Abstract
We have previously demonstrated that subcutaneous and intra-abdominal adipose tissue show different patterns of expression for developmental genes (Shox2, En1, Tbx15 Hoxa5, Hoxc8, and Hoxc9), and that the expression level of Tbx15 and Hoxa5 in humans correlated with the level of obesity and fat distribution. To further explore the role of these developmental genes in adipose tissue, we have characterized their expression in different adipose depots in mice, and studied their regulation in obesity and by fasting. Developmental and adipogenic gene expression was compared in two subcutaneous and three intra-abdominal white adipose tissue (WAT) depots as well as brown adipose tissue (BAT) from lean or obese mice in a fed or fasting state. Each of these six adipose depots display a unique pattern of developmental gene expression, whereas expression of adipogenic transcription factors PPARγ2 C/EBPα, β, and δ showed constant expression levels in all depots. Expression levels of developmental genes were similar in obese (ob/ob and high-fat diet (HFD)) and lean mice in most depots. Fasting systematically decreased expression of Hoxc8, PPARγ2, and increased C/EBPδ in both lean and ob/ob mice, but produced only variable changes in the expression of other developmental and adipogenic genes. These data indicate that each fat depot has a unique developmental gene expression signature, which is largely independent of nutritional state. This finding further supports a fundamental role of developmental genes in fat distribution and the development and/or function of specific adipose tissue depots.
INTRODUCTION
The distribution of white adipose tissue (WAT) not only varies considerably between species but also between individuals of the same species (1). In humans, variations of WAT distribution are of particular importance due to their association with metabolic disorders. Individuals with increased intra-abdominal/visceral fat are at high risk of type 2 diabetes and metabolic syndrome, whereas those with increased sub-cutaneous fat are at little or no risk of metabolic disease (2). Understanding what controls adipose distribution and why these different fat distributions are differentially linked to metabolic complications is therefore of considerable importance (1).
Recently, we and others have begun to gain insight into differences in adipose depots at a molecular level through studies of gene expression (3–6). By comparing gene expression patterns of adipocytes and stromovascular fraction from epididymal (intra-abdominal) and subcutaneous depots, we have identified a potentially important role for fundamental developmental and patterning genes in control of adipose tissue distribution and function. Thus, among the most differentially expressed genes were a number of homeobox genes including homeobox C9 (Hoxc9), homeobox C8 (Hoxc8) and homeobox A5 (Hoxa5), short stature homeobox 2 (Shox2), and engrailed 1 (En1), as well as T-box15 (Tbx15), which belongs to the family of T-box genes known to play a major role in mesoderm development in vertebrates. In rodents, Shox2, En1, Tbx15, and Hoxc9 were more highly expressed in subcutaneous adipose tissue, whereas Hoxa5 and Hoxc8 were more highly expressed in epididymal adipose tissue. These transcription factors also showed differences of expression between subcutaneous and intra-abdominal WAT in humans, with a differential expression >1,000-fold in many cases (5). In humans, expression of Tbx15 and Hoxa5, as well as the developmental patterning gene glypican-4, correlated with BMI and waist-hip-ratio (WHR), reflecting the level of obesity and body fat distribution.
Although considerable attention has focused on subcutaneous vs. visceral obesity, adipose tissue is located in many other regions of the body, which may have different functions and be of different developmental origins (1). For example, intra-abdominal fat in rodents and humans consists of several different depots, including perigonadal (epididymal in males and periovarian in females), perirenal, omental, and mesenteric fat. In addition, different normal individuals show considerable variation in subcutaneous fat accumulation in different areas of the body. Conversely, individuals with various forms of partial lipodystrophy exhibit a loss of subcutaneous fat from different regions of the body (7). Finally, there is brown adi-pose tissue (BAT), which accumulates in the interscapular area in rodents and in the cervical and mediastinal areas in humans. BAT is clearly functionally distinct from WAT, serving as a site of energy expenditure rather than energy storage (8).
