Abstract
Orosensory perception of dietary fat varies in individuals, thus influencing nutritional status. Several studies associated fat detection and preference with CD36 or 6-n-propylthiouracil (PROP) sensitivity. Other studies have not confirmed the latter association. We analyzed the relationship between orosensory perception of oleic acid, two CD36 variants, and PROP tasting. Thresholds of oleic acid perception were assessed in 64 subjects using a modification of the three-alternative forced-choice procedure. Subjects were classified for PROP taster status and genotyped for TAS2R38 and CD36 (SNPs: rs1761667 and rs1527483). Subjects homozygous for GG of the rs1761667 polymorphism showed higher sensitivity to oleic acid than AA subjects. The capability to detect oleic acid was directly associated with TAS2R38 or PROP responsiveness. PROP non-tasters had a lower papilla density than tasters, and those with genotype GG of the rs1761667 polymorphism had lower oleic acid thresholds than PROP non-tasters with genotype AA. In conclusion, results showed a direct association between orosensory perception of oleic acid and PROP tasting or rs1761667 polymorphism of CD36, which play a significant role in PROP non-tasters, given their low number of taste papillae. Characterization of individual capability to detect fatty acids may have important nutritional implications by explaining variations in human fat preferences.
Keywords: orosensory perception of oleic acid, CD36, PROP genotype and phenotype
1. Introduction
Over the last decade, evidence has been presented of the multiple roles of dietary fatty acids as regulators of energy and lipid metabolism, and their effects on human health and disease outcomes [1]. Therefore, the potential capability to discriminate dietary fatty acids selectively and quantitatively may have important implications for nutritional status and health of individuals. In this context, studies aimed at analyzing fat perception are important to understand how individuals make choices about fat-rich foods in terms of the quality and quantity that they ingest [2].
In humans, orosensory perception of dietary fat clearly depends on multiple factors, including textural and odorant properties, as traditionally thought [3]. Recently, the taste component’s significant involvement in dietary lipid detection has been proposed as a sixth primary sensory quality of the gustatory system [4]. The gustatory contribution to fatty acid perception has been further shown by different studies in which the olfactory component was excluded by stopping nasal airflow, or its texture was disguised by using sonicated emulsions [2,3,5,6]. Long-chain fatty acids seem to be primarily responsible for dietary fat gustatory perception in the oral cavity [7,8], despite dietary lipids being mainly comprised of triglycerides. However, the importance of the hydrolysis of triglycerides via a lingual lipase to release free fatty acids when it comes to the orosensory detection of fat has been shown both in rodents [9] and in humans [5]. The initial stages of the signal transduction mechanisms proposed for the gustatory perception of lipids have been studied in several animal models [10,11], including the interaction of fatty acids with the plasma membrane glycoprotein CD36 [11,12,13,14,15,16]. In humans, the presence of CD36 protein in gustatory papillae has been documented [17] as the primary long-chain fatty acid receptor in taste bud cells, and so has its role in the orosensory perception of dietary lipid and fat preference [5,18].
A multitude of variations have been shown for oral sensitivity to fat in humans [6,19,20], and many factors can contribute to them. Among them, three common variants in the CD36 gene—rs1761667, rs1527483 and rs3840546—have been associated with oral sensitivity to and preference for fat (the first two), and obesity (the third) [5,18]. Several pieces of evidence have been reported concerning the effect of the polymorphism rs1761667 on CD36 protein expression levels, which could explain variations in orosensory perception of fats [21,22,23]. Recently, it has been shown that variations in oleic acid taste sensitivity could be related to variations in general taste sensitivity as indicated by differences in the expression of salivary proteins, such as carbonic anhydrase 6, which have been associated with taste perception [24]. Within this context, it would be of great interest to further explain variability in fat sensitivity by analyzing the associations of the CD36 variants that affect oral fat perception and preference with other genes known to be involved in taste variability. The genetic ability to taste the bitterness of 6-n-propylthiouracil (PROP) is one of best-known examples of taste variability, and has been used as a general index of oral chemosensory perception since it is associated with the perception of a wide range of oral stimuli [25]. The ability to taste PROP is associated with haplotypes of the gene that expresses the PROP-binding bitter receptor, TAS2R38, which explains most PROP phenotypic variance [26]. Also, oral sensitivity to PROP is related to expression of specific salivary proteins [27,28]. Moreover, a polymorphism in the carbonic anhydrase 6 gene has been shown to affect PROP sensitivity by acting on cell growth and fungiform papillae maintenance, thus providing an explanation for why PROP super-tasters, who have a high number of papillae, are more responsive to a broad range of stimuli [29].
Given the nutritional value of dietary lipids, the relationship between PROP taster status and perception or liking of fat is of particular interest, and has been extensively investigated, albeit with controversial results [30]. Most studies have shown a direct association between PROP sensitivity and fat perception, and an inverse correlation with the liking of fat: PROP non-taster subjects gave lower taste intensity ratings for linoleic acid [2], had a lower ability to distinguish fat and creaminess in foods [31,32,33,34,35,36], and a higher preference for dietary fat [32,37,38], compared with PROP taster subjects. However, other reports showed no associations between PROP sensitivity and these variables [39,40]. On the other hand, the role of PROP status in salt perception is primarily focused on the analysis of NaCl [41], while evidence suggesting that this phenotype can influence the perception or selection of salty foods is so far lacking. Some studies suggested that PROP related sensory differences may not be limited to taste, but extended to the olfactory system [42], and that PROP status may influence the perception of foods via their aromas or flavors [32,43].
Based on these considerations, investigations on the role of PROP status and CD36 in fat perception and preferences could better characterize the genetic contribution to fat ingestion and shed a light on potential links to dietary fat nutritional aspects. Here, we analyzed the relationship between the oral threshold for multimodal oral perception to oleic acid, the major liposoluble nutrient in the human diet [44], the two common variants (rs1761667 and rs1527483) in the CD36 gene, and PROP tasting (genotype and phenotype) as factors that may influence oral perception of dietary fats. Oleic acid orosensory detection thresholds were measured in subjects genotyped for CD36 and TAS2R38 polymorphisms and classified for their PROP taster status by having the fat stimulus presented in impregnated filter paper disks.
As mentioned, fats are complex stimuli that provide taste, olfactory, and textural cues, and are further influenced by different physical states (liquid, solid, and semi-solid). These considerations, together with the hydrophobicity of tastants and their high susceptibility to oxidation processes, justify the multiplicity of methods for measuring oral fat perception, all of which have limitations [19,20,32,34,45] such as complicated, lengthy and cumbersome procedures that test subjects must undergo. This fact could represent a factor contributing to the large individual differences that have been reported for oral fat perception, which is why it is important to find an effective, simple and reliable method to be applied in basic research studies [45].
