Abstract
The current study is the first to use magnetoencephalography (MEG) to examine how individuals with social anxiety disorder (SAD) process emotional facial expressions (EFEs). We expected that, compared to healthy controls (HCs), participants with SAD will show an early (<200 ms post-stimulus) over-activation in the insula and the fusiform gyrus (FG, associated with the N170/M170 component), and later (>200 ms post-stimulus) over-activation in the dorsolateral prefrontal cortex (DLPFC). Individuals with SAD (n = 12) and healthy controls (HCs, n = 12) were presented with photographs of facial displays during MEG recording. As compared to the HC group, the SAD group showed a reduced M170 (right FG under-activation around 130–200 ms); early reduced activation in the right insula, and lower insular sensitivity to the type of EFE displayed. In addition, the SAD group showed a late over-activation in the right DLPFC. This unique EFE processing pattern in SAD suggests an early under-activation of cortical areas, possibly related to reduced emphasis on high spatial frequency information and greater early emphasis on low spatial frequency information. The late DLPFC over-activation in the SAD group may correlate to failures of cognitive control in this disorder. The importance of a temporal perspective for the understanding of facial processing in psychopathology is underlined.
Abbreviations: AFNI, analysis of functional neuroimages; BDI, Beck Depression Inventory; DLPFC, dorsolateral prefrontal cortex; EEG, electroencephalography; EFE, emotional facial expressions; FG, fusiform gyrus; FMRI, functional magnetic resonance imaging; FNE, fear of negative evaluation; HC, healthy control; HSF, high spatial frequency; LSAS, Liebowitz Social Anxiety Scale; LSF, low spatial frequency; MEG, magnetoencephalography; SA, social anxiety; SAD, social anxiety disorder; SAM, synthetic aperture modeling; TMS, transcranial magnetic stimulation
Keywords: Social anxiety, Magnetoenchephalography, Facial processing, Cognitive control, Regulation
Highlights
-
•
This study is the first to use MEG to study social anxiety disorder (SAD).
-
•
SADs and controls viewed emotional facial expressions during MEG.
-
•
Compared to controls, SADs showed reduced M170 (early fusiform gyrus activity).
-
•
SADs presented a late over-activation in the right dorsolateral prefrontal cortex.
-
•
The late frontal over-activity may correlate to failures of cognitive control in SAD.
1. Introduction
Social anxiety disorder (SAD) is the most common anxiety disorder in the community, with an estimated life-time prevalence rate as high as 13% (Furmark, 2002). Individuals with social anxiety (SA) are agonized by the potential risk of performing inadequately in social situations or showing overt signs of nervousness with resultant embarrassment or humiliation (American-Psychiatric-Association, 1994). The processing of emotional facial expressions (EFEs) is an important aspect in social functioning, as they enable people to quickly infer other persons' thoughts, feelings, intentions and motivations (e.g., Said et al., 2011). Such nonverbal aspects of human interaction are especially relevant for individuals suffering from SA, for whom social evaluation is a primary concern.
Over the years, neuroimaging paradigms have been recruited to the study of EFE processing in SAD. Face processing entails several neural systems (Vuilleumier and Pourtois, 2007). First, the limbic system, including the amygdala and insula regions, processes coarse, low spatial frequency (LSF) information from the face (Vuilleumier et al., 2003). LSF information is important mainly for decoding emotional expressions (Langner et al., 2009). The amygdala and insula are involved in the detection of emotional, social or threatening stimuli (Anderson et al., 2003; Calder et al., 2001; Schienle et al., 2002), and their activity is modulated by the type of facial expression (Adolphs, 2002). MEG studies have shown that the activity of both areas is observed during the early stages of emotional face processing: the amygdala around 40 milliseconds (ms) (Garvert et al., 2014) and the insula around 150 ms post-stimulus onset (Bayle and Taylor, 2010; Chen et al., 2009). Moreover, Luo et al. (2007) found early event related synchronizations (ERS) in response to fearful faces in the amygdala at around 30 ms, and for angry expressions at around 150 ms.
This quick limbic processing pathway may be partly independent of a second, slower system involving the extrastriate visual cortex, including the fusiform gyrus (FG) (Vuilleumier et al., 2002; Vuilleumier et al., 2003; Williams et al., 2004). The “fusiform face area” (Kanwisher et al., 1997) in the FG has a key role in face perception, and is a part of a specialized neural system for face processing (Kanwisher and Yovel, 2006). This second stage of processing extracts finer and more elaborate high spatial frequency (HSF) features (Vuilleumier et al., 2003), important for precise recognition of identity and more detailed analysis of facial traits, such as age (Alorda et al., 2007; Winston et al., 2003). This slower processing of faces in the FG is usually associated with the N170 electroencephalography (EEG) component, or M170 in magnetoencephalography (MEG) (Taylor et al., 2011). The N170/M170 face-selective component (Bentin et al., 1996) indexes the late stages of structural encoding of faces which include a configurational analysis of whole faces. As such, the N170 is maximal to face stimuli that are optimal for face recognition and identification (Eimer, 2000); and is correlated with successful face categorization and identification (Liu et al., 2002).
In addition to the abovementioned systems, the processing of EFEs also requires top-down mechanisms, aimed at inhibiting emotional reactions to threatening stimuli and associated with prefrontal activation (Davidson, 2002; Ochsner and Gross, 2005). The dorsolateral prefrontal cortex (DLPFC) is important in this aspect as it initiates emotion regulation by inhibiting the amygdala (Siegle et al., 2007). The importance of the DLPFC region in the ability to disengage attention from faces was illustrated when transcranial magnetic stimulation (TMS) of the right prefrontal cortex resulted in impaired disengagement from angry faces, associated with decreased activation within the right DLPFC (De Raedt et al., 2010). In addition, a recent study showed that participants with high rumination scores (brooders) display higher DLPFC activity when attempting to disengage attention from negative EFEs (Vanderhasselt et al., 2011), suggesting that brooders need to recruit more attentional control (manifest as the DLPFC activity) in order to successfully disengage from negative information. MEG studies suggest that the frontal involvement in EFE processing arrives rather late in the processing stream: at around 250 ms post-stimulus (in Taylor et al., 2011); or around 160–210 ms (in Luo et al., 2007).
Haxby et al. (2000, 2002) described the EFE processing system as comprised of a core system which includes face-specific areas (including the FG and superior temporal sulcus, STS) which perform the visual analysis of faces; and an extended neural system, aimed at extracting important social information from faces, such as temporary mood states and intentions, as well as more stable personality characteristics. This extended system is comprised of brain structures which are involved in other functions, such as directing attention (e.g., frontal areas) or emotional processing (such as the amygdala and insula) (Haxby et al., 2002).
Due to the importance of facial expressions in social interaction and in SA, various studies explored the neural correlates of EFE processing in SAD, using both fMRI and EEG. Findings from fMRI studies have consistently shown that SADs present enhanced activation in limbic areas (such as the amygdala and insula) when viewing threatening faces (Evans et al., 2008; Gentili et al., 2008; Stein et al., 2002; Straube et al., 2005), as well as neutral ones (Birbaumer et al., 1998; Cooney et al., 2006). In contrast, the findings regarding the role of FG in face processing yielded a conflicting pattern of results using both fMRI and EEG methodologies. First, using fMRI, Straube et al. (2004) found that participants with SAD exhibited stronger FG activation compared to healthy controls (HCs), during categorization of face pictures as schematic or photographic, and also during free viewing of angry, happy and neutral faces (Straube et al., 2005). On the other hand, Gentili et al. (2008) found weaker activation in the left FG in SADs (compared to HCs), when performing a one-back repetition detection task based on face identity. Similarly, Beaton et al. showed in two studies that shy participants present weaker right FG activation to faces (compared to non-shy controls), when judging the faces' familiarity (Beaton et al., 2009) or gender (Beaton et al., 2010). The role of the FG in processing of facial expressions in SAD is therefore not yet clearly understood. Importantly, due to the low temporal resolution of fMRI, these studies cannot offer temporal information regarding the timing of the limbic over-activity or the FG activation.