In this study, we have expanded our characterization of the developmental differences in adipose depots by exploring the site-specific pattern of expression of different developmental genes six different fat depots. These include two subcutaneous WAT depots: the flank (Flk) representing the lower body, and interscapular area of the back (Bck), representing the upper body; three intra-abdominal WAT depots epididymal (Epd), perirenal (Prr), and mesenteric (Msn); and interscapular BAT. In addition, in these same depots, we have assessed the expression of the major adipogenic transcription factors, Cebpb, Cebpd, Cebpa, and Pparg2, and determined the effects of obesity and feeding vs. fasting on developmental gene expression.
METHODS AND PROCEDURES
Animals and diets
All animals were maintained on a 12 h-light–dark cycle in a temperature-controlled room and divided in groups, each consisting of five mice (n = 5). Mice in random fed groups were allowed ad libitum access to water and food. Mice in fasted groups were subjected to a 16-h overnight fast before killing. Standard low-fat chow diet (LFD) containing 22% calories from fat, 23% from protein, and 55% from carbohydrates (Mouse Diet 9F 5020; PharmaServ), and high-fat diet (HFD) containing 60% calories from fat, 20% from protein, and 20% from carbohydrates (Open Source Diet D12492, Research Diet) were used for this study. All animals were killed by cervical dislocation between 9 and 11 am. Animal care and manipulations were approved by the Animal Care Committee of Joslin Diabetes Center and were in accordance with the National Institutes of Health guidelines.
Adipose tissue dissection
Five different white adipose depots (two subcutaneous and three intra-abdominal) and interscapular BAT were harvested from each mouse, using the following procedure. The skin, but not the muscle wall underneath, was cut transversely around the abdomen of the animal. The skin was then pulled posteriorly toward the groin and anteriorly toward the head, revealing subcutaneous adipose depots. The flank (Flk) subcutaneous white adipose depot extending from the dorsal region around each hip and projecting into the inguinal region was the first to be harvested. In the various experiments this depot weighted from 360 mg in lean fed mice to 2.29 g per mouse in ob/ob fed mice. The back (Bck) subcutaneous white adipose depot (210 mg–2.26 g per mouse) located in the interscapular region was then dissected and separated from the BAT (80–340 mg per mouse) located underneath. To harvest intra- abdominal depots, the peritoneal muscle wall was then cut along the ventral mid-line from the groin to the chin and from the start of this first incision downwards to the knee on both sides of the animal. Epididymal (Epd) white adipose depot (400 mg–2.17 g per mouse) located around both testes was carefully separated from the epididymis. The intra-abdominal perirenal (Prr) white adipose depot (120–670 mg per mouse), which is attached dorsally to both kidneys, was then dissected. Finally, the intra-abdominal mesenteric (Msn) adipose depot was dissected (180–940 mg per mouse). For this, the intestines comprising the duodenum, jejunum, ileum, and cecum were uncoiled and the fat located at their surface and around the stomach was gently scraped away and harvested.
RNA extraction and quantitative PCR
Total RNA from each adipose tissue was extracted by using RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA) and treated with DNase I. RNA concentration was measured using ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Total RNA (1 μg) was reverse- transcribed with random hexamers using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Framingham, MA). Primers for quantitative real-time PCR are listed in (Supplementary Table S1 online). Quantitative real-time PCR was performed using either the 7700 or 7900HT Fast Real-Time PCR System (Applied Biosystems) with standard curve method and normalized to 18 S ribosomal RNA.
RESULTS
Interdepot expression comparison of developmental genes
We (5) and others (3,4,6) have previously shown that fat taken from subcutaneous and intra-abdominal depots in mice and humans exhibit differences in gene expression, and among these some of the most striking are the differences in expression of developmental genes, such as Hoxa5, Hoxc8, Hoxc9, Shox2, En1, and Tbx15. To determine the extent to which the expression of these developmental genes differed in other fat depots, we isolated adipose tissue from five WAT depots and the interscapular BAT of fed C57BL/6 mice and analyzed expression of each gene by quantitative real-time PCR.