2. Experimental Section
2.1. Subjects
Sixty-four non-smoking Caucasian subjects (23 males, 41 females, age 27.6 ± 0.85 years) from Sardinia, Italy were recruited according to standard procedures. All were normal weight with a body mass index (BMI) ranging from 18.6 to 25.3 kg/m2, had maintained a stable weight in the previous 3 months, and did not follow a diet or take medications that might interfere with taste function. Subjects with extreme scores for restraint and/or disinhibition and/or perceived hunger, assessed by the Three-Factor Eating Questionnaire, were excluded from the study [46]. Normogeusia for four of the basic taste qualities (sweet, sour, salty, and bitter) was verified in all subjects by taste strips (Bunghart Messtechnik, Wedel, Germany). Subjects were informed about the procedure and the aim of the study. All approved and signed an informed consent form. The Ethical Committee of the University Hospital of Cagliari approved the study procedures (Protocol No. 451/09, 15 October 2009; Amendment No. 8, 29 November 2010), which were performed in accordance with the latest revision of the Declaration of Helsinki.
2.2. Study Design
All subjects (n = 64) were tested in three sessions separated by 1-month periods. They were assessed for PROP taster status in the first two sessions, while in the third session, sensitivity to oleic acid flavor was assessed. All were requested to refrain from eating, drinking (except water), and using oral care products or chewing gum for at least 8 h prior to testing. Women were tested on the sixth/seventh day of their menstrual cycle to avoid oral sensitivity changes due to the estrogen phase [47,48,49,50]. A group of subjects (n = 36) were also tested for sensitivity to oleic acid esterified with glycerol (triolein) in a fourth session.
All solutions used for the assessments were prepared the day before each session and stored in a refrigerator until 1 h before testing. Stimuli were presented at room temperature.
2.3. PROP Taster Status
Assignment of each subject to a PROP taster group (super-taster, medium taster, or non-taster) was performed using the three-solution test according to Tepper et al. [51], which has been validated in several studies [27,28,52,53,54]. Briefly, the taste intensity rating for three suprathreshold PROP (0.032, 0.32, and 3.2 mmol/L) and sodium chloride (NaCl; 0.01, 0.1, 1.0 mol/L) solutions was collected in each subject, by using the Labeled Magnitude Scale [55], which gave subjects the freedom to rate the PROP bitterness relative to the “strongest imaginable” oral stimulus they had ever experienced in their life. Each stimulation was followed by oral rinsing with spring water. The order of presentation of the taste stimuli (PROP or NaCl) in 10 mL samples was reversed in the two sessions. Concentrations were tested in a random order, and the interstimulus interval was set at 60 s. The mean rating of the two replicates was calculated, and functions of perceived taste intensity for PROP and NaCl for each subject were generated from the results [51]. Subjects who gave lower intensity ratings to PROP than to NaCl were classified as PROP non-tasters, those who gave similar ratings to the two stimuli were classified as medium tasters, and those who gave higher ratings to PROP than to NaCl were classified as super-tasters.
Identification and count of the fungiform papillae in a circle area (6 mm in diameter) of the tip of the anterior tongue surface in PROP taster and non-taster subjects were performed according to Melis et al. [29].
2.4. Oleic Acid Threshold Assessments
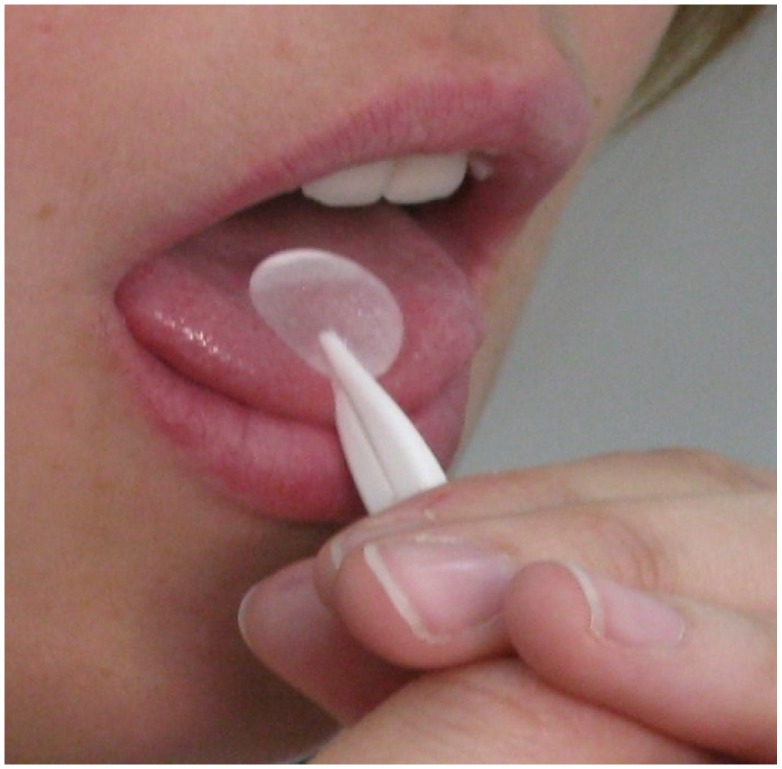
The threshold for oleic acid multimodal oral perception was assessed in each subject, in the absence of nose clips, by a modification of the staircase method implemented in a three-alternative forced-choice procedure [5], where stimuli were presented to subjects by means of filter paper disks (1.5 cm diameter) (Figure 1). Filter paper disks were impregnated with 10 μL of a mixture of oleic acid and mineral oil, with oleic acid ranging from 0.0015 to 10 μL (pure). Subjects were asked to place the paper disk on the center of their tongue, keep it in the mouth for 10 s and then spit it out. Each subject was presented with three samples: two contained only mineral oil (control) and one the amount of oleic acid under evaluation. They were instructed to savor each disk in order to facilitate the release of the stimulus. The interstimulus interval was set at 60 s. Subjects were instructed to taste the three samples, without swallowing, and to indicate the sample that was different. They rinsed their mouth with deionized water before and after tasting each sample. The procedure continued with the presentation of a new concentration only when the subject reported having no perception. The oleic acid concentration presented was increased after a single incorrect response and reduced after two correct responses in a row. A reversal was considered to have occurred at points where the concentration sequence changed direction. The procedure was terminated when four reversals occurred. The threshold concentration was calculated as the mean value of the four reversals.
Figure 1.
Photograph showing a stimulus presentation in the fatty acid flavor threshold assessment. The stimulation was performed by positioning, on the anterior part of the tongue, the paper disk impregnated with 10 μL of a mixture of oleic acid (or triolein) and mineral oil with the former ranging from 0.0015 to 10 μL (pure).
