. Exp. Pharmacol. 208, 79–94. [DOI] [PubMed] [Google Scholar]
- 64.Hooper L. V., Littman D. R., Macpherson A. J. (2012) Interactions between the microbiota and the immune system. Science 336, 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chadwick V. S., Mellor D. M., Myers D. B., Selden A. C., Keshavarzian A., Broom M. F., Hobson C. H. (1988) Production of peptides inducing chemotaxis and lysosomal enzyme release in human neutrophils by intestinal bacteria in vitro and in vivo. Scand. J. Gastroenterol. 23, 121–128. [DOI] [PubMed] [Google Scholar]
- 66.Nast C. C., LeDuc L. E. (1988) Chemotactic peptides. Mechanisms, functions, and possible role in inflammatory bowel disease. Dig. Dis. Sci. 33 (3Suppl) 50S–57S. [DOI] [PubMed] [Google Scholar]
- 67.Campbell E. L., Serhan C. N., Colgan S. P. (2011) Antimicrobial aspects of inflammatory resolution in the mucosa: a role for proresolving mediators. J. Immunol. 187, 3475–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rabiet M. J., Huet E., Boulay F. (2007) The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie 89, 1089–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Molloy M. J., Grainger J. R., Bouladoux N., Hand T. W., Koo L. Y., Naik S., Quinones M., Dzutsev A. K., Gao J. L., Trinchieri G., Murphy P. M., Belkaid Y. (2013) Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe 14, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. (1981) The kinetics of neutrophil activation. The response to chemotactic peptides depends upon whether ligand-receptor interaction is rate-limiting. J. Biol. Chem. 256, 9909–9914. [PubMed] [Google Scholar]
- 71.Lu D. J., Takai A., Leto T. L., Grinstein S. (1992) Modulation of neutrophil activation by okadaic acid, a protein phosphatase inhibitor. Am. J. Physiol. 262, C39–C49. [DOI] [PubMed] [Google Scholar]
- 72.Higashijima T., Uzu S., Nakajima T., Ross E. M. (1988) Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J. Biol. Chem. 263, 6491–6494. [PubMed] [Google Scholar]
- 73.Weingarten R., Ransnäs L., Mueller H., Sklar L. A., Bokoch G. M. (1990) Mastoparan interacts with the carboxyl terminus of the alpha subunit of Gi. J. Biol. Chem. 265, 11044–11049. [PubMed] [Google Scholar]
- 74.Parkos C. A., Colgan S. P., Bacarra A. E., Nusrat A., Delp-Archer C., Carlson S., Su D. H., Madara J. L. (1995) Intestinal epithelia (T84) possess basolateral ligands for CD11b/CD18-mediated neutrophil adherence. Am. J. Physiol. 268, C472–C479. [DOI] [PubMed] [Google Scholar]
- 75.Springer T. A. (1990) Adhesion receptors of the immune system. Nature 346, 425–434. [DOI] [PubMed] [Google Scholar]