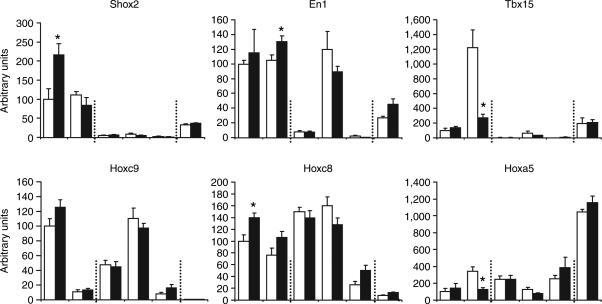
As previously noted (5), Shox2 displayed a 15- to 20-fold higher level of expression in flank as compared to intra- abdominal epididymal fat. Shox2 expression was also relatively high in the subcutaneous back depot and relatively low in intra-abdominal perirenal fat. Interestingly, expression of Shox2 was 10 times lower yet in intra-abdominal mesenteric fat, whereas BAT had a similar level of Shox2 as the Epd and Prr depots (Figure 1a).
Figure 1.
Expression pattern of developmental genes and adipogenic transcription factors in the different fat depots of C57BL/6 mice. (a) White adipose tissues from two subcutaneous fat depots—one from the flank (Flk) and the other from the interscapular area of the back surrounding the brown fat pad (Bck), three intra-abdominal fat depots from epididymal fat (Epd), perirenal fat (Prr) and mesenteric fat (Msn) and interscapular brown adipose tissue (BAT) were collected from 14-week-old male C57BL/6 mice under random feeding condition. Total RNA was extracted, and cDNA was synthesized. Gene expression of developmental genes Hoxa5, Hoxc8, Hoxc9, Shox2, En1, and Tbx15 was quantified by real-time PCR using the standard curve method, with expression in flank depot assigned a value of 100 arbitrary units. Five independent samples each from a different mouse (n = 5) were each analyzed in duplicate or triplicate. Differences between all depots were statistically analyzed by one-way analysis of variance with the Games-Howell post hoc test. The bar indicates significant difference (P < 0.05) between depots. Gene expression levels in two subcutaneous fat (Flk, Bck) and BAT are shown by the open bars, and the gene expressions in three intra-abdominal fat depots (Epd, Prr, Msn) are shown by the filled bars. Each fat depot shows a unique pattern of distribution. (b) Gene expression of four adipogenic transcription factors of Cebpb, Cebpd, Cebpa, and Pparg2 were assessed by quantitative real-time PCR using the same methods and samples indicated above.
Engrailed 1 (En1) is also highly expressed in subcutaneous fat, with equal levels of expression in both flank and back WAT. Among intra-abdominal depots, on the other hand, there was a wide range of expression with high levels in the Prr depot and low to very low levels in Epd and Msn WAT and BAT.
Tbx15 has been previously identified as being highly expressed in subcutaneous flank fat (5), but the expression was even higher in subcutaneous back fat (Figure 1a). Tbx15 was also highly expressed in Prr, with much lower expression in the other intra-abdominal depots, Epd and Msn, as well as in BAT.
Of the three Hox genes, Hoxc9 was the only gene more highly expressed in subcutaneous Flk than intra- abdominal Epd with about a twofold difference in mRNA levels. However, this differential expression relation between subcutaneous and intra-abdominal fat was not observed for other depots. Indeed, the highest level of expression for Hoxc9 was in the perirenal depot, while a much lower level was observed in subcutaneous Bck and intra-abdominal mesenteric depots. The lowest level of Hoxc9 expression was in BAT, which was approximately tenfold lower than in the surrounding WAT (Bck) and Msn fat.
In contrast to Hoxc9, Hoxc8, and Hoxa5 are intra-abdominal dominant genes (5). Hoxc8 was expressed at high level in two intra-abdominal fat depots (Epd and Prr), whereas it expression was lower in mesenteric fat and in subcutaneous Bck. As with Hoxc9, expression of Hoxc8 in BAT was the lowest of any depot studied.
As previously reported (5), Hoxa5 was most highly expressed in Epd and also relatively highly expressed in other intra-abdominal depots (Prr and Msn) as compared to lower levels in subcutaneous fat Flk and Bck (Figure 1a). Interestingly however, Hoxa5 expression was highest in BAT, with levels over threefold above those of the surrounding WAT.
These data are summarized schematically in Table 1. From this it is apparent that each fat depot has a unique pattern of expression of developmental genes with high levels of Shox2 in the subcutaneous flank depot; high levels of En1 and Tbx15 in flank, back and perirenal depots; high levels of Hoxc9 in flank, epididymal and perirenal depots, but not in back and mesenteric depot; high levels of Hoxc8 in the epididymal and perirenal depots and lowest level in BAT; and high levels of Hoxa5 in epididymal fat and BAT, with low expression in both subcutaneous (flank and interscapular) depots.