Second, using EEG, a similarly inconclusive pattern emerged with the face-specific N170 component found as weaker, stronger or equally powerful in participants with SAD as compared to HCs (Kolassa and Miltner, 2006; Mueller et al., 2009; Muhlberger et al., 2009). These discrepancies may stem from the focus of participants' attention in the different tasks: Kolassa et al. (2006) found stronger N170 amplitudes in participants with SAD (compared to HCs), but only on tasks in which emotional expression was task-relevant (emotion categorization task), but not when it was task-irrelevant (gender categorization). A weaker N170 in participants with SAD (compared to HCs) was found using another variant of an emotion-irrelevant task (dot-probe, in which two faces are presented, Mueller et al., 2009). Another factor which may have affected the N170 is the type of facial stimuli: all studies which found no effects of SA on the N170 used artificial faces, whether exclusively (Kolassa, 2009; Kolassa et al., 2007), or alongside natural faces (Muhlberger et al., 2009). Due to the diverse findings, it is thus still unclear whether SA affects the amplitude of the N170/M170, but it seems that relevant variables which may modulate this component are the type of task, type of faces (artificial or natural) and whether a single face or multiple faces are presented.
In addition to these functional findings, recent studies also suggest the existence of structural brain abnormalities in SAD as compared to HCs. Differences in gray matter morphometry and cortical thickness have been observed in various brain areas of individuals with SAD (although results are somewhat mixed, see review by Bruhl et al., 2014). Interestingly, studies also point to abnormalities in the connectivity or interaction of different brain areas in SAD. As compared to HCs, individuals with SAD show reduced volume of the left uncinate fasciculus, which connects frontal and temporal areas, including the amygdala (Baur et al., 2013); and show reduced connectivity between limbic areas (anterior insula) and prefrontal regions (dorsal anterior cingulate cortex) (Klumpp et al., 2012).
The processing of EFEs in SAD has also been studied using behavioral methods. These studies consistently suggest that individuals with SAD experience difficulty disengaging from threatening stimuli (Amir et al., 2003), as well as ignoring irrelevant emotional information from faces (Gilboa-Schechtman et al., 2004) or words (Grant & Beck, 2006; Mattia et al., 1993). Eye tracking studies also revealed that people with SAD exhibit disengagement difficulties from EFEs (Buckner et al., 2010; Schofield et al., 2012). As compared to HCs, individuals with SAD also show longer fixation duration at EFEs during the first 1000 ms of stimulus exposure (Wieser et al., 2009). In addition, individuals with SAD initially direct their gaze more frequently at angry faces rather than neutral faces when shown angry–neutral face pairs (Gamble and Rapee, 2010; Schofield et al., 2012). While behavioral and eye-tracking studies depict a unique pattern of EFE processing in SA, these methodologies have not enabled a clear understanding of the moment-by-moment unfolding of these processes.
In summary, research efforts spanning a variety of methodologies have been aimed at uncovering the EFE processing patterns in individuals with SAD. Specifically, the main questions have been whether, as compared to HCs, individuals with SAD (a) show greater early sensitivity to facial display of threat; and (b) do they show later elaborate processing or avoidance of threatening expressions. An examination of the temporal course of face processing is likely to shed light on these questions. This is the main focus of the present study.
1.1. Overview of the present study
We chose to use MEG technology, which provides excellent temporal resolution (in the order of milliseconds) and good spatial resolution with source modeling methods. Despite these advantages, MEG has never been used in the study of SA before.
Participants diagnosed with SAD and HCs were presented with photographs of facial displays and asked to categorize the faces according to gender. Our decision to use a task in which emotional expression is task irrelevant was driven by two considerations. First, we believe that gender categorization tasks are ecologically valid, as in many interactions emotion identification is an implicit, rather than explicit task. Second, we reasoned that using such a task would maximize our chances to observe individual differences in EFE processing. Whereas both socially anxious and non-anxious people would focus on emotion when task directions explicitly instructed them to do so, only anxious individuals may focus on emotion even in the absence of task instructions.
The stimuli included facial displays varying both in expressions (happy and angry); as well as facial postures (arrogant, neutral and submissive). The latter dimension was chosen as social dominance cues have been suggested to play an important role in SA (Gilboa-Schechtman and Shachar, 2013; Trower et al., 1990). We focused on three regions of interest, tapping both limbic and cortical aspects of face-processing: the right FG, involved in structural encoding of faces; the insula, a limbic region involved in emotion processing; and the DLPFC, a frontal region involved in attention regulation. Three hypotheses were examined: The early insular over-activation in SAD hypothesis: Consistent with the early insular activation studies, we predicted that during the early stages (<200 ms) of the trial, participants with SAD will show an over-activation in the insula, (compared to HCs). The early FG over-activation in SAD hypothesis: Consistent with the neural over-activation to EFEs found in fMRI research and enhanced N170 found in some EEG studies, we predicted that participants with SAD will show an over-activation in the FG during the early stages (<200 ms) of the trial (compared to HCs). The late DLPFC over-activation in SAD hypothesis: Consistent with the disengagement difficulty findings emerging from behavioral, eye-tracking, and some imaging studies, we predicted that in late stages of the trial (>200 ms) participants with SAD will show increased DLPFC activation (compared to HCs), especially for threatening faces.
2. Methods
2.1. Participants
Participants were 12 (4 female) right-handed adults who met DSM-IV criteria for current SAD and 12 (4 female) demographically matched healthy controls with no history of any DSM-IV psychiatric disorders. The participants were recruited from a public mental health clinic, or through advertisements within Bar Ilan University and the internet. All participants provided written informed consent in accordance with the Bar Ilan University Review Board guidelines. All participants were diagnosed using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995). The interviewers were graduate students in clinical psychology who received training for the administration of the SCID prior to the study.
Inclusion criteria for the SAD group included (a) a primary diagnosis of SAD according to DSM-IV criteria, (b) age between 18 and 65, (c) right handedness. Exclusion criteria included (a) past or current diagnosis of schizophrenia, (b) history of neurological disorders, or (c) metal implants in the head or body.
In addition to the primary diagnosis of SAD, individuals in the clinical group received diagnoses of major depressive disorder (n = 2), generalized anxiety disorder (n = 1), obsessive–compulsive disorder (n = 2), and agoraphobia (n = 1). Demographic data of each group is presented in Table 1.
Table 1.
Means (and standard deviations) of demographic data and t values of between-group tests for each variable.
| HC group n = 12 |
SAD group n = 12 |
t (23) | |
|---|---|---|---|
| Age | 29.1 (6.3) | 29.7 (6.9) | −0.12 n.s. |
| % females | 33% | 33% | |
| LSAS | 14.1 (9.3) | 74.3 (26.3) | −7.4*** |
| FNE | 7.5 (5.9) | 25.3 (4.5) | −8.2*** |
| BDI | 2.5 (2.3) | 14.5 (11.6) | −3.5** |
Note. LSAS — Liebowitz Social Anxiety Scale, FNE — Fear of Negative Evaluation, BDI — Beck Depression Inventory.
p < 0.01.
p < 0.001.
2.2. Self-report measures
Participants completed questionnaires assessing social anxiety and avoidance (Liebowitz Social Anxiety Scale self-report, LSAS-SR, Liebowitz, 1987), fear of social evaluation (Fear of Negative Evaluation questionnaire, FNE, Watson and Friend, 1969), and depressive symptoms (Beck Depression Inventory, BDI, Beck et al., 1961). As shown in Table 1, compared with healthy controls, participants with SAD reported greater social anxiety (LSAS-SR), fear of negative evaluation (FNE) and depressive symptoms (BDI).
2.3. Stimuli
A specialized stimulus set was created for the present study. As previously mentioned, we sought to create a set of facial displays varying both in emotion as well as social dominance. Because head tilt (a powerful social dominance cue, Mignault and Chaundhuri, 2003) is not included in commonly used facial database sets (e.g., NIMSTIM, KDEF) a new set of stimuli was compiled. The set consisted of photographs of 5 male and 5 female faces, each displaying 5 facial displays: neutral, angry, happy, arrogant (head tilted upward, direct eye gaze) and submissive (head tilted downward, eyes gazing down). Because hair is an obvious sign of gender, photographs were edited to remove hair in order to render the stimuli less discriminable (for a similar procedure see Goshen-Gottstein and Ganel, 2000). The stimuli subtended a visual angle of 19° × 21°. The visual complexity of the stimuli was assessed by 16 participants, who were requested to rate the complexity of each picture, using a 7-point scale (where 1 = very simple and 7 = very complex) (see Janssen et al., 2011). The mean visual complexity of the pictures was 3.59 (SD = 0.43).
