Abstract
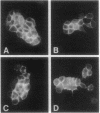
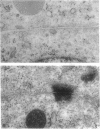
E-cadherin is expressed in both the ZR-75-1-Tx and the ZR-75-1-Ro sublines of ductal breast carcinoma cells and is concentrated at cell-cell borders as shown by immunocytochemical examination. Free cell borders generally show no or little staining. The localized decrease in E-cadherin expression observed after interleukin 6 (IL-6) treatment of either subline correlates with the increase in free cell borders as IL-6 causes cell-cell separation. As we previously reported, many IL-6-treated ZR-75-1-Tx cells round up and detach from the substratum while ZR-75-1-Ro cells remain adherent and display prominent processes. The results are consistent with the view that E-cadherin expression is not responsible for the marked difference in the IL-6-induced phenotypes in these cell lines, although the localized decrease may play a role in cell-cell separation. ZR-75-1-Tx cells are deficient in desmosomes and show a wider intercellular space than ZR-75-1-Ro cells. Alternative mechanisms involving different aspects of the interlinked cytoskeletal and cell adhesion structures are considered to account for the IL-6-induced antimorphogenetic effect.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens J., Mareel M. M., Van Roy F. M., Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989 Jun;108(6):2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Vakaet L., Friis R., Winterhager E., Van Roy F., Mareel M. M., Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993 Feb;120(3):757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Weidner K. M., Frixen U. H., Schipper J. H., Sachs M., Arakaki N., Daikuhara Y., Birchmeier W. The role of E-cadherin and scatter factor in tumor invasion and cell motility. EXS. 1991;59:109–126. doi: 10.1007/978-3-0348-7494-6_8. [DOI] [PubMed] [Google Scholar]
- Boyer B., Dufour S., Thiery J. P. E-cadherin expression during the acidic FGF-induced dispersion of a rat bladder carcinoma cell line. Exp Cell Res. 1992 Aug;201(2):347–357. doi: 10.1016/0014-4827(92)90283-e. [DOI] [PubMed] [Google Scholar]
- Boyer B., Thiery J. P. Cyclic AMP distinguishes between two functions of acidic FGF in a rat bladder carcinoma cell line. J Cell Biol. 1993 Feb;120(3):767–776. doi: 10.1083/jcb.120.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer B., Tucker G. C., Vallés A. M., Franke W. W., Thiery J. P. Rearrangements of desmosomal and cytoskeletal proteins during the transition from epithelial to fibroblastoid organization in cultured rat bladder carcinoma cells. J Cell Biol. 1989 Oct;109(4 Pt 1):1495–1509. doi: 10.1083/jcb.109.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Gundersen G. G. Stabilization of post-translational modification of microtubules during cellular morphogenesis. Bioessays. 1991 Jun;13(6):285–293. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Andreola S., Clemente C., D'Amato L., Rilke F. Vimentin and p53 expression on epidermal growth factor receptor-positive, oestrogen receptor-negative breast carcinomas. Br J Cancer. 1988 Apr;57(4):353–357. doi: 10.1038/bjc.1988.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Shulman L. M., Revel M. IL-6 receptors and sensitivity to growth inhibition by IL-6 in clones of human breast carcinoma cells. J Biol Regul Homeost Agents. 1991 Oct-Dec;5(4):125–136. [PubMed] [Google Scholar]
- Damsky C. H., Richa J., Solter D., Knudsen K., Buck C. A. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983 Sep;34(2):455–466. doi: 10.1016/0092-8674(83)90379-3. [DOI] [PubMed] [Google Scholar]
- Douglas W. H., Elser J. E. A method for in situ embedding of cultured cells grown on plastic surfaces. In Vitro. 1972 Jul-Aug;8(1):26–29. doi: 10.1007/BF02617940. [DOI] [PubMed] [Google Scholar]
- Frixen U. H., Behrens J., Sachs M., Eberle G., Voss B., Warda A., Löchner D., Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991 Apr;113(1):173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Iguchi T., Kawashima T., Bao Z. Z., Yacky C., Boettiger D., Horwitz A. F. Repression of integrin beta 1 subunit expression by antisense RNA. Cell Struct Funct. 1991 Jun;16(3):241–249. doi: 10.1247/csf.16.241. [DOI] [PubMed] [Google Scholar]
- Hirano S., Nose A., Hatta K., Kawakami A., Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987 Dec;105(6 Pt 1):2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukoulis G. K., Virtanen I., Korhonen M., Laitinen L., Quaranta V., Gould V. E. Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991 Oct;139(4):787–799. [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Lin A. N., Leong I., Carter D. M. Junctional epidermolysis bullosa keratinocytes in culture display adhesive, structural, and functional abnormalities. J Invest Dermatol. 1991 Nov;97(5):849–861. doi: 10.1111/1523-1747.ep12491525. [DOI] [PubMed] [Google Scholar]