2.5. Molecular Analyses
Subjects were genotyped for two CD36 single nucleotide polymorphisms (SNPs), rs1761667 (G/A) located at the −31,118 promoter region of exon 1A and rs1527483 (C/T) located at intron 11. DNA was extracted from saliva samples using the QIAamp® DNA Mini Kit (QIAGEN S.r.l., Milano, Italy) according to the manufacturer’s instructions. Purified DNA concentration was estimated by measurements at an optical density of 260 nm. A polymerase chain reaction (PCR) was employed to amplify the CD36 gene region, including the two polymorphisms. The primers were synthesized by Invitrogen (50 nmol scale, desalted) (Europrim, Invitrogen Cambridge, UK). Molecular analyses were performed using PCR followed by restriction enzyme analysis of the fragments obtained according to Banerjee et al. [56], and as briefly described below. To genotype the CD36 rs1761667 polymorphism, a 190-bp fragment was amplified with forward 5’-CAAATCACAATCTATTCAAGACCA-3’ and reverse 5’-TTTTGGGAGAAATTCTGAAGAG-3’ primers. DNA was amplified using EuroTaq thermostable DNA polymerase (EuroClone S.p.A., Pero (MI), Italy). Thermal cycles of amplification were carried out in a Personal Eppendorf Master cycler (Eppendorf, Hamburg, Germany). The amplification protocol included an initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, and then extension at 72 °C for 30 s. A final extension was carried out at 72 °C for 5 min. The PCR products were digested with the HhaI restriction enzyme (Thermo Scientific Inc, Waltham, MA, USA) that recognizes GCG^C site and cut best at 37 °C for 4–16 h. To genotype the CD36 rs1527483 polymorphism, the following primer set was used to amplify a 252-bp fragment, the forward 5’-CGCTACAACAATTTTATAGATTTTGAC-3’ and reverse 5’-TGAAATAAAAATAATCTTGTCGATGA-3’ primers. DNA was amplified using EuroTaq thermostable DNA polymerase (EuroClone S.p.A., Italy). The amplification protocol included an initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and then extension at 72 °C for 30 s. A final extension was carried out at 72 °C for 5 min. The thermal cycles of amplification were performed in a Personal Eppendorf Master cycler (Eppendorf, Hamburg, Germany). The amplified samples were digested with the TaqI restriction enzyme (Thermo Scientific) that recognizes T^CGA site and cut if incubated at 65 °C for 5–16 h. Digestion products were analyzed by electrophoresis on a 2% agarose gel and the DNA bands were visualized by ethidium bromide staining and ultraviolet light to score the deletion. PCR 50 bp Low Ladder DNA was used as a molecular mass marker (GeneRuler™-Thermo Scientific).
Subjects were also genotyped for three SNPs at base pairs 145 (C/G), 785 (C/T), and 886 (G/A) of TAS2R38. This gene expresses the receptor that binds the chemical moiety of the bitter thiourea compounds, such as phenylthiocarbamide or PROP [26]. The three SNPs of TAS2R38 result in three amino acid substitutions (Pro49Ala, Ala262Val, and Val296Ile) and give rise to two major haplotypes, the dominant taster variant (PAV) and the recessive non-taster variant (AVI), and three rare (AAI, PVI, and AAV). Molecular analyses of TAS2R38 were performed using PCR techniques followed by the sequencing of the fragments obtained according to Calò et al. [52]. Individuals with rare haplotypes were excluded.
2.6. Data Analyses
Three-way analysis of variance (ANOVA) was used to compare PROP intensity ratings with NaCl intensity ratings across PROP taster groups (super-tasters, medium tasters, and non-tasters). The Fisher method was used to test genotype distribution and allele frequencies of the two CD36 SNPs according to PROP status. Threshold differences to oleic acid flavor related to genotypes of the two CD36 SNPs were evaluated by one-way ANCOVA using papilla density as covariate. One-way ANOVA was also used to compare threshold differences to oleic acid flavor related to PROP tasting (genotype and phenotype), fungiform papilla density (number/cm2) between PROP tasters and non-tasters, and PROP bitterness intensity ratings according to TAS2R38 locus. Two-way ANOVA was used to compare the threshold differences to oleic acid flavor related to genotypes of the two CD36 SNPs between super-tasters, medium tasters, and non-tasters, or PAV/PAV, PAV/AVI, and AVI/AVI subjects. Post hoc comparisons were conducted with the Fisher least significant difference (LSD) test. Statistical analyses were conducted using STATISTICA for WINDOWS (version 7; StatSoft Inc., Tulsa, OK, USA). p-Values ≤ 0.05 were considered significant.
3. Results
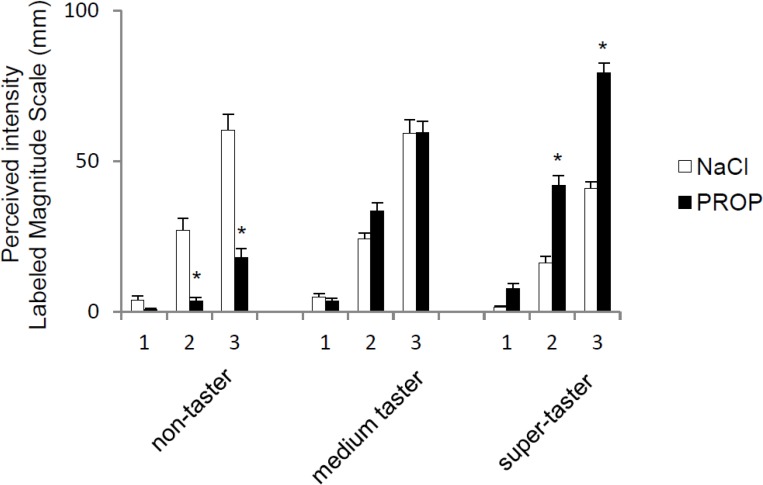
Based on their PROP taster group assignments, 33% of the subjects were non-tasters (n = 21); 42% were medium tasters (n = 27); and 25% were super-tasters (n = 16). ANOVA revealed a significant three-way interaction of taster group × solution type × concentration on the intensity ratings (F(4,366) = 17.587; p < 0.0001) (Figure 2). Post hoc comparisons showed that non-tasters gave lower intensity ratings to the two highest PROP concentrations compared with the two highest NaCl concentrations, respectively (p < 0.0001). Medium tasters gave similar ratings to PROP and NaCl at all concentrations. Super-tasters gave higher ratings to 0.32 and 3.2 mmol/L PROP compared with the two highest NaCl concentrations, respectively (p < 0.0001).
Figure 2.
Relationship between perceived taste intensity and stimulus concentration in PROP non-tasters (n = 21), medium tasters (n = 27), and super-tasters (n = 16). All values are mean (±SEM). The numbers 1, 2, and 3 on the x-axis correspond to three NaCl and PROP solutions (NaCl: 0.01, 0.1, 1.0 mol/L) and (PROP: 0.032, 0.32, and 3.2 mmol/L). * Significant difference between PROP and the corresponding sodium chloride concentration (p < 0.0001; Tukey Post hoc test subsequent three-way ANOVA).