The validity of these stimuli was examined in a pilot sample (n = 12). These raters were requested to indicate how angry, happy, arrogant, submissive and neutral each face appeared to be, from 1 (“not at all”) to 7 (“very much”). The stimuli included in the MEG study were only those for which the highest rated attribute was compatible with the expression presented, e.g., only angry faces which received the highest “angry” ratings (compared to their ratings on the other attributes — arrogant, happy, submissive or neutral). The group means for each type of facial expressions are presented in Table 2. As can be seen from the Table, displays in each category were highest on the appropriate dimension (shown in bold).
Table 2.
Means and standard deviations (in parentheses) of facial display ratings. The numbers in boldface signify the highest rated attribute for each facial expression.
| Display type | Angry | Happy | Dominant | Submissive | Neutral |
|---|---|---|---|---|---|
| Angry | 5.10 (0.77) | 1.18 (0.13) | 3.93 (0.78) | 1.52 (0.36) | 1.72 (0.29) |
| Dominant | 2.98 (0.82) | 1.61 (0.62) | 4.88 (0.46) | 1.69 (0.23) | 2.99 (0.57) |
| Happy | 1.04 (0.66) | 6.12 (0.69) | 3.44 (0.41) | 1.53 (0.27) | 1.62 (0.35) |
| Neutral | 2.36 (0.58) | 1.53 (0.71) | 3.03 (0.48) | 1.83 (0.36) | 4.99 (0.38) |
| Submissive | 1.53 (0.31) | 1.20 (0.13) | 1.80 (0.37) | 4.93 (0.31) | 2.83 (0.42) |
2.4. Gender categorization task
While in the MEG, participants categorized faces according to gender. Each trial began with fixation cross presented for 800 or 1200 ms. This interval was selected in order to allow sufficient time for brain activity to return to baseline between trials. On each trial, a face stimulus was presented for 1000 ms. During this time interval participants were prevented from responding in order to reduce motor artifacts. Consequently, a question mark (‘?’) appeared on the screen, and participants were requested to indicate the gender of the face. The participant's response terminated the trial. Each facial stimulus was presented 10 times, resulting in 500 trials. The trials were presented in random order. The stimuli were presented in 3 blocks, with 2 short resting periods between them.
2.5. Procedure
Before the MEG measurement, each participant's scalp surface was digitized using a 3D digitizer (Polhemus, 3Space/Fastrack, USA). Five coils were attached to the participant's scalp in order to record the head position relative to the MEG sensor. Head position was recorded prior and following the MEG session in order to rule out excessive movement. None of the participants exceeded the maximal movement allowed which was 0.4 cm. The experiment was run with the participants in supine position. A photosensitive diode on the screen recorded the exact onset time of visual stimuli.
After registration of the head position, instructions for the gender-categorization task were presented on the screen. Participants were requested to refrain, as much as possible, from moving their head and from blinking during the experiment. A response box was used to record manual responses. MEG was recorded continuously with a 0.1–600 Hz bandwidth at a sampling rate of 1017.25 Hz using a whole-head 248 magnetometer array (4-D Neuroimaging, Magnes 3600 WH).
2.6. MEG analyses
Data were analyzed offline, as follows:
2.6.1. Preprocessing
Power-line noise was removed using an extra channel which recorded the 50 Hz signal from the power outlet. Calculating the average 50 Hz cycle on every MEG channel allowed cleaning the power line noise without a notch-filter (Tal and Abeles, 2013).
2.6.2. Sensor level analyses
We first observed the data in the sensor level. Using Fieldtrip toolbox (Oostenveld et al., 2011) for Matlab (Mathworks, Natic, MA), data were segmented into epochs starting 150 ms before stimulus presentation and up to 850 ms following stimulus onset. Event-related fields (ERFs) were measured relative to a 150–0 ms pre-stimulus baseline period. The waveforms were then low-pass filtered with an offline cutoff of 50 Hz and the baseline was adjusted by subtracting the mean amplitude of the pre-stimulus period (150 ms) of each trial from all the data points in the segment. Jump and motor artifacts were removed using Fieldtrip toolbox algorithms. Spatial independent component analysis (ICA) was applied in order to clean the eye-movement and heartbeat artifacts. Trials containing additional artifacts were visually rejected (Monroe et al., 2013). Across all participants, the mean number of trials that were retained after clean-up was 484.5 (96.9% of the trials), with a standard deviation of 11.7 trials (2.3%). For each participant, the trials of each condition were time-locked and averaged to form five ERF waveforms, one for each facial expression.
In order to determine the times-of-interest for the whole sample, we averaged these ERF waveforms across participants. This produced an overall visualization of the time-course of activity which enabled us to pinpoint the time-windows of activation (the times-of-interest, see Results section).
2.6.3. Synthetic aperture modeling (SAM)
In order to estimate the neural sources of the activation patterns, we used SAM beamforming analysis (Robinson and Vrba, 1999). The weights for each voxel were derived from a traditional covariance matrix calculated for all trials with a window beginning 150 ms prior to stimulus onset and ending 850 ms after stimulus onset.
First, we created a source image of event-related phase-locked activity based on the averaged activity in virtual channels at each voxel location (SAMerf procedure, Robinson, 2004). Since SAMerf localizes the evoked response, which tends to be mainly low frequency, a frequency band of 1–40 Hz was used. A template anatomical scan was resized to the individual head shape of each participant, and then spatially normalized to the Talairach brain atlas (Talairach and Tournoux, 1998) using AFNI (Analysis of Functional NeuroImages, Cox, 1996). The following regions of interest (ROIs) were selected using labels from AFNI's atlas: fusiform gyrus, insula and DLPFC (Brodmann areas 9 and 46). Clusters containing at least 20 neighboring statistically significant (p < 0.05) voxels in the ROIs were identified and virtual sensors of the voxels with maximal activity in each cluster were entered to the analysis.
2.7. Statistical analysis
Grand averages of the SAM analysis were computed and a statistical comparison between the conditions was conducted using AFNI, in each of the chosen times-of-interest (detailed in the Results section). For each ROI and time-window in the hypotheses, we performed 2 × 5 repeated measures ANOVAs with group (SAD, HC) as a between-subject variable, and facial expression (angry, happy, arrogant, submissive, and neutral) as a within-subject variable. The significance threshold was set at 0.05, Greenhouse–Geisser correction for sphericity was applied to within-subject effects when needed and reported significance values reflect the correction. In order to assure the robustness of the analyses, a non-parametric permutation test was applied for each analysis. Each analysis was performed 1000 times after randomizing group and conditions, and the distribution of the F values was calculated yielding a critical value which was determined as the top 5% of the distribution. The F values of the original sample were compared to the critical values of the permutation tests. The advantage of this approach is that the distribution upon which statistical inference is based is estimated directly from the randomization, rather than depending on a priori assumptions. The same approach was applied for post-hoc tests investigating the source of the interaction effects. All of the reported results withstood the permutation tests.
In addition to the specific hypothesis-driven analyses, the rest of the ROIs and time-windows were tested in a similar manner to seek for other unexpected results, and are reported when appropriate.
3. Results
3.1. Behavioral and self-report data
The behavioral data of one participant was removed as he consistently confused the two response buttons (i.e., used the “Male” button as “Female” and vice versa). The SAD and HC groups did not significantly differ in accuracy or decision latency (accuracy: t(21) = 0.05, n.s.; RT: t(21) = –0.98, n.s.). In the SAD group, accuracy was 97.67% (SD = 0.02%) and the mean decision latency was 441 ms (SD = 108), and in the HC group accuracy was 97.71% (SD = 0.02%) and mean decision latency was 390 ms (SD = 138). Depression levels (assessed using the BDI) were uncorrelated with the participants' neural activation, and were therefore not included in further analyses.