Table 1.
Comparison of interdepot expression of developmental genes and adipogenic transcription factors
| White adipose tissue (WAT) |
||||||
|---|---|---|---|---|---|---|
| Gene | Flank | Back | Epididymal | Perirenal | Mesenteric | BAT |
| Shox2 | +++ | ++ | + | + | - | + |
| En1 | +++ | +++ | + | +++ | - | + |
| Tbx15 | ++ | +++ | + | ++ | - | + |
| Hoxc9 | +++ | ++ | +++ | +++ | ++ | - |
| Hoxc8 | ++ | ++ | +++ | +++ | ++ | + |
| Hoxa5 | + | + | +++ | ++ | ++ | +++ |
| Cebpb | + | + | + | + | + | +++ |
| Cebpd | ++ | +++ | +++ | +++ | ++ | + |
| Cebpa | ++ | ++ | ++ | ++ | ++ | ++ |
| Pparg2 | ++ | ++ | ++ | ++ | ++ | ++ |
Interdepot comparison in gene expression of adipogenic transcription factors
For comparison to the differential expression of the developmental genes, we performed quantitative PCR analysis on two early adipogenic transcription factors, C/EBPβ (Cebpb) and C/EBPδ (Cebpd), and two late adipogenic transcription factors, C/EBPα (Cebpa) and PPARγ2 (Pparg2). Cebpb expression was relatively uniform for all five WAT depots, but at a much higher level in BAT. Cebpd expression, on the other hand, was much lower in BAT as compared to WAT, and among WAT depots, Cebpd was more highly expressed in Bck, Epd and Prr compared to Flk or Msn depots, although the differences were small. Both of the major late adipogenic transcription factors showed similar levels of expression in all of the depots studied, with Cebpa levels slightly higher in flank, epididymal and perirenal depots while Pparg2 mRNA levels were slightly higher in all three intra-abdominal fat depots and BAT (Figure 1b and Table 1).
Gene expression in genetic and diet-induced obese mice
In humans the expression level of Hoxa5, Tbx15, and glypican-4 correlates closely with the extent of obesity and the pattern of fat distribution (5). To study the potential link for these patterns of developmental gene expression with obesity in rodents, we compared expression in each fat depot between lean C57Bl/6 mice and ob/ob mice under fed conditions. In general, most of the developmental genes showed only modest changes in expression (less than twofold) comparing obese to lean animals, and thus most of the depots in ob/ob mice retained the unique pattern of developmental genes observed in lean C57Bl/6 mice (Figure 2b). There was significantly lower expression of Shox2 in epididymal and perirenal fat (65 and 53% decrease, respectively) of ob/ob mice as compared to lean mice; 53% decreased expression of En1 in perirenal fat; and moderately decreased expression of Hoxc9, Hoxc8, and Hoxa5 in epididymal and perirenal fat of ob/ob mice compared to lean controls (Figure 2a). There was also a suggestion of decreased expression of Tbx15 in interscapular subcutaneous WAT of ob/ob mice as compared to lean mice; however, this difference did not reach statistical significance. By contrast, the expression of En1, Tbx15, and Hoxa5 was increased 2- to 3.4-fold in BAT of ob/ob mice. Taking all of these modest changes into account, only the interdepot pattern of Hoxc8 expression, which was observed in lean mice with higher levels in intra-abdominal vs. subcutaneous fat, was lost in ob/ob mice (Figure 2a).
Figure 2.
Comparison of expression of the developmental genes and adipogenic transcription factors in the different fat depots of C57BL/6 and ob/ob mice. (a) Total RNA was extracted from the six different fat depots in 10-week-old male ob/ob mice (on a C57BL/6 background) under random feeding conditions, and gene expressions of Hoxa5, Hoxc8, Hoxc9, Shox2, En1, and Tbx15 assessed as described in the legend of Figure 1 and compared to that in lean C57BL/6 mice. Note that the unique distribution of the developmental transcription factors observed in lean mice was conserved in ob/ob mice, with the exception of Hoxc8. (b) Gene expressions of the adipogenic transcription factors, Cebpb, Cebpd, Cebpa, and Pparg2 in ob/ob vs. lean mice. Gene expression in adipose tissue of C57BL/6 mice (open columns) and ob/ob mice (filled columns) was assessed as described in Figure 1b. Differences in gene expression in the same depot between C57BL/6 mice and ob/ob mice from five independent samples each from a different mouse (n = 5) were analyzed by Student's t-test. *statistical significance at P < 0.05.