Molecular analysis at the three SNPs of the TAS2R38 locus identified 13 subjects who were PAV homozygous, 31 were heterozygous, and 20 were AVI homozygous. PROP bitterness intensity ratings (3.2 mM) were strongly associated with TAS2R38 genotypes (F(2, 61) = 57.808; p < 0.000001). PROP bitterness ratings were lower in individuals with the AVI/AVI diplotype of TAS2R38 (16.52 ± 2.72) than in individuals with PAV/PAV (68.01 ± 5.22) and PAV/AVI diplotype (65.94 ± 3.45) (p < 0.00011; Tukey Post hoc test). No differences in bitterness intensity ratings between PAV/PAV and PAV/AVI subjects were found (p > 0.05).
Molecular analysis showed that the observed allele frequencies at each of the two CD36 SNPs (rs1761667 and rs1527483) were as follows: for the rs1761667 (A/G) polymorphism, 7 subjects were homozygous AA, 38 were heterozygous, and 19 were homozygous GG, while for the rs1527483 (C/T) polymorphism, 57 subjects were homozygous CC, 7 were heterozygous, and no subject was homozygous TT. No differences were found among the three PROP taster groups based on the genotype distribution and allele frequency of the two polymorphisms of the CD36 gene (χ2 < 1.358; p > 0.061; Fisher method) (Table 1).
Table 1.
Genotype distributions and allele frequencies of rs1761667 and rs1527483 polymorphisms of CD36 according to PROP taster status.
| Total | PROP Status | p-Value * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Super-Taster | Medium Taster | Non-Taster | |||||||
| n | % | n | % | n | % | n | % | ||
| rs1761667 | |||||||||
| Genotype | |||||||||
| GG | 19 | 29.69 | 5 | 31.25 | 9 | 33.33 | 5 | 28.80 | 0.425 |
| AG | 38 | 59.37 | 11 | 68.75 | 15 | 55.56 | 12 | 57.15 | |
| AA | 7 | 10.94 | 0 | 0 | 3 | 11.11 | 4 | 19.05 | |
| Allele | |||||||||
| G | 76 | 59.37 | 21 | 65.62 | 33 | 61.12 | 22 | 52.38 | 0.507 |
| A | 52 | 40.63 | 11 | 35.38 | 21 | 38.88 | 20 | 47.62 | |
| rs1527483 | |||||||||
| Genotype | |||||||||
| CC | 57 | 89.06 | 16 | 100 | 25 | 92.59 | 16 | 76.19 | 0.061 |
| CT | 7 | 10.94 | 0 | 0 | 2 | 7.41 | 5 | 23.81 | |
| TT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Allele | |||||||||
| C | 121 | 94.53 | 32 | 100 | 52 | 96.30 | 37 | 88.09 | 0.072 |
| T | 7 | 5.47 | 0 | 0 | 2 | 3.70 | 5 | 11.91 | |
* p-Value derived from Fisher method (n = 64).
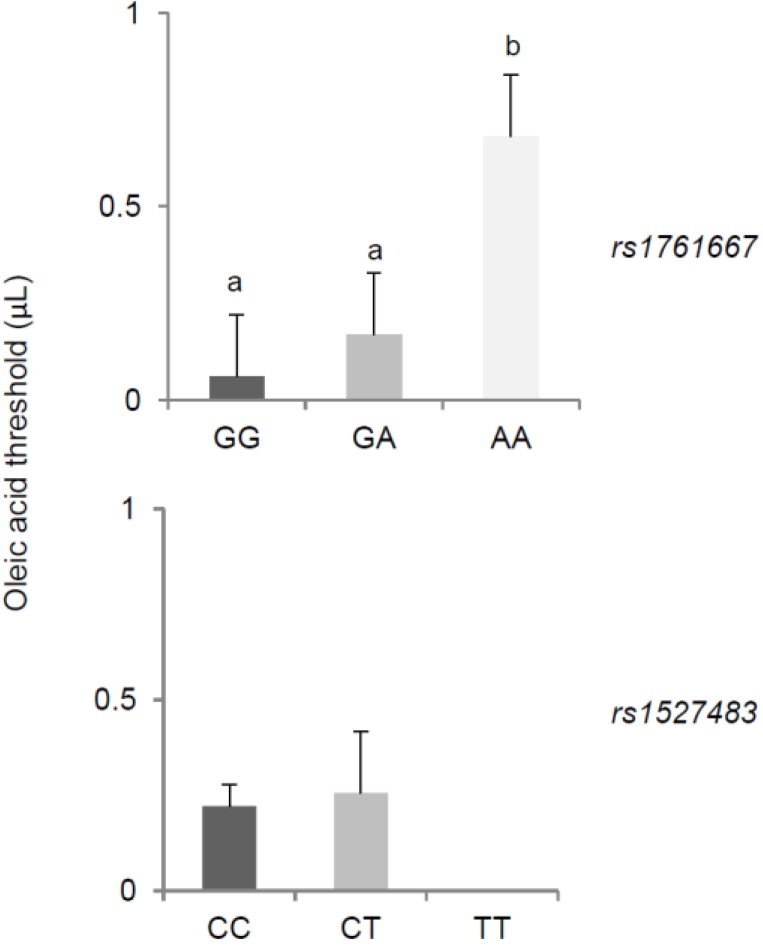
Mean values (±SEM) of flavor threshold for oleic acid in individuals with genotype GG, GA, and AA of rs1761667 locus and genotype CC, CT, and TT of rs1527483 locus of CD36 are shown in Figure 3. Pairwise comparison subsequent to one-way ANCOVA showed that subjects homozygous for the G-allele of the rs1761667 polymorphism exhibited a 5-fold lower threshold for oleic acid than homozygous AA subjects (p = 0.041, Fisher LSD test) (upper graph). No changes associated with the rs1527483 polymorphism were found (lower graph).
Figure 3.
Mean values (±SEM) of the flavor threshold for oleic acid in individuals with genotype GG, GA, and AA of rs1761667 locus and genotype CC, CT, and TT of rs1527483 locus of CD36. Different letters indicate a significant difference (p < 0.033, Fisher LSD test subsequent to one-way ANCOVA).