3.2. Sensor level analyses
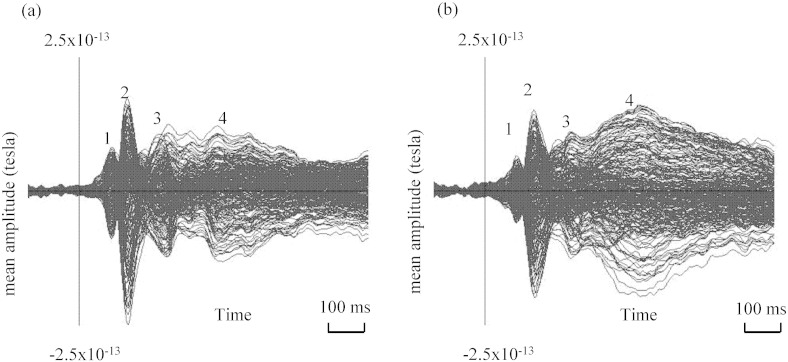
As shown in Fig. 1, sensor-level analyses revealed four main time-windows of activity (times-of-interest): (1) 70–130 ms, (2) 130–200 ms, which encompassed the M170 component, (3) 200–300 ms, and (4) 300–500 ms. These time-windows corresponded with previous research, which identified the early visual components at around 100 ms, the M170 component at around 150 ms, and late components from 200 onwards. Fig. 1a and b presents the grand-averaged MEG data, with all sensors overlaid, for the SAD and HC groups separately.
Fig. 1.
Grand-averaged MEG data for (a) HC group and (b) SAD group, with all sensors overlaid, showing the four times-of-interest in our study: (1) 70–130 ms, (2) 130–200 ms, (3) 200–300 ms, and (4) 300–500 ms.
3.3. The early (<200 ms) insular over-activation in SAD hypothesis
We found no differences between groups in the insula in the 70–130 ms time-window. In contrast to our prediction, in the 130–200 ms time window we found a main effect of Group, with the HC group showing stronger insular activation than the SAD group (F(1,22) = 7.79, p < 0.01, η2 = 0.17). This main effect was qualified by an Expression × Group interaction (F(2,21) = 3.72, p < 0.01, η2 = 0.11). Post-hoc analyses revealed that the HC group showed a significant effect of Expression in the insula (F(4,44) = 4.36, p < 0.01, η2 = 0.28), such that happy faces elicited stronger activation as compared to angry (t(11) = 2.99, p < 0.01), submissive (t(11) = 2.85, p < 0.05) and neutral faces (t(11) = 2.91, p < 0.01); and arrogant faces elicited stronger activation compared to neutral ones (t(11) = 3.85, p < 0.01). In the SAD group, however, there was no significant difference in activation to the various facial displays (F(4, 44) = 0.36, p = 0.82).
3.4. The early (<200 ms) FG over-activation in SAD hypothesis
We found no differences between groups in the FG in the 70–130 ms time-window. Again, in contrast to our prediction, in the right FG around 130–200 ms, the HC group showed stronger M170 than the SAD group (main effect of Group, F(1,22) = 8.88, p < 0.01, η2 = 0.13). An Expression × Group interaction (F(2,21) = 3.78, p < 0.01, η2 = 0.14) revealed that HCs showed greater M170 than did individuals with SAD for angry and happy faces, t(22) = 2.31, p < 0.05, t(22) = 2.11, p < 0.05, respectively; but not for arrogant, submissive or neutral faces (all p′s > 0.05).
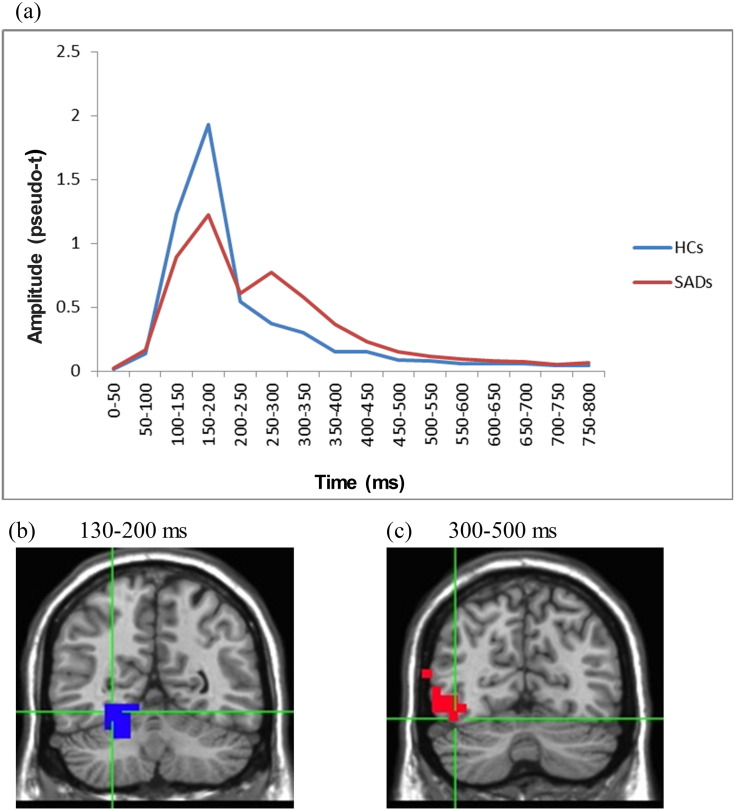
Interestingly, post-hoc analyses showed that in the later time-window of 200–300 ms, this pattern was reversed, with the SAD group showing stronger right FG activation compared to the HC group (main effect of Group, F(1,22) = 6.56, p < 0.05, η2 = 0.25). As can be seen in Fig. 2, this pattern continued in the next time-window (300–500 ms) as well (main effect of Group, F(1,22) = 8.67, p < 0.01, η2 = 0.24).
Fig. 2.
Different patterns of activation in the right FG for the SAD and HC groups (averaged across facial expressions). a: Time-course of activation for the two groups. For reasons of clarity, this graphic presentation shows a continuous time-line, whereas the statistical analyses were performed on distinct time-windows. b and c: The SAD vs. HC contrast at the 130–200 ms and 300–500 ms time-windows in neighboring right FG areas. Red color depicts areas with significantly greater activity for SAD, blue color depicts areas with significantly less activity for SAD (p < 0.05).
3.5. The DLPFC late (>200 ms) over-activation in SA hypothesis
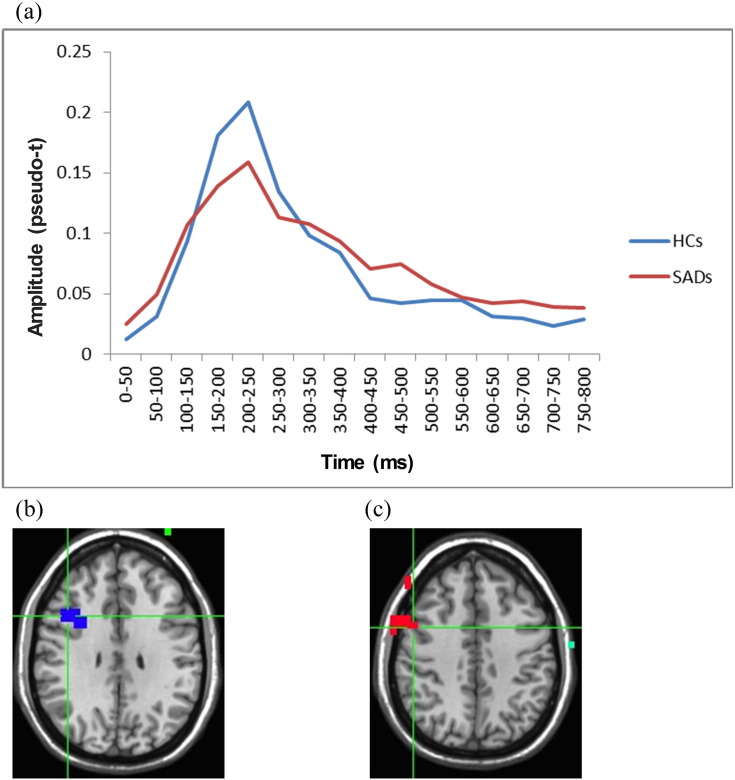
In the right DLPFC, in the 200–300 ms time-window, we found that the HC group showed greater activation than the SAD group (effect of Group, F(1,22) = 12.83, p < 0.01, η2 = 0.21), as can be seen from Fig. 3. In the 300–500 ms time-window, however, the pattern was reversed and individuals with SAD showed greater activity compared to HCs (effect of Group, F(1,22) = 11.85, p < 0.01, η2 = 0.22).