Obesity also produced only modest changes in the expression of the various adiopogenic transcription factors tested. Cebpb expression was increased in the three intra-abdominal fat depots of ob/ob mice compared to control, but by only 30–90%. Cebpd expression in back, epididymal and perirenal depots of ob/ob mice was decreased by 30–40%. On the other hand, Cebpd was increased by 2.2-fold in BAT of ob/ob mice, but the levels in BAT were still much lower than in any of the WAT depots. Cebpa expression was slightly, but significantly, increased in flank and perirenal (~1.2-fold) of the obese mice and was increased 2.4-fold in mesenteric fat. Interdepot expression of Pparg2 was relatively modest when comparing obese with lean mice with a 50% increase in Bck and a 36% decrease in Prr in ob/ob mice (Figure 2b).
In addition to the genetically obese ob/ob model, we also compared the expression of these developmental genes in the fat depots of lean C57Bl6 mice fed with standard diet (LFD) and C57Bl6 mice subjected to a HFD. After 7 weeks, mice on a HFD gained substantially more weight than mice on a LFD (33.2 g ± 1.3 vs. 23.4 g ± 1.0, P value < 0.001). As expected, HFD was also associated with a significant threefold increase in weight of all fat depots when compared to those from mice on a LFD (data not shown). Again, very few changes in expression were observed among the different fat depots when mice on a LFD were compared with mice on a HFD (Figure 3). There was a significant increase in expression of Shox2 and HoxC8 in flank fat of mice on a HFD (2.1-fold and 1.4-fold, respectively). In the Bck depot, expression of Tbx15 and HoxA5 decreased 60–80% with HFD, whereas a modest, but still significant, change of expression was observed for En1 (24% increase). Expression of HoxC8 was increased 63% in BAT of mice on a HFD, but this was not affected in all other depots tested. Finally, no significant differences in expression of Pparg2, Cebpa, Cebpb, and Cebpd were observed in any of the fat depots when comparing mice on a LFD to those on a HFD (data not shown).
Figure 3.
Comparison of expression of the developmental genes the different fat depots of C57BL/6 mice in LFD vs. HFD. Three-week old C57Bl6 male mice were fed for 7 weeks with a normal chow diet (LFD, opened columns) or with a high-fat diet (HFD, filled columns). Gene expression of Shox2, Tbx15, En1, Hoxc9, Hoxc8, and Hoxa5 was measured in the six different fat depots of both LFD and HFD mice by quantitative RT-PCR. Note that the unique distribution of the developmental transcription factors observed in lean LFD mice was conserved in obese HFD mice. Differences in gene expression before and after fasting in the same depot from five independent samples each from a different mouse (n = 5) were analyzed by Student's t-test (*significance at P < 0.05).
Gene expression and feeding status in C57B6 mice and ob/ob mice
All of the above data were gathered on mice in the random fed state. To determine the effect of acute food deprivation on the expression of developmental genes, a group of C57B6 lean mice and ob/ob were fasted overnight before killing (Figure 4). For most of the developmental genes, there were no signifi-cant differences between expression in the fed and fasted states (Figure 4a,b). Shox2 continued to exhibit higher levels of expression in subcutaneous depots, but did show small, but significant decreases in the Flk and Epd depots in lean mice.
Figure 4.