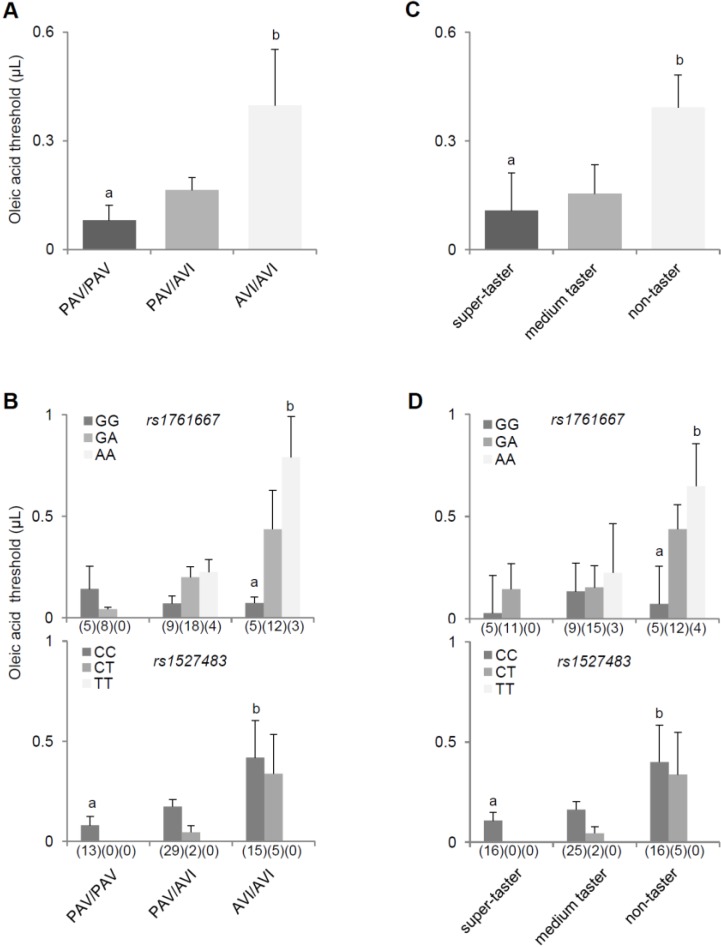
The relationships between the threshold for oleic acid flavor, PROP genotype, and phenotype and the two polymorphisms (rs1761667 and rs1527483) of CD36 are shown in Figure 4. In particular, Figure 4A shows the mean threshold values (±SEM) for oleic acid in subjects with genotype PAV/PAV, PAV/AVI, and AVI/AVI of TAS2R38. Post hoc comparison subsequent to one-way ANOVA showed that AVI/AVI subjects exhibited a 5-fold higher oleic acid threshold than PAV/PAV subjects (p = 0.035, Fisher LSD). Threshold values for heterozygous subjects were intermediate. Figure 4B shows the threshold values (mean ± SEM) for oleic acid in the same TAS2R38 genotype groups according to the two polymorphisms of CD36. Post hoc comparison subsequent to two-way ANOVA highlighted that subjects homozygous AVI/AVI for TAS2R38 with a genotype homozygous for the G-allele of the rs1761667 polymorphism had an 11-fold lower threshold for oleic acid than subjects with the same TAS2R38 genotype (AVI/AVI), but with a homozygous AA genotype of the rs1761667 polymorphism (p = 0.018, Fisher LSD) (Figure 4B, upper graph), and subjects with genotype CC of the rs1527483 polymorphism of CD36 who were homozygous for the taster variant in TAS2R38 (PAV) showed a 5-fold lower threshold than subjects with the same genotype (CC) for this locus of CD36 who were homozygous for the non-taster variant in TAS2R38 (AVI) (p = 0.037, Fisher LSD) (Figure 4B, lower graph). Figure 4C shows mean values (±SEM) of threshold for oleic acid in PROP super-tasters, medium tasters, and non-tasters. Post hoc comparison subsequent to one-way ANOVA showed that PROP non-tasters exhibited a 3.6-fold higher threshold for oleic acid than super-tasters (p = 0.042, Fisher LSD). Finally, PROP non-tasters with a genotype homozygous for the G-allele of the rs1761667 polymorphism of CD36 had a 9-fold lower threshold for oleic acid than PROP non-tasters with genotype AA of the same locus (p = 0.042, Fisher; LSD subsequent to two-way ANOVA) (Figure 4D, upper graph), and PROP super-tasters with genotype CC of rs1527483 polymorphism of CD36 showed a 4-fold lower threshold than PROP non-tasters with the same genotype for this locus of CD36 (p = 0.048, Fisher LSD; LSD subsequent to two-way ANOVA) (Figure 4D, lower graph). No other differences relating to the two polymorphisms were found.
Figure 4.
Relationships between flavor threshold to oleic acid, PROP genotype and phenotype, and the two polymorphism (rs1761667 and rs1527483) of CD36. (A) Mean values (±SEM) of the oleic acid threshold in subjects with genotype PAV/PAV (n = 13), PAV/AVI (n = 31), and AVI/AVI (n = 20) of TAS2R38. Different letters indicate a significant difference (p = 0.035, Fisher LSD test subsequent to one-way ANOVA); (B) Mean values (±SEM) of the oleic acid threshold according to TAS2R38 and rs1761667 (upper graph) or rs1527483 polymorphism (lower graph) of CD36. Different letters indicate a significant difference (p < 0.037; Fisher LSD test subsequent to two-way ANOVA); (C) Mean values (±SEM) of the oleic acid threshold in PROP super-tasters (n = 16), medium tasters (n = 27), and non-tasters (n = 21). Different letters indicate a significant difference (p = 0.042, Fisher LSD test subsequent to one-way ANOVA); (D) Mean values (±SEM) of the oleic acid threshold according to PROP taster status and CD36 rs1761667 (upper graph) or rs1527483 polymorphism (lower graph). Different letters indicate a significant difference (p < 0.048; Fisher LSD test subsequent to two-way ANOVA). The numbers in parenthesis below each bar indicate the number of subjects.
The density of the fungiform papillae on the tip of the anterior part of the tongue of PROP non-tasters (52.19 ± 9.45) was lower than that of tasters (92.32 ± 14.27) (p = 0.046, one-way ANOVA).
4. Discussion
In the light of the evidence collected over the last decade on the nutritional value of dietary lipids [1], and given the wide individual variations in fat consumption and preference [18,57], the present data provide new insights into characterization of individual capability to detect dietary fatty acids in order to identify factors involved in the choice patterns for fat consumption in humans.
Our results show that the paper screening test, a method already used for classifying individuals by PROP taster status [58], is an effective and quick technique and can also be used for assessing oral perception of fatty acids, as well as circumventing the problems related to stimulus hydrophobicity and to the production of homogeneous and stable oil-in-water emulsions. We have been inspired by the elegant approach used by Ebba et al. [2], who measured, by means of a scaling method, the chemosensory response to lipid molecules delivered to the oral cavity by edible taste strips. As stated by Prescott, the identification and response to tastants related to food requires the integration, cognitively “constructed”, of functionally united, although anatomically separated, systems (primarily gustatory and olfactory), which can be seen as a functionally distinct sense, the flavor [59]. This is particularly true for the orosensory perception of dietary fats, which clearly depends on activation of taste, olfactory (with aromatic cues) and somatosensory (with textural cues) systems. Therefore, we used threshold measurements to determine individual capability to simultaneously detect the diverse components of orosensory perception of oleic acid, as it happens when it is eaten with food. However, in our study, the contribution of the somatosensory component due to textural cues of the stimulus was eliminated, or greatly minimized, by comparing three paper disks with the same oil content, two only impregnated with mineral oil (control) and one with oleic acid in mineral oil. The threshold values determined with this method were about 10-fold lower (ranging from 0.001 μL to 2.25 μL) than those obtained using texture controlled emulsions [6,19,60].