Fig. 3.
Different patterns of activation in the right DLPFC for the SAD and HC groups (averaged across facial expressions). a: Time-course of activation for the two groups. For reasons of clarity, this graphic presentation shows a continuous time-line, whereas the statistical analyses were performed on distinct time-windows. b and c: The SAD vs. HC contrast at the 200–300 ms and 300–500 ms time-windows, respectively. Red color depicts areas with significantly greater activity for SAD, blue color depicts areas with significantly less activity for SAD (p < 0.05).
4. Discussion
Using the high temporal resolution of MEG, we examined the neural activation during the processing of facial displays varying in emotion and in social dominance, for individuals with SAD and for HCs. The pattern of neural activation during face processing in individuals with SAD versus HCs, was characterized by an initial under-activation, reflected in a decreased right FG (M170), insula and right DLPFC activation; followed by a later over-activation in the right FG (200–500 ms) and the right DLPFC (from 300 ms onwards).
4.1. The early (<200 ms) processing of facial displays
In contrast to our predictions, we found a reduced M170 (right FG activation in the 130–200 ms time window), as well as lower insular and DLPFC activation, in the SAD compared to the HC group. These results dovetail the findings of studies indicating an attenuation of early neural components during face processing of individuals with high, versus low, levels of trait anxiety (Frenkel and Bar-Haim, 2011; Walentowska and Wronka, 2012). Furthermore, the reduced M170 in individuals with SAD in our study supports Mueller et al.'s (2009) finding of a reduced N170 in socially anxious individuals. Existing fMRI findings regarding the role of the FG in face processing in SA have been inconsistent. Some fMRI studies found an under-activation of the FG in individuals with SAD (Gentili et al., 2008) and in shy people (Beaton et al., 2009; Beaton et al., 2010). Pujol et al. (2009), for example, found that the FG activity was negatively correlated with social anxiety severity, particularly for fearful faces. Other fMRI studies, however, found an over-activation of the FG in SAD (Straube et al., 2004; Straube et al., 2005). The discrepancy between these findings could be a result of fMRI's relatively low-temporal resolution. If the right FG indeed shows a complex pattern of initial under-activation and a later over-activation (as found in our study), paradigms which are not temporally sensitive could yield contrasting results. Thus, in fMRI acquisition the results of a dynamic process would be averaged to a single mean value. Clearly, temporal resolution is essential to examine the temporal unfolding of such processes.
An interesting perspective on our results regarding the FG early under-activation in SAD comes from studies of the different uses of low spatial frequency (LSF) and high spatial frequency (HSF) information. LSF information is quickly processed and is important for decoding emotional expressions (Langner et al., 2009); while HSF processing is slower and extracts finer details important for precise recognition of identity, age, etc. (Alorda et al., 2007; Winston et al., 2003). Langner et al. (2009) found that whereas both individuals with high and low SA used HSF information for judging facial emotion, they differed in the use of LSF information: participants with high SA relied more heavily on LSF information in their judgments, compared to low SA participants. In general, both the amygdala and the P1 component are preferentially responsive to LSF rather than to HSF facial information (Vuilleumier et al., 2003; Winston et al., 2003). In contrast, the FG activity and the N170 component are greater with HSF faces than LSF (Nakashima et al., 2008; Vuilleumier et al., 2003). We suggest that the reduced M170 found in our study may be related to differential use of LSF information in SADs. It is possible that in the early stages of processing (in which LSF is usually the main focus (Vlamings et al., 2009)), individuals with high SA put more emphasis on LSF information (connected to amygdala activation), and less emphasis on HSF information (connected to fusiform activation), resulting in a reduced M170. From an evolutionary perspective, as SA is hypothesized to act as an early warning system for potential attacks from dominant group members (Trower et al., 1990), it seems adaptive for socially anxious individuals to focus their early processing resources on LSF information, which is crucial for quick decoding of EFEs (Alorda et al., 2007; Winston et al., 2003).
In addition, the reduced M170 found in the SAD group in our study, could be linked to the early DLPFC under-activation in this group. The DLPFC has shown an early involvement in bottom-up visual processes, as early as 125 ms post-stimulus (Katsuki and Constantinidis, 2012).
In the insula region we found that the HC group showed stronger activation (compared to the SAD group), and further analyses showed that the HC group showed insular sensitivity to the type of facial expression displayed, whereas the SAD group did not. A study about the effects of trait anxiety on face processing produced somewhat parallel results. Frenkel and Bar-Haim (2011) found that in a non-anxious group, the Late Positive Potential (LPP) amplitude increased as a function of fear intensity conveyed by the facial stimuli, whereas in the anxious group the LPP amplitude was not significantly modulated by stimulus fear intensity. The authors suggested that this anxiety-related insensitivity of the LPP might compromise threat evaluation in anxious individuals, making them more prone to ongoing fear responses in the face of very subtle threat cues.
Combined, our results did not support the early over-activation hypotheses (in the FG or the insula). However, deeper brain areas, such as the amygdala, could not be adequately imaged with our analyses. It is possible that an enhanced sensitivity of SADs to threat, as part of the limbic quick pathway of EFE processing, could be discovered using other imaging techniques and analyses.
4.2. The late (>200 ms) processing of facial displays
A late over-activation of both the right FG (>200 ms) and the right DLPFC (>300 ms) was found in SAD as compared to HCs. This pattern may indicate a late recruitment of the FG in order to compensate for the initial under-activation. Importantly, the DLPFC late over-activation is implicated in attentional control in the processing of EFEs, and with the ability to disengage from angry faces (De Raedt et al., 2010) and from fear related stimuli (Fales et al., 2008). Thus, the DLPFC late over-activation observed in our study is consistent with behavioral findings of disengagement difficulties from threatening cues in SAD (Amir et al., 2003). Our results suggest that over-activation of the DLPFC may underlie the failure to control attention during EFE processing in SAD.
The unusual EFE processing pattern in SAD, may be linked to the purported brain structure and connectivity abnormalities identified in SAD (Bruhl et al., 2014). As prefrontal areas have been shown to modulate limbic activity during emotion regulation (Banks et al., 2007), the abnormal connectivity (or de-coupling, as Bruhl et al. (2014) suggest) may underlie a reduced frontal regulation of limbic activity in SAD. Klumpp et al. (2012) showed that the insular hyper-reactivity for fearful faces in individuals with SAD, compared to controls, involves reduced connectivity with a prefrontal region implicated in cognitive control and emotion regulation. It is possible that such de-coupling between frontal and limbic areas in SAD results in early reduced activation (including a reduced M170), followed by a compensatory frontal over-activation.
4.3. Therapeutic and diagnostic implications
A specific temporal pattern of facial display processing in SAD was identified in our study. Other studies also reveal face-emotion processing impairments which are characteristic of different disorders (Bediou et al., 2012; Isaac and Lincoln, 2011; Pierce et al., 2001). Recent research suggests the possibility of employing specific neural patterns of EFE processing in various disorders as potential biomarkers and diagnostic tools (Isaac, 2012). Biomarkers may help to improve diagnosis and prognosis and aid the development of personalized treatments and evaluation of treatment outcomes (Walsh et al., 2011). In addition, our study revealed a frontal over-activation in SAD which suggests a difficulty in inhibiting the processing of faces, i.e., problems in cognitive control. These findings support the recent promising results from cognitive bias modification paradigms, which aim to train and modify selective attention through practice on a computerized task (MacLeod and Mathews, 2012). Such training programs have shown effectiveness in reducing SA symptoms (Amir et al., 2009), and this reduction was accompanied by facilitated attention disengagement from threat.
4.4. Limitations
In closing, some limitations of our study should be noted. First, our sample included a modest number of participants. Although comparable to other neuroimaging studies of clinical populations (e.g., Blair et al., 2011; Gentili et al., 2008; Hahn et al., 2011), this sample size limits chances to identify existing differences between the SAD and the HC groups. In addition, our study included mostly male participants. As gender may have an effect on social cues processing in general and in SAD patients specifically (see Arrais et al., 2010), we believe a replication of our findings using a more balanced gender distribution will provide informative data.