Effect of fasting on gene expression of developmental and adipogenic transcription factors genes in C57BL/6 mice and ob/ob Mice. Expression of the six developmental transcription factors Shox2, En1, Tbx15, Hoxc9, Hoxc8 Hoxa5 (a,b) and four adipogenic transcription factors Cebpb, Cebpd, Cebpa, and Pparg2 (c,d), were measured in six different fat depots before and after fasting in both lean C57BL/6 mice (a,c) and ob/ob mice (b,d). Differences in gene expression before and after fasting in the same depot were analyzed by Student's t-test. Note that Hoxc8 in white fat pads shown decreased trend by fasting in both C57BL/6 mice and ob/ob mice. Expression of each gene in the different fat depots is indicated as open circles (Flk), filled circles (Bck), open squares (Epd), filled squares (Prr), open triangles (Msn), open diamonds (BAT). Differences in gene expression before and after fasting in the same depot from five independent samples each from a different mouse (n = 5) were analyzed by Student's t-test (*significance at P < 0.05).
On the other hand, Shox2 was significantly increased in BAT (1.6-fold increase) in fasted lean mice, but not in fasted ob/ob mice. Fasting also induced modest changes in expression of En1 with a decrease of 54% in epididymal fat and increases in perirenal and BAT of 1.4- and 1.7-fold increase in fasted lean mice (Figure 4a) with no change in ob/ob mice (Figure 4b). Tbx15 expression was not changed by fasting in lean mice in any depot, whereas ob/ob mice showed an increased expression of 1.6-fold in BAT only (Figure 4b). The one major change in expression of any developmental gene during fasting was a 7.8-fold increase in Hoxc9 expression in mesenteric fat of ob/ob mice, allowing this gene to reach a similar level as observed in epididymal and perirenal depots (Figure 4b). Hoxc8 was significantly decreased in flank, epididymal and perirenal fat (39–62% decrease), and tended to be lower in the back and mesenteric depots following fasting of lean and ob/ob mice (Figure 4a,b). Hoxa5 increased expression in intra-abdominal fat depots by 1.5- to 2.2-fold in fasted lean mice (Figure 4a), but no change was observed in fasted ob/ob mice (Figure 4b)
In contrast to the relatively modest and variable changes in the developmental genes with fasting, many of the adipogenic transcription factor genes showed consistent and significant changes (Figure 4). Thus, Cebpd was significantly increased by 1.4- to 3.0-fold in all depots fat depot (except for epididymal) during fasting in lean mice. Cebpd expression also significantly increased in all depots of fasted ob/ob mice by 1.5- to 2.1-fold. On the other hand, expression of Cebpa decreased during fasting in all WAT of lean mice and ob/ob mice, while it increased slightly in BAT. Pparg2 expression decreased in all fat depots during fasting of lean mice and ob/ob mice by 61–81%. Cebpb showed more mixed results, with a ~50% decrease in the flank and BAT and a 1.4-fold increase in perirenal fat of fasting C57B6 lean mice. These changes were not observed in ob/ob mice during fasting.
DISCUSSION
There is a growing body of evidence to suggest that adipose tissue in different depots exhibits different properties and may have different developmental lineages (1). These differences have been most carefully documented by comparison of epididymal and subcutaneous flank fat in rodents and omental vs. subcutaneous abdominal fat from humans and include differences in patterns of gene expression (5,9), differences in rates of lipogenesis and lipolysis (10), and differences in the ability of preadipocytes from these depots to differentiate when placed in culture (11–14). Evidence that these differences may be developmental includes the fact that a number of differences in gene expression involve developmental genes and fat distribution is known to have a strong hereditary component (reviewed in ref. 1)).
In this study, we expanded our view of differences in adipose depots by systematically comparing six different fat depots (five white and one brown) for expression level of six fundamental developmental transcription factors, including Shox2, En1, Tbx15, Hoxc9, Hoxc8, and Hoxa5. The six adipose tissue depots included two subcutaneous WAT– one from dorsal, lower body fat (flank) and the other from dorsal, upper body fat (interscapular back WAT). The three intra-abdominal WAT depots included the epididymal fat pad, as well as perirenal and mesenteric fat depots. In addition, we assessed gene expression in interscapular BAT, allowing direct comparisons of WAT and BAT taken from the same anatomical area.