The major aim of the current study was to determine and characterize factors involved in individual capability to detect dietary fatty acids. A primary finding is that the orosensory perception of fatty acids is directly associated with PROP responsiveness and with the polymorphism rs1761667 in CD36, which seem to play a significant role, mostly in PROP non-taster subjects. In agreement with evidence reported on the effect of this polymorphism on CD36 protein expression levels, all showing that the homozygous AA condition reduces CD36 expression [21,22,23], we found that subjects homozygous for the G-allele of the rs1761667 polymorphism showed a higher capability to detect oleic acid than homozygous AA subjects, who should have a reduced CD36 expression level, while heterozygous subjects showed intermediate sensitivity. This result confirms recent data obtained from obese subjects [5], even though the threshold values we found were much lower (about 30-fold), probably because we measured the overall response to taste and olfactory components of orosensory perception of oleic acid, in contrast to Pepino et al. [5], who completely excluded the olfactory component.
The relationship between PROP taster status and fat perception has been extensively investigated with controversial results [30]. Our data support a direct relationship between fat multimodal oral perception and the genetic ability to perceive the bitter taste of PROP [2]. In fact, we found that the lowest capability to orally detect oleic acid was associated with the lowest responsiveness to PROP (in PROP non-tasters) or with the genotype homozygous for the non-taster variant of TAS2R38 (AVI/AVI), while the highest sensitivity to oleic acid was associated with the highest responsiveness to PROP (in PROP super-tasters) or the genotype homozygous for the taster variant of TAS2R38 (PAV/PAV). Several studies showed that PROP non-tasters have a lower density of fungiform papillae than PROP tasters [29,61,62]. Ebba et al. [2] hypothesized that the increased ability to taste linoleic acid exhibited by PROP tasters, compared with non-tasters, could be ascribed to the difference in density of fungiform papillae between these two groups. Indeed, our data showed a lower density of fungiform papillae on the anterior part of the tongue of PROP non-tasters, who also exhibit a lower capability to detect oleic acid compared with PROP tasters. In addition, albeit that no differences were found among the three PROP taster groups based on the genotype distribution and allele frequency of the rs1761667 polymorphism of CD36, a comparative analysis indicated that the effect of the rs1761667 polymorphism of the CD36 gene on the capability to detect oleic acid was only present in PROP non-tasters or AVI/AVI subjects. In fact, PROP non-tasters (or AVI/AVI subjects) with a genotype homozygous for the G-allele of the rs1761667 polymorphism had a lower oleic acid threshold than PROP non-tasters with the homozygous AA genotype. Instead, no effects of this polymorphism of CD36 on oleic acid perception were found in PROP taster subjects. This finding suggests that a high expression level of CD36 in gustatory cells seems to be a determining factor for detecting dietary fat only in subjects who have a low density of taste papillae.
Recent data have shown that a minor allele of rs1527483 polymorphism of CD36 was associated with increased ratings of fat content in salad dressings in African Americans [18]. The very low frequency that we found for the T-allele may explain why no change in capability to detect oleic acid orally, in relation to the rs1527483 polymorphism, was found in our population sample. On the other hand, the higher threshold exhibited by PROP non-tasters (or AVI/AVI subjects) with a genotype homozygous for the C-allele of the rs1527483 polymorphism of CD36, with respect to super-tasters (or PAV/PAV subjects) with the same genotype for this locus of CD36, may be due to the difference in papilla density between the two PROP taster groups.
5. Conclusions
The present study provides evidence that the paper screening test is a quick, easy, and low-cost method for assessing the orosensory perception of dietary fats. In addition, our findings extend our knowledge about the characterization of individual capability to detect dietary fats in the oral cavity by showing that the polymorphism rs1761667 in CD36, which influences protein expression levels, plays a crucial role mostly in PROP non-tasters, given their low number of taste papillae. A better understanding of individual orosensory capability could lead to the recognition of the wide variation in human fat preferences and consumption patterns, and thus may have important implications for the nutritional status and health of individuals.
Acknowledgments
This study was supported by the Contribution to Research of Cagliari University (CAR2014), Regione Autonoma della Sardegna L.R. 7/2007, 2012 (grant number CRP 59490), and Fondazione Banco di Sardegna (2014). The authors thank the volunteers, whose contributions made this study possible. We also thank Caterina Chillotti for running the clinical trials.