Second, the current study used a gender-categorization task in which the emotion dimension was task-irrelevant. Imaging studies show differential neural responses when attention is explicitly directed to facial expressions (e.g., in an emotion categorization task), compared to when emotion processing is task-relevant (Fusar-Poli et al., 2009; Monroe et al., 2013). Some studies (e.g., Lichtenstein-Vidne et al., 2012) suggest that emotional stimuli do not capture attention in an unconditional manner, and they interfere with other cognitive processes only when they are relevant to the task. Future studies may examine the ways in which task-relevance of the emotional information affects facial processing in non-clinical and clinical populations.
Third, the MEG preparation and scan process include physical contact between the experimenter and the participant, and closed-circle camera monitoring of the participant. These conditions may be more stressful for the socially anxious than for controls, and could affect their performance and neural activity. Future studies may benefit from measuring state anxiety before and after the MEG procedure, as well as from the inclusion of a non-socially anxious clinical control group. Inclusion of other clinical study groups could also add to our understanding of the specificity of face processing patterns in social anxiety.
Fourth, based on the fMRI literature we used ROI-based analysis. Clearly, MEG data offer rich possibilities for additional data analyses using whole brain recording and bottom-up techniques. Future research may profitably explore these advantages.
Fifth, in the present study, neutral faces served as baseline stimuli. However, socially anxious individuals may judge neutral faces as threatening (Birbaumer et al., 1998). Future studies may use a more “neutral” baseline, such as inverted or scrambled faces. Finally, our facial stimuli consisted of five different expressions, out of which only one presented an averted gaze (submissive faces). Gaze direction is an important social cue which modulates ERPs to faces at very early stages (100 ms after stimulus) (Itier et al., 2007). The role of gaze processing in SA is still unclear as only few studies explored the neural correlates of gaze processing in this disorder (Schmitz et al., 2012; Schneier et al., 2009). Using other facial stimuli with another type of gaze aversion (e.g., sideways) could further the understanding of face and gaze processing in SA.
4.5. Conclusions
The current study offers a first examination of the temporal unfolding of face processing in SAD using MEG. Individuals with SAD exhibited an early under-activation of cortical areas involved in structural encoding of faces. This pattern could be related to greater emphasis in SA in early processing stages on LSF information (connected to amygdala activation), and less emphasis on HSF information (connected to fusiform activation). We also found that participants with SAD exhibited a late frontal over-activation, in areas which appear to be involved in cognitive control. These results rhyme with behavioral findings which implicate attentional deficits in disengaging from negative information as the key mechanism for impairments in the regulation of emotion (Joormann and D'Avanzato, 2010). This dynamic pattern of activation highlights the importance of adopting a temporal perspective in understanding individual differences in the processing of facial expressions.
Conflicts of interest
None.
Funding
This work was supported by the Israel Foundation Trustees Program for the Advancement of Research in the Social Sciences grant number 29, awarded to Sharon Riwkes and by the Israeli Science Foundation Grant number 455-2010 awarded to Eva Gilboa-Schechtman.
Acknowledgments
The authors would like to thank Yuval Harpaz, PhD, from the Gonda Multidisciplinary Brain Research Center at Bar-Ilan University, for his help with the acquisition and analysis of the MEG data; and to Prof. David Cohen for his generous advice and assistance with the recording setup.
Contributor Information
Sharon Riwkes, Email: sharonriwkes@gmail.com.
Abraham Goldstein, Email: goldsa@mail.biu.ac.il.
Eva Gilboa-Schechtman, Email: gilboae@mail.biu.ac.il.
References
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behav. Cogn. Neurosci. Rev. 2002;1(1):21–62. doi: 10.1177/1534582302001001003. 17715585 [DOI] [PubMed] [Google Scholar]
- Alorda C., Serrano-Pedraza I., Campos-Bueno J.J., Sierra-Vázquez V., Montoya P. Low spatial frequency filtering modulates early brain processing of affective complex pictures. Neuropsychologia. 2007;45(14):3223–3233. doi: 10.1016/j.neuropsychologia.2007.06.017. 17681356 [DOI] [PubMed] [Google Scholar]
- American-Psychiatric-Association . Diagnostic and Statistical Manual of Mental Disorders. fourth edition. Author; Washington, DC: 1994. [Google Scholar]
- Amir N., Beard C., Taylor C.T., Klumpp H., Elias J., Burns M., Chen X. Attention training in individuals with generalized social phobia: a randomized controlled trial. J. Consult. Clin. Psychol. 2009;77(5):961–973. doi: 10.1037/a0016685. 19803575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N., Elias J., Klumpp H., Przeworski A. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behav. Res. Ther. 2003;41(11):1325–1335. doi: 10.1016/s0005-7967(03)00039-1. 14527531 [DOI] [PubMed] [Google Scholar]
- Anderson A.K., Christoff K., Panitz D., De Rosa E., Gabrieli J.D. Neural correlates of the automatic processing of threat facial signals. J. Neurosci. 2003;23(13):5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. 12843265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrais K., Machado-de-Sousa J., Trzesniak C., Filho A., Ferrari M., Osório F. Social anxiety disorder women easily recognize fearfull, sad and happy faces: The influence of gender. J. Psychiatry Res. 2010;44(8):535–540. doi: 10.1016/j.jpsychires.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. 18985136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V., Brühl A.B., Herwig U., Eberle T., Rufer M., Delsignore A., Hanggi J. Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: a quantitative fiber tractography study. Hum. Brain Mapp. 2013;34(2):437–446. doi: 10.1002/hbm.21447. 22076860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle D.J., Taylor M.J. Attention inhibition of early cortical activation to fearful faces. Brain Res. 2010;1313:113–123. doi: 10.1016/j.brainres.2009.11.060. 20004181 [DOI] [PubMed] [Google Scholar]
- Beaton E.A., Schmidt L.A., Schulkin J., Antony M.M., Swinson R.P., Hall G.B. Different fusiform activity to stranger and personally familiar faces in shy and social adults. Soc. Neurosci. 2009;4(4):308–316. doi: 10.1080/17470910902801021. 19322727 [DOI] [PubMed] [Google Scholar]
- Beaton E.A., Schmidt L.A., Schulkin J., Hall G.B. Neural correlates of implicit processing of facial emotions in shy adults. Pers. Individ. Dif. 2010;49(7):755–761. [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J.E., Erbaugh J.K. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. 13688369 [DOI] [PubMed] [Google Scholar]
- Bediou B., Brunelin J., d'Amato T., Fecteau S., Saoud M., Hénaff M.A., Krolak-Salmon P. A comparison of facial emotion processing in neurological and psychiatric conditions. Front. Psychol. 2012;3:98. doi: 10.3389/fpsyg.2012.00098. 22493587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S., Allison T., Puce A., Perez E., McCarthy G. Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. 20740065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N., Grodd W., Diedrich O., Klose U., Erb M., Lotze M., Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9(6):1223–1226. doi: 10.1097/00001756-199804200-00048. 9601698 [DOI] [PubMed] [Google Scholar]
- Blair K.S., Geraci M., Otero M., Majestic C., Odenheimer S., Jacobs M., Blair R.J., Pine D.S. Atypical modulation of medial prefrontal cortex to self-referential comments in generalized social phobia. Psychiatry Res. 2011;193(1):38–45. doi: 10.1016/j.pscychresns.2010.12.016. 21601433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl A.B., Delsignore A., Komossa K., Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev. 2014;47C:260–280. doi: 10.1016/j.neubiorev.2014.08.003. 25124509 [DOI] [PubMed] [Google Scholar]