We find that each fat depot has a unique pattern of developmental gene expression. Among all genes studied, Shox2 appeared to be more specifically expressed in subcutaneous WAT with higher levels of expression in flank and back WAT than in any of the three intra-abdominal depots. En1 and Tbx15, which were originally identified as a subcutaneous dominant genes based on comparison of flank to epididymal fat (1) also exhibits relatively high expression in perirenal fat, but interestingly was at much lower levels in mesenteric fat. Indeed, Tbx15 and En1 appear to have a pattern of expression more related to the dorsal-ventral location of the WAT depot with high levels in flank, back and perirenal depots compared to ventrally located mesenteric and epididymal WAT. On the other hand, Hoxc9 shows higher levels of expression in epididymal and perirenal fat, but is not highly expressed in mesenteric or subcutaneous WAT, whereas Hoxa5 is more highly expressed in all intra-abdominal depots compared to subcutaneous depots, but is even more highly expressed in BAT. Hox genes are organized in four clusters (HoxA, HoxB, HoxC, and HoxD) that encode conserved transcription factors expressed along the antero-posterior axis of vertebrates that serve as determinants of embryonic cell fate (15). Thus, it is not surprising that Hoxa5 is the most highly expressed in BAT, and Hoxc8, and Hoxc9 are more highly expressed in flank, perirenal and epididymal fat. But none of these Hox genes present a strongly delineated anterior-posterior pattern of expression as usually observed during embryonic development. In this regard, it is interesting to note that BAT and the subcutaneous inter-scapular WAT which surrounds it present very different patterns of expression for all the developmental transcription factors tested. Indeed, Shox2, En1, Tbx15, Hoxc9, Hoxc8 are all much more highly expressed in subcutaneous interscapular WAT compared to BAT which it surrounds, whereas Hoxa5 expression is much higher in BAT than interscapular WAT or any other WAT tested. These results reinforce the theory that even if BAT and WAT share some common characteristics, they almost certainly arise from different precursor cells (1). In contrast to these rather marked differences in expression of developmental genes, the adipogenic transcription factors, Cebpb, Cebpd, Cebpa, and Pparg2 show relatively modest differences in expression, and the pattern of expression does not correspond to the expression pattern of any of the developmental genes tested.
The unique patterns of depot-specific expression of Shox2, En1, Tbx15, Hoxc9, Hoxc8, and Hoxa5 in various adipose tissues of mice suggests involvement of these genes in the positioning of these different depots in the body. Myoblasts taken from different positions along the antero-posterior axis showed a specific expression pattern of Hox genes (16). Similarly, genome-wide gene expression profiling study of primary fibroblasts from 43 anatomical sites spanning the human body has also indicated that Hoxa5 and Tbx15 as position-specific transcription factors (17).
The role played by Hox and other embryonic developmental genes in the control of adipose tissue development or function remains unclear. In our previous studies of human adipose tissue we demonstrated differences in expression of Tbx15, Hoxa5 and the inositol glycan linked membrane protein glypican-4 as related to BMI and WHR. To determine whether this might be cause or effect of obesity and fat distribution in this study we compared the expression of these developmental genes in each fat depot in lean and ob/ob mice on the same C57Bl/6 background in both the fed and fasted states. In general, the interdepot gene expression patterns of developmental genes in the six fat depots from ob/ob mice or mice with HFD induced obesity were similar to those from their respective controls. Thus, in humans, Tbx15 expression is 10,00-fold higher in visceral fat than subcutaneous fat and expression in visceral fat decreases by >90% of overweight and obese individuals (BMI >25) and also correlates negatively with WHR. Conversely, Hoxa5 expression in humans positively correlates with BMI and WHR in both visceral and subcutaneous adi-pose tissue (5). By contrast in both obese rodent models (ob/ob and HFD mice), the expression of Hoxa5 was not affected in most of the fat depots tested, and the expression of Tbx15 was significantly decreased only in interscapular white adipose depot of mice in HFD, although the same trend was observed in ob/ob mice. Thus for both Tbx15 and Hoxa5 the relationships to BMI and WHR appear to represent primary differences in expression related to BMI and WHR as opposed to changes secondary to obesity or increased food intake.