Author Contributions
I.T.B. conceived and designed the experiments; M.M., P.M., and G.S. performed the experiments; M.M., R.C., and I.T.B. analyzed the data; P.M., R.C., and I.T.B contributed reagents/materials/analysis tools; M.M., R.C., and I.T.B wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Smit L.A., Mozaffarian D., Willett W. Review of fat and fatty acid requirements and criteria for developing dietary guidelines. Ann. Nutr. Metab. 2009;55:44–55. doi: 10.1159/000228995. [DOI] [PubMed] [Google Scholar]
- 2.Ebba S., Abarintos R.A., Kim D.G., Tiyouh M., Stull J.C., Movalia A., Smutzer G. The examination of fatty acid taste with edible strips. Physiol. Behav. 2012;106:579–586. doi: 10.1016/j.physbeh.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattes R.D. Is there a fatty acid taste? Annu. Rev. Nutr. 2009;29:305–327. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattes R.D. Fat Taste in Humans: Is It a Primary? In: Montmayeur J.P., le Coutre J., editors. Fat Detection: Taste, Texture, and Post Ingestive Effects. CRC Press; Boca Raton, FL, USA: 2010. pp. 167–193. [Google Scholar]
- 5.Pepino M.Y., Love-Gregory L., Klein S., Abumrad N.A. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 2012;53:561–566. doi: 10.1194/jlr.M021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalé-Rush A., Burgess J.R., Mattes R.D. Multiple routes of chemosensitivity to free fatty acids in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1206–G1212. doi: 10.1152/ajpgi.00471.2006. [DOI] [PubMed] [Google Scholar]
- 7.Fukuwatari T., Shibata K., Iguchi K., Saeki T., Iwata A., Tani K., Sugimoto E., Fushiki T. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol. Behav. 2003;78:579–583. doi: 10.1016/S0031-9384(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 8.Smith J.C., Fisher E.M., Maleszewski V., McClain B. Orosensory factors in the ingestion of corn oil/sucrose mixtures by the rat. Physiol. Behav. 2000;69:135–146. doi: 10.1016/S0031-9384(00)00197-9. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T., Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R447–R454. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]
- 10.Cartoni C., Yasumatsu K., Ohkuri T., Shigemura N., Yoshida R., Godinot N., le Coutre J., Ninomiya Y., Damak S. Taste Preference for Fatty Acids Is Mediated by GPR40 and GPR120. J. Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan N.A., Besnard P. Oro-sensory perception of dietary lipids: New insights into the fat taste transduction. Biochim. Biophys. Acta. 2009;1791:149–155. doi: 10.1016/j.bbalip.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Martin C., Chevrot M., Poirier H., Passilly-Degrace P., Niot I., Besnard P. CD36 as a lipid sensor. Physiol. Behav. 2011;105:36–42. doi: 10.1016/j.physbeh.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J.P., Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Investig. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahimi A., Abumrad N.A. Role of CD36 in membrane transport of long-chain fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:139–145. doi: 10.1097/00075197-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard D., Laugerette F., Darcel N., el-Yassimi A., Passilly-Degrace P., Hichami A., Khan N.A., Montmayeur J.P., Besnard P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 16.El-Yassimi A., Hichami A., Besnard P., Khan N.A. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 2008;283:12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 17.Ozdener M.H., Subramaniam S., Sundaresan S., Sery O., Hashimoto T., Asakawa Y., Besnard P., Abumrad N.A., Khan N.A. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 2014;146:995–1005. doi: 10.1053/j.gastro.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller K.L., Liang L.C., Sakimura J., May D., van Belle C., Breen C., Driggin E., Tepper B.J., Lanzano P.C., Deng L., et al. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring) 2012;20:1066–1073. doi: 10.1038/oby.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart J.E., Feinle-Bisset C., Golding M., Delahunty C., Clifton P.M., Keast R.S. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 2010;104:145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- 20.Mattes R.D. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem. Senses. 2009;34:145–150. doi: 10.1093/chemse/bjn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin C., Passilly-Degrace P., Gaillard D., Merlin J.F., Chevrot M., Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: Impact on spontaneous fat preference. PLoS One. 2011;6:e24014. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma X., Bacci S., Mlynarski W., Gottardo L., Soccio T., Menzaghi C., Iori E., Lager R.A., Shroff A.R., Gervino E.V., et al. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum. Mol. Genet. 2004;13:2197–2205. doi: 10.1093/hmg/ddh233. [DOI] [PubMed] [Google Scholar]
- 23.Madden J., Carrero J.J., Brunner A., Dastur N., Shearman C.P., Calder P.C., Grimble R.F. Polymorphisms in the CD36 gene modulate the ability of fish oil supplements to lower fasting plasma triacyl glycerol and raise HDL cholesterol concentrations in healthy middle-aged men. Prostaglandins Leukot. Essent. Fat. Acids. 2008;78:327–335. doi: 10.1016/j.plefa.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Mounayar R., Morzel M., Brignot H., Tremblay-Franco M., Canlet C., Lucchi G., Ducoroy P., Feron G., Neyraud E. Salivary markers of taste sensitivity to oleic acid: A combined proteomics and metabolomics approach. Metabolomics. 2014;10:688–696. doi: 10.1007/s11306-013-0602-1. [DOI] [PubMed] [Google Scholar]
- 25.Tepper B.J. Nutritional implications of genetic taste variation: The role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 2008;28:367–388. doi: 10.1146/annurev.nutr.28.061807.155458. [DOI] [PubMed] [Google Scholar]
- 26.Kim U.K., Jorgenson E., Coon H., Leppert M., Risch N., Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 27.Cabras T., Melis M., Castagnola M., Padiglia A., Tepper B.J., Messana I., Tomassini Barbarossa I. Responsiveness to 6-n-propylthiouracil (PROP) is associated with salivary levels of two specific basic proline-rich proteins in humans. PLoS One. 2012;7:e30962. doi: 10.1371/journal.pone.0030962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melis M., Aragoni M.C., Arca M., Cabras T., Caltagirone C., Castagnola M., Crnjar R., Messana I., Tepper B.J., Barbarossa I.T., et al. Marked increase in PROP taste responsiveness following oral supplementation with selected salivary proteins or their related free amino acids. PLoS One. 2013;8:e59810. doi: 10.1371/journal.pone.0059810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melis M., Atzori E., Cabras S., Zonza A., Calò C., Muroni P., Nieddu M., Padiglia A., Sogos V., Tepper B.J., et al. The gustin (CA6) gene polymorphism, rs2274333 (A/G), as a mechanistic link between PROP tasting and fungiform taste papilla density and maintenance. PLoS One. 2013;8:e74151. doi: 10.1371/journal.pone.0074151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tepper B.J., Banni S., Melis M., Crnjar R., Tomassini Barbarossa I. Genetic sensitivity to the bitter taste of 6-n-Propylthiouracil (PROP) and Its Association with Physiological Mechanisms Controlling Body Mass Index (BMI) Nutrients. 2014;6:3363–3381. doi: 10.3390/nu6093363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy V., Lucchina L., Bartoshuk L. Genetic variation in taste: Potential biomarker for cardiovascular disease risk? In: Prescott J., Tepper B.J., editors. Genetic Variations in Taste Sensitivity: Measurement, Significance and Implications. Dekker; New York, NY, USA: 2004. pp. 195–228. [Google Scholar]