- Luna E. J., Hitt A. L. Cytoskeleton--plasma membrane interactions. Science. 1992 Nov 6;258(5084):955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Mareel M. M., Van Roy F. M., De Baetselier P. The invasive phenotypes. Cancer Metastasis Rev. 1990 Jul;9(1):45–62. doi: 10.1007/BF00047588. [DOI] [PubMed] [Google Scholar]
- Murakami M., Hibi M., Nakagawa N., Nakagawa T., Yasukawa K., Yamanishi K., Taga T., Kishimoto T. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science. 1993 Jun 18;260(5115):1808–1810. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Botti C., Mottolese M., Bigotti A., Segatto O. Changes in expression of alpha 6/beta 4 integrin heterodimer in primary and metastatic breast cancer. Br J Cancer. 1992 Aug;66(2):318–322. doi: 10.1038/bjc.1992.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro P., Gómez M., Pizarro A., Gamallo C., Quintanilla M., Cano A. A role for the E-cadherin cell-cell adhesion molecule during tumor progression of mouse epidermal carcinogenesis. J Cell Biol. 1991 Oct;115(2):517–533. doi: 10.1083/jcb.115.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M., Cardillo M. R., Hanby A., Stamp G. W. Integrins and their accessory adhesion molecules in mammary carcinomas: loss of polarization in poorly differentiated tumors. Hum Pathol. 1992 Oct;23(10):1159–1166. doi: 10.1016/0046-8177(92)90034-z. [DOI] [PubMed] [Google Scholar]
- Raymond W. A., Leong A. S. Co-expression of cytokeratin and vimentin intermediate filament proteins in benign and neoplastic breast epithelium. J Pathol. 1989 Apr;157(4):299–306. doi: 10.1002/path.1711570406. [DOI] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Neveu M., Liang P., Sotiropoulou G. Identification by differential display of alpha 6 integrin as a candidate tumor suppressor gene. FASEB J. 1993 Jul;7(10):964–970. doi: 10.1096/fasebj.7.10.8344495. [DOI] [PubMed] [Google Scholar]
- Schipper J. H., Frixen U. H., Behrens J., Unger A., Jahnke K., Birchmeier W. E-cadherin expression in squamous cell carcinomas of head and neck: inverse correlation with tumor dedifferentiation and lymph node metastasis. Cancer Res. 1991 Dec 1;51(23 Pt 1):6328–6337. [PubMed] [Google Scholar]
- Schmidt J. W., Piepenhagen P. A., Nelson W. J. Modulation of epithelial morphogenesis and cell fate by cell-to-cell signals and regulated cell adhesion. Semin Cell Biol. 1993 Jun;4(3):161–173. doi: 10.1006/scel.1993.1020. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y., Hirohashi S. Expression of E- and P-cadherin in gastric carcinomas. Cancer Res. 1991 Apr 15;51(8):2185–2192. [PubMed] [Google Scholar]
- Sommers C. L., Walker-Jones D., Heckford S. E., Worland P., Valverius E., Clark R., McCormick F., Stampfer M., Abularach S., Gelmann E. P. Vimentin rather than keratin expression in some hormone-independent breast cancer cell lines and in oncogene-transformed mammary epithelial cells. Cancer Res. 1989 Aug 1;49(15):4258–4263. [PubMed] [Google Scholar]
- Taga T., Kishimoto T. Cytokine receptors and signal transduction. FASEB J. 1992 Dec;6(15):3387–3396. doi: 10.1096/fasebj.6.15.1334470. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Tamm I., Cardinale I., Krueger J., Murphy J. S., May L. T., Sehgal P. B. Interleukin 6 decreases cell-cell association and increases motility of ductal breast carcinoma cells. J Exp Med. 1989 Nov 1;170(5):1649–1669. doi: 10.1084/jem.170.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Cardinale I., Sehgal P. B. Interleukin-6 and 12-O-tetradecanoyl phorbol-13-acetate act synergistically in inducing cell-cell separation and migration of human breast carcinoma cells. Cytokine. 1991 May;3(3):212–223. doi: 10.1016/1043-4666(91)90019-a. [DOI] [PubMed] [Google Scholar]
- Tamm I., Kikuchi T., Cardinale I., Krueger J. G. Cell-adhesion-disrupting action of interleukin 6 in human ductal breast carcinoma cells. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3329–3333. doi: 10.1073/pnas.91.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg T., Zick Y., Dror R., Sabanay I., Gilon C., Levitzki A., Geiger B. The effect of tyrosine-specific protein phosphorylation on the assembly of adherens-type junctions. EMBO J. 1992 May;11(5):1733–1742. doi: 10.1002/j.1460-2075.1992.tb05225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. L., Handel L. M., Nelson W. J. Elevated expression of pp60c-src alters a selective morphogenetic property of epithelial cells in vitro without a mitogenic effect. Mol Cell Biol. 1988 Feb;8(2):632–646. doi: 10.1128/mcb.8.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner K. M., Behrens J., Vandekerckhove J., Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990 Nov;111(5 Pt 1):2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock M. J., Buck C. A., Bechtol K. B., Damsky C. H. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem. 1987 Jul;34(3):187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- Yamashita J., Ogawa M., Nomura K., Matsuo S., Inada K., Yamashita S., Nakashima Y., Saishoji T., Takano S., Fujita S. Interleukin 6 stimulates the production of immunoreactive endothelin 1 in human breast cancer cells. Cancer Res. 1993 Feb 1;53(3):464–467. [PubMed] [Google Scholar]