There are a number of modest alterations in developmental gene expression in obese vs. lean mice, which were specific to the model tested. There was a twofold increase in Shox2 expression in the subcutaneous flank fat of mice in HFD and a modest reduction in Shox2 expression in epididymal and perirenal fat of ob/ob mice. Likewise, En1 expression was decreased in perirenal fat and increased BAT of ob/ob mice but not HFD mice. In addition, Hoxc9, Hoxc8, and Hoxa5 expression were decreased in both epididymal and perirenal fat of ob/ob mice, whereas in HFD mice Hoxc9 expression was not changed in any depot, Hoxc8 expression only increased in subcutaneous flank and BAT, and Hoxa5 was decreased only in subcutaneous interscapular WAT. Because ob/ob mice are leptin deficient due to a mutation in the leptin gene, it is possible that the different patterns observed between ob/ob and HFD obese mice represent some effect of leptin on expression of these transcription factors. Furthermore, characterization of the expression pattern of these developmental transcription factors in response to leptin administration will be necessary to directly address this possibility.
It is interesting to note that there were only modest changes in expression in either control or ob/ob mice in the fed vs. fasted states. These in vivo findings are consistent with previous results where human preadipocytes taken from different depots of lean or obese patients and cultured for many generations show differences in expression of developmental genes similar to those observed from freshly isolated cells from the same depots (9). Interestingly, Hoxc8 expression, which we find to be decreased in epididymal and perirenal fat of ob/ob mice, is also decreased by >75% in preadipocytes cultured from omental depots of obese humans (9).
In conclusion, these results demonstrate that each fat depot has a unique pattern of development gene expression and that these differences are largely independent of the state of obesity and feeding/fasting. Taken together with previous data that similar differences in developmental gene expression are observed in the stromovascular fraction from different depots (5) and in cultured human preadipocytes from different depots (9), it seems likely that these developmental genes play a role in depot-specific differences in adipocyte differentiation or function. Exactly how this might occur remains to be determined. Recently, cross talk has been demonstrated between Hoxa10 and Wnt signaling in endometrium (18), and Wnt signaling is known to also play an important role in control of preadipocyte differentiation (19). In endometrial stromal cells, HOXA10 has also been shown to interact with the FOXO transcription factor FKHR in the regulation of IGFBP-1 expression (20), and FoxO transcription factors are also involved in adipocyte differentiation (21). It is possible that these developmental genes could therefore directly or indirectly influence the nature of the adipose tissue which develops in each depot. Defining the exact role of these developmental genes in adipose tissue development and function remains an important challenge.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Diabetes Genome Anatomy Project (DGAP) (DK60837) and NIH grant DK82659, the NIH-funded Joslin Diabetes and Endocrinology Center grant DK34834, and the Mary K. Iacocca Professorship fund.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 3.Vohl MC, Sladek R, Robitaille J, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 4.Cantile M, Procino A, D'Armiento M, Cindolo L, Cillo C. HOX gene network is involved in the transcriptional regulation of in vivo human adipogenesis. J Cell Physiol. 2003;194:225–236. doi: 10.1002/jcp.10210. [DOI] [PubMed] [Google Scholar]
- 5.Gesta S, Blüher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal AK, Garg A. Genetic disorders of adipose tissue development, differentiation, and death. Annu Rev Genomics Hum Genet. 2006;7:175–199. doi: 10.1146/annurev.genom.7.080505.115715. [DOI] [PubMed] [Google Scholar]
- 8.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 9.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 10.Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 11.Adams M, Montague CT, Prins JB, et al. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100:3149–3153. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djian P, Roncari AK, Hollenberg CH. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J Clin Invest. 1983;72:1200–1208. doi: 10.1172/JCI111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauner H, Entenmann G. Regional variation of adipose differentiation in cultured stromal-vascular cells from the abdominal and femoral adipose tissue of obese women. Int J Obes. 1991;15:121–126. [PubMed] [Google Scholar]
- 14.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 15.Gehring WJ. Homeo boxes in the study of development. Science. 1987;236:1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- 16.Biressi S, Molinaro M, Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev Biol. 2007;308:281–293. doi: 10.1016/j.ydbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daikoku T, Song H, Guo Y, et al. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol. 2004;18:1238–1250. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- 19.Prestwich TC, Macdougald OA. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007;19:612–617. doi: 10.1016/j.ceb.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JJ, Taylor HS, Akbas GE, et al. Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol Reprod. 2003;68:24–30. doi: 10.1095/biolreprod.102.009316. [DOI] [PubMed] [Google Scholar]
- 21.Nakae J, Kitamura T, Kitamura Y, et al. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.