- 32.Hayes J.E., Duffy V.B. Revisiting sugar-fat mixtures: Sweetness and creaminess vary with phenotypic markers of oral sensation. Chem. Senses. 2007;32:225–236. doi: 10.1093/chemse/bjl050. [DOI] [PubMed] [Google Scholar]
- 33.Prescott J., Bartoshuk L.M., Prutkin J. 6-n-Propylthiouracil tasting and the perception of nontaste oral sensations. In: Prescott J., Tepper B.J., editors. Genetic Variation in Taste Sensitivity. Marcel Dekker; New York, NY, USA: 2004. pp. 89–104. [Google Scholar]
- 34.Tepper B.J., Nurse R.J. Fat perception is related to PROP taster status. Physiol. Behav. 1997;61:949–954. doi: 10.1016/S0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 35.Hayes J.E., Duffy V.B. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol. Behav. 2008;95:77–87. doi: 10.1016/j.physbeh.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkmeyer S.V., Tepper B.J. Understanding creaminess perception of dairy products using free-choice profiling and genetic responsivity to 6-n-propylthiouracil. Chem. Senses. 2003;28:527–536. doi: 10.1093/chemse/28.6.527. [DOI] [PubMed] [Google Scholar]
- 37.Tepper B.J., Nurse R.J. PROP taster status is related to fat perception and preference. Ann. N. Y. Acad. Sci. 1998;855:802–804. doi: 10.1111/j.1749-6632.1998.tb10662.x. [DOI] [PubMed] [Google Scholar]
- 38.Duffy V.B., Bartoshuk L.M. Food acceptance and genetic variation in taste. J. Am. Diet. Assoc. 2000;100:647–655. doi: 10.1016/S0002-8223(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 39.Drewnowski A., Henderson S.A., Barratt-Fornell A. Genetic sensitivity to 6-n-propylthiouracil and sensory responses to sugar and fat mixtures. Physiol. Behav. 1998;63:771–777. doi: 10.1016/S0031-9384(97)00540-4. [DOI] [PubMed] [Google Scholar]
- 40.Drewnowski A., Henderson S.A., Cockroft J.E. Genetic sensitivity to 6-n-propylthiouracil has no influence on dietary patterns, body mass indexes, or plasma lipid profiles of women. J. Am. Diet. Assoc. 2007;107:1340–1348. doi: 10.1016/j.jada.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Bartoshuk L.M., Duffy V.B., Lucchina L.A., Prutkin J., Fast K. PROP (6-n-propylthiouracil) supertasters and the saltiness of NaCl. Ann. N. Y. Acad. Sci. 1998;855:793–796. doi: 10.1111/j.1749-6632.1998.tb10660.x. [DOI] [PubMed] [Google Scholar]
- 42.Yackinous C., Guinard J.X. Relation between PROP taster status and fat perception, touch, and olfaction. Physiol. Behav. 2001;72:427–437. doi: 10.1016/S0031-9384(00)00430-3. [DOI] [PubMed] [Google Scholar]
- 43.Mattes R.D. 6-n-Propylthiouracil taster status: Dietary modifier, marker or misleader? In: Prescott J., Tepper B.J., editors. Genetic Variation in Taste Sensitivity. Marcel Dekker; New York, NY, USA: 2004. pp. 229–250. [Google Scholar]
- 44.Sette S., le Donne C., Piccinelli R., Arcella D., Turrini A., Leclercq C., Group I.-S.S. The third Italian National Food Consumption Survey, INRAN-SCAI 2005-06—Part 1: Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011;21:922–932. doi: 10.1016/j.numecd.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Liang L.C., Sakimura J., May D., Breen C., Driggin E., Tepper B.J., Chung W.K., Keller K.L. Fat discrimination: A phenotype with potential implications for studying fat intake behaviors and obesity. Physiol. Behav. 2012;105:470–475. doi: 10.1016/j.physbeh.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stunkard A.J., Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 47.Than T.T., Delay E.R., Maier M.E. Sucrose threshold variation during the menstrual cycle. Physiol. Behav. 1994;56:237–239. doi: 10.1016/0031-9384(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 48.Alberti-Fidanza A., Fruttini D., Servili M. Gustatory and food habit changes during the menstrual cycle. Int. J. Vitam. Nutr. Res. 1998;68:149–153. [PubMed] [Google Scholar]
- 49.Glanville E.V., Kaplan A.R. Taste perception and the menstrual cycle. Nature. 1965;205:930–931. doi: 10.1038/205930a0. [DOI] [PubMed] [Google Scholar]
- 50.Pal T., Bhattacharyya A.K. Cyclic changes in salivary lactate dehydrogenase, peroxidase and leucine aminopeptidase during menstrual cycle. Indian J. Exp. Biol. 1989;27:695–698. [PubMed] [Google Scholar]
- 51.Tepper B.J., Christensen C.M., Cao J. Development of brief methods to classify individuals by PROP taster status. Physiol. Behav. 2001;73:571–577. doi: 10.1016/S0031-9384(01)00500-5. [DOI] [PubMed] [Google Scholar]
- 52.Calò C., Padiglia A., Zonza A., Corrias L., Contu P., Tepper B.J., Barbarossa I.T. Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiol. Behav. 2011;104:1065–1071. doi: 10.1016/j.physbeh.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Padiglia A., Zonza A., Atzori E., Chillotti C., Calò C., Tepper B.J., Barbarossa I.T. Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am. J. Clin. Nutr. 2010;92:539–545. doi: 10.3945/ajcn.2010.29418. [DOI] [PubMed] [Google Scholar]
- 54.Rankin K.M., Godinot N., Christensen C.M., Tepper B.J., Kirkmeyer S.V. Assessment of different methods for 6-n-propylthiouracil status classification. In: Prescott J., Tepper B.J., editors. Genetic Variation in Taste Sensitivity. Marcel Dekker; New York, NY, USA: 2004. pp. 63–88. [Google Scholar]
- 55.Green B.G., Shaffer G.S., Gilmore M.M. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem. Senses. 1993;18:683–702. doi: 10.1093/chemse/18.6.683. [DOI] [Google Scholar]
- 56.Banerjee M., Gautam S., Saxena M., Bid H.K., Agrawal C.G. Association of CD36 gene variants rs1761667 (G > A) and rs1527483 (C > T) with Type 2 diabetes in North Indian population. Int. J. Diabetes Mellit. 2010;2:179–183. doi: 10.1016/j.ijdm.2010.08.002. [DOI] [Google Scholar]
- 57.Keller K.L., Olsen A., Cravener T.L., Bloom R., Chung W.K., Deng L., Lanzano P., Meyermann K. Bitter taste phenotype and body weight predict children’s selection of sweet and savory foods at a palatable test-meal. Appetite. 2014;77:113–121. doi: 10.1016/j.appet.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao L., Kirkmeyer S.V., Tepper B.J. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol. Behav. 2003;78:625–633. doi: 10.1016/S0031-9384(03)00057-X. [DOI] [PubMed] [Google Scholar]
- 59.Prescott J. Chemosensory learning and flavour: Perception, preference and intake. Physiol. Behav. 2012;107:553–559. doi: 10.1016/j.physbeh.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Poette J., Mekoué J., Neyraud E., Berdeaux O., Renault A., Guichard E., Genot C., Feron G. Fat sensitivity in humans: Oleic acid detection threshold is linked to saliva composition and oral volume. Flavour Fragr. J. 2014;29:39–49. doi: 10.1002/ffj.3177. [DOI] [Google Scholar]
- 61.Yeomans M.R., Prescott J., Gould N.J. Acquired hedonic and sensory characteristics of odours: Influence of sweet liker and propylthiouracil taster status. Q. J. Exp. Psychol. (Hove) 2009;62:1648–1664. doi: 10.1080/17470210802557793. [DOI] [PubMed] [Google Scholar]
- 62.Shahbake M., Hutchinson I., Laing D.G., Jinks A.L. Rapid quantitative assessment of fungiform papillae density in the human tongue. Brain Res. 2005;1052:196–201. doi: 10.1016/j.brainres.2005.06.031. [DOI] [PubMed] [Google Scholar]