- Buckner J.D., Maner J.K., Schmidt N.B. Difficulty disengaging attention from social threat in social anxiety. Cognit. Ther. Res. 2010;34(1):99–105. doi: 10.1007/s10608-008-9205-y. 20182655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder A.J., Lawrence A.D., Young A.W. Neuropsychology of fear and loathing. Nat. Rev. Neurosci. 2001;2(5):352–363. doi: 10.1038/35072584. 11331919 [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Dammers J., Boers F., Leiberg S., Edgar J.C., Roberts T.P., Mathiak K. The temporal dynamics of insula activity to disgust and happy facial expressions: a magnetoencephalography study. Neuroimage. 2009;47(4):1921–1928. doi: 10.1016/j.neuroimage.2009.04.093. 19442746 [DOI] [PubMed] [Google Scholar]
- Cooney R.E., Atlas L.Y., Joormann J., Eugène F., Gotlib I.H. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res. 2006;148(1):55–59. doi: 10.1016/j.pscychresns.2006.05.003. 17030117 [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. 8812068 [DOI] [PubMed] [Google Scholar]
- Davidson R.J. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol. Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. 11801232 [DOI] [PubMed] [Google Scholar]
- De Raedt R., Leyman L., Baeken C., Van Schuerbeek P., Luypaert R., Vanderhasselt M.A., Dannlowski U. Neurocognitive effects of HF-rTMS over the dorsolateral prefrontal cortex on the attentional processing of emotional information in healthy women: an event-related fMRI study. Biol. Psychol. 2010;85(3):487–495. doi: 10.1016/j.biopsycho.2010.09.015. 20923694 [DOI] [PubMed] [Google Scholar]
- Eimer M. The face-specific N170 component reflects late stages in the structural encoding of faces. Neuroreport. 2000;11(10):2319–2324. doi: 10.1097/00001756-200007140-00050. 10923693 [DOI] [PubMed] [Google Scholar]
- Evans K.C., Wright C.I., Wedig M.M., Gold A.L., Pollack M.H., Rauch S.L. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress. Anxiety. 2008;25(6):496–505. doi: 10.1002/da.20347. 17595018 [DOI] [PubMed] [Google Scholar]
- Fales C.L., Barch D.M., Rundle M.M., Mintun M.A., Snyder A.Z., Cohen J.D., Sheline Y.I. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol. Psychiatry. 2008;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. 17719567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Structured Clinical Interview for DSM-IVAxis I Disorders. patient edition. Biometric Research Department; New York, USA: 1995. [Google Scholar]
- Frenkel T.I., Bar-Haim Y. Neural activation during the processing of ambiguous fearful facial expressions: an ERP study in anxious and nonanxious individuals. Biol. Psychol. 2011;88(2–3):188–195. doi: 10.1016/j.biopsycho.2011.08.001. 21846487 [DOI] [PubMed] [Google Scholar]
- Furmark T. Social phobia: overview of community surveys. Acta Psychiatr. Scand. 2002;105(2):84–93. doi: 10.1034/j.1600-0447.2002.1r103.x. 11939957 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S., Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009;34(6):418–432. 19949718 [PMC free article] [PubMed] [Google Scholar]
- Gamble A.L., Rapee R.M. The time-course of attention to emotional faces in social phobia. J. Behav. Ther. Exp. Psychiatry. 2010;41(1):39–44. doi: 10.1016/j.jbtep.2009.08.008. 19781689 [DOI] [PubMed] [Google Scholar]
- Garvert M.M., Friston K.J., Dolan R.J., Garrido M.I. 102P2. 2014. Subcortical Amygdala Pathways Enable Rapid Face Processing; pp. 309–316.25108179 (Neuroimage). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C., Gobbini M.I., Ricciardi E., Vanello N., Pietrini P., Haxby J.V., Guazzelli M. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with social phobia and healthy subjects. Brain Res. Bull. 2008;77:286–292. doi: 10.1016/j.brainresbull.2008.08.003. 18771714 [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E., Ben Artzi E., Jeczemien P., Marom S., Hermesh H. Depression impairs the ability to ignore the emotional aspects of facial expressions: evidence from the Garner task. Cogn. Emot. 2004;18(2):209–231. doi: 10.1080/02699930341000176a. [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E., Shachar-Lavie I. More than a face: a unified theoretical perspective on nonverbal social cues in social anxiety. Front. Hum. Neurosci. 2013;7:904. doi: 10.3389/fnhum.2013.00904. 24427129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen-Gottstein Y., Ganel T. Repetition priming for familiar and unfamiliar faces in a sex-judgment task: evidence for a common route for the processing of sex and identity. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26(5):1198. doi: 10.1037//0278-7393.26.5.1198. [DOI] [PubMed] [Google Scholar]
- Grant D.M., Beck J.G. Attentional biases in social anxiety and dysphoria: does comorbidity make a difference? J. Anxiety Disord. 2006;20(4):520–529. doi: 10.1016/j.janxdis.2005.05.003. 16023323 [DOI] [PubMed] [Google Scholar]
- Hahn A., Stein P., Windischberger C., Weissenbacher A., Spindelegger C., Moser E., Kasper S., Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–889. doi: 10.1016/j.neuroimage.2011.02.064. 21356318 [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends Cogn. Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. 10827445 [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. Human neural systems for face recognition and social communication. Biol. Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. 11801231 [DOI] [PubMed] [Google Scholar]
- Isaac L. Facing the future: face-emotion processing deficits as a potential biomarker for various psychiatric and neurological disorders. Front. Psychol. 2012;3:171. doi: 10.3389/fpsyg.2012.00171. 22701441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac L., Lincoln A. Featural versus configural face processing in a rare genetic disorder: Williams syndrome. J. Intellect. Disabil. Res. 2011;55(11):1034–1042. doi: 10.1111/j.1365-2788.2011.01426.x. 21554469 [DOI] [PubMed] [Google Scholar]
- Itier R.J., Alain C., Kovacevic N., McIntosh A.R. Explicit versus implicit gaze processing assessed by ERPs. Brain Res. 2007;1177:79–89. doi: 10.1016/j.brainres.2007.07.094. 17916340 [DOI] [PubMed] [Google Scholar]
- Janssen N., Pajtas P., Caramazza A. A set of 150 pictures with morphologically complex English compound names: norms for name agreement, familiarity, image agreement, and visual complexity. Behav. Res. 2011;43(2):478–490. doi: 10.3758/s13428-011-0065-0. [DOI] [PubMed] [Google Scholar]
- Joormann J., D'Avanzato C. Emotion regulation in depression: examining the role of cognitive processes. Cogn. Emot. 2010;24(6):913–939. 20300538 [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. 9151747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N., Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2006;361(1476):2109–2128. doi: 10.1098/rstb.2006.1934. 17118927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F., Constantinidis C. Early involvement of prefrontal cortex in visual bottom-up attention. Nat. Neurosci. 2012;15(8):1160–1166. doi: 10.1038/nn.3164. 22820465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H., Angstadt M., Phan K.L. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol. Psychol. 2012;89(1):273–276. doi: 10.1016/j.biopsycho.2011.10.010. 22027088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I.T. Interpretive bias in social phobia: an ERP study with morphed emotional schematic faces. Cognition & Emotion. 2009;23(1):69–95. [Google Scholar]
- Kolassa I.T., Kolassa S., Musial F., Miltner W.H.R. Event-related potentials to schematic faces in social phobia. Cognition & Emotion. 2007;21(8):1721–1744. [Google Scholar]
- Kolassa I.T., Miltner W.H.R. Psychophysiological correlates of face processing in social phobia. Brain Res. 2006;1118(1):130–141. doi: 10.1016/j.brainres.2006.08.019. 16970928 [DOI] [PubMed] [Google Scholar]
- Langner O., Becker E.S., Rinck M. Social anxiety and anger identification: bubbles reveal differential use of facial information with low spatial frequencies. Psychol. Sci. 2009;20(6):666–670. doi: 10.1111/j.1467-9280.2009.02357.x. 19422621 [DOI] [PubMed] [Google Scholar]
- Lichtenstein-Vidne L., Henik A., Safadi Z. Task relevance modulates processing of distracting emotional stimuli. Cogn. Emot. 2012;26(1):42–52. doi: 10.1080/02699931.2011.567055. 21598126 [DOI] [PubMed] [Google Scholar]
- Liebowitz M.R. Social phobia. In: Ban T.A., Pichot P., Poldinger W., editors. Modern Problems of Pharmacopsychiatry. Karger; Basel: 1987. [Google Scholar]
- Liu J., Harris A., Kanwisher N. Stages of processing in face perception: an MEG study. Nat. Neurosci. 2002;5(9):910–916. doi: 10.1038/nn909. 12195430 [DOI] [PubMed] [Google Scholar]
- Luo Q., Holroyd T., Jones M., Hendler T., Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34(2):839–847. doi: 10.1016/j.neuroimage.2006.09.023. 17095252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C., Mathews A. Cognitive bias modification approaches to anxiety. Annu. Rev. Clin. Psychol. 2012;8:189–217. doi: 10.1146/annurev-clinpsy-032511-143052. 22035241 [DOI] [PubMed] [Google Scholar]
- Mattia J.I., Heimberg R.G., Hope D.A. The revised Stroop color-naming task in social phobics. Behav. Res. Ther. 1993;31(3):305–313. doi: 10.1016/0005-7967(93)90029-t. 8476405 [DOI] [PubMed] [Google Scholar]
- Mignault A., Chaundhuri A. The many faces of a neutral face: head tilt and perception of dominance and emotion. J. Nonverbal Behav. 2003;27(2):111–132. [Google Scholar]
- Monroe J.F., Griffin M., Pinkham A., Loughead J., Gur R.C., Roberts T.P., Christopher Edgar J. The fusiform response to faces: explicit versus implicit processing of emotion. Hum. Brain Mapp. 2013;34(1):1–11. doi: 10.1002/hbm.21406. 21932258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E.M., Hofmann S.G., Santesso D.L., Meuret A.E., Bitran S., Pizzagalli D.A. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychol. Med. 2009;39(7):1141–1152. doi: 10.1017/S0033291708004820. 19079826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlberger A., Wieser M.J., Herrmann M.J., Weyers P., Tröger C., Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. J. Neural Transm. 2009;116(6):735–746. doi: 10.1007/s00702-008-0108-6. 18784899 [DOI] [PubMed] [Google Scholar]
- Nakashima T., Goto Y., Abe T., Kaneko K., Saito T., Makinouchi A., Tobimatsu S. Electrophysiological evidence for sequential discrimination of positive and negative facial expressions. Clin. Neurophysiol. 2008;119(8):1803–1811. doi: 10.1016/j.clinph.2008.04.014. 18538630 [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. 15866151 [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. 21253357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K., Müller R.A., Ambrose J., Allen G., Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124(10):2059–2073. doi: 10.1093/brain/124.10.2059. 11571222 [DOI] [PubMed] [Google Scholar]
- Pujol J., Harrison B.J., Ortiz H., Deus J., Soriano-Mas C., López-Solà M., Cardoner N. Influence of the fusiform gyrus on amygdala response to emotional faces in the non-clinical range of social anxiety. Psychol. Med. 2009;39(7):1177–1187. doi: 10.1017/S003329170800500X. 19154647 [DOI] [PubMed] [Google Scholar]
- Robinson S.E. Localization of event-related activity by SAM(erf) Neurol Clin Neurophysiol. 2004;2004:109. 16012649 [PubMed] [Google Scholar]
- Robinson S.E., Vrba J. Functional neuroimaging by synthetic aperture magnetometry (SAM) In: Yoshimoto K.M., [!(%xInRef|ce:surname)!] T., Kuriki S., Karibe H., Nakasoto N., editors. Recent Advances in Biomagnetism. Tohoku University; Senai: 1999. [Google Scholar]
- Said C.P., Haxby J.V., Todorov A. Brain systems for assessing the affective value of faces. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2011;366(1571):1660–1670. doi: 10.1098/rstb.2010.0351. 21536552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A., Stark R., Walter B., Blecker C., Ott U., Kirsch P., Vaitl D. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. 2002;13(16):2023–2026. doi: 10.1097/00001756-200211150-00006. 12438918 [DOI] [PubMed] [Google Scholar]
- Schmitz J., Scheel C.N., Rigon A., Gross J.J., Blechert J. You don't like me, do you? Enhanced ERP responses to averted eye gaze in social anxiety. Biol. Psychol. 2012;91(2):263–269. doi: 10.1016/j.biopsycho.2012.07.004. 22820039 [DOI] [PubMed] [Google Scholar]
- Schneier F.R., Kent J.M., Star A., Hirsch J. Neural circuitry of submissive behavior in social anxiety disorder: a preliminary study of response to direct eye gaze. Psychiatry Res. 2009;173(3):248–250. doi: 10.1016/j.pscychresns.2008.06.004. 19628377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield C.A., Johnson A.L., Inhoff A.W., Coles M.E. Social anxiety and difficulty disengaging threat: evidence from eye-tracking. Cogn Emot. 2012;26(2):300–311. doi: 10.1080/02699931.2011.602050. 21970428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G.J., Thompson W., Carter C.S., Steinhauer S.R., Thase M.E. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol. Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. 17027931 [DOI] [PubMed] [Google Scholar]
- Stein M.B., Goldin P.R., Sareen J., Zorrilla L.T., Brown G.G. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch. Gen. Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. 12418936 [DOI] [PubMed] [Google Scholar]
- Straube T., Kolassa I.T., Glauer M., Mentzel H.J., Miltner W.H. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol. Psychiatry. 2004;56(12):921–930. doi: 10.1016/j.biopsych.2004.09.024. 15601601 [DOI] [PubMed] [Google Scholar]
- Straube T., Mentzel H.J., Miltner W.H. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52(3):163–168. doi: 10.1159/000087987. 16137995 [DOI] [PubMed] [Google Scholar]
- Tal I., Abeles M. Cleaning MEG artifacts using external cues. J. Neurosci. Methods. 2013;217(1–2):31–38. doi: 10.1016/j.jneumeth.2013.04.002. 23583420 [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical; New York, NY.: 1998. [Google Scholar]
- Taylor M.J., Bayless S.J., Mills T., Pang E.W. Recognising upright and inverted faces: MEG source localisation. Brain Res. 2011;1381:167–174. doi: 10.1016/j.brainres.2010.12.083. 21238433 [DOI] [PubMed] [Google Scholar]
- Trower P., Gilbert P., Sherling G. Social anxiety, evolution, and self-presentation: an interdisciplinary perspective. In: Leitenberg H., editor. Handbook of Social and Evaluation Anxiety. Plenum Press; New York and London: 1990. pp. 11–45. [Google Scholar]
- Vanderhasselt M.A., Kühn S., De Raedt R. Healthy brooders employ more attentional resources when disengaging from the negative: an event-related fMRI study. Cogn. Affect. Behav. Neurosci. 2011;11(2):207–216. doi: 10.3758/s13415-011-0022-5. 21373973 [DOI] [PubMed] [Google Scholar]
- Vlamings P.H., Goffaux V., Kemner C. Is the early modulation of brain activity by fearful facial expressions primarily mediated by coarse low spatial frequency information? J. Vis. 2009;9(5):12.1–13. doi: 10.1167/9.5.12. 19757890 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Clarke K., Husain M., Driver J., Dolan R.J. Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia. 2002;40(12):2156–2166. doi: 10.1016/s0028-3932(02)00045-3. 12208011 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat. Neurosci. 2003;6(6):624–631. doi: 10.1038/nn1057. 12740580 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45(1):174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. 16854439 [DOI] [PubMed] [Google Scholar]
- Walentowska W., Wronka E. Trait anxiety and involuntary processing of facial emotions. Int. J. Psychophysiol. 2012;85(1):27–36. doi: 10.1016/j.ijpsycho.2011.12.004. 22210124 [DOI] [PubMed] [Google Scholar]
- Walsh P., Elsabbagh M., Bolton P., Singh I. In search of biomarkers for autism: scientific, social and ethical challenges. Nat. Rev. Neurosci. 2011;12(10):603–612. doi: 10.1038/nrn3113. 21931335 [DOI] [PubMed] [Google Scholar]
- Watson D., Friend R. Measurement of social-evaluative anxiety. J. Consult. Clin. Psychol. 1969;33(4):448–457. doi: 10.1037/h0027806. 5810590 [DOI] [PubMed] [Google Scholar]
- Wieser M.J., Pauli P., Weyers P., Alpers G.W., Mühlberger A. Fear of negative evaluation and the hypervigilance-avoidance hypothesis: an eye-tracking study. J. Neural Transm. 2009;116(6):717–723. doi: 10.1007/s00702-008-0101-0. 18690409 [DOI] [PubMed] [Google Scholar]
- Williams M.A., Morris A.P., McGlone F., Abbott D.F., Mattingley J.B. Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. J. Neurosci. 2004;24(12):2898–2904. doi: 10.1523/JNEUROSCI.4977-03.2004. 15044528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J.S., Vuilleumier P., Dolan R.J. Effects of low-spatial frequency components of fearful faces on fusiform cortex activity. Curr. Biol. 2003;13(20):1824–1829. doi: 10.1016/j.cub.2003.09.038. 14561410 [DOI] [PubMed] [Google Scholar]