Abstract
Background:
Renal ischemia-reperfusion (RIR) is the main cause of renal failure. The incidence of RIR injury seems to be gender-related due to female sex hormone; estrogen. This study was designed to investigate the protective role of estrogen against RIR injury in male and ovariectomized female rats.
Methods:
Thirty-nine Wistar rats were used in this study as male and ovariectomized female rats in the sham-operated, RIR, and estradiol-treated plus RIR groups. The RIR was induced by clamping the renal vessels for 45 min and then 24 h of reperfusion. All animals finally were sacrificed for the measurements.
Results:
The serum levels of creatinine and blood urea nitrogen and kidney tissue damage score significantly increased in both male and female RIR rats (P < 0.05). Estradiol however significantly attenuated theses parameters (P < 0.05) toward normal levels in female (P < 0.05), but not in male rats. Kidney weight increased in both genders and estradiol intensified it in the male rats (P < 0.05). Uterus weight was increased by estradiol in female rats (P < 0.05) and testis weight did not alter in male rats.
Conclusions:
Estradiol demonstrated a protective role against RIR injury in female rats; however, estradiol as an antioxidant could not protect the male kidney from RIR injury.
Keywords: Estrogen, gender, rat, renal ischemia-reperfusion
INTRODUCTION
Acute renal failure is an important disturbance in many patients[1] and renal ischemia-reperfusion (RIR) injury is the most common cause of acute renal failure. RIR injury induces renal dysfunction characterized by increasing levels of creatinine (Cr) and blood urea nitrogen (BUN).[2,3,4] In addition, studies have shown that the incidence of renal diseases is gender-related, and males exhibit a more rapid reduction in renal function than females.[5,6] Mature male rats are more vulnerable to RIR injury than female rats, and females have higher survival rates than males.[7] Sex hormones; testosterone and estrogen play an important role in this phenomenon.[8,9,10,11,12,13] Studies on experimental animal models have shown that male and female sex hormones affect renal injury.[13,14,15,16,17,18] In addition, it is reported that estradiol replacement therapy attenuates renal dysfunction in diabetic ovariectomized female rats.[19] Estradiol therapy in brain injury in rats provides a protective effect against ischemia[20,21] and improves spatial learning and memory.[21] In addition, administration of estradiol ameliorates liver dysfunction in aging male rats[22] while estradiol did not protect the kidney against confers protection nephrotoxicity.[23,24,25] To the best of our knowledge, the role of estradiol in RIR injury in males and females was not compared yet. Accordingly, this study was designed to determine whether estradiol could prevent RIR-induced renal injury in male and ovariectomized female rats.
METHODS
Animals
Sixteen adult female (weighing 175 ± 5.2 g) and 19 adult male (weighing 222.4 ± 8.6 g) Wistar rats (Animal Center, Isfahan University of Medical Sciences, Isfahan, Iran) were used in this study. The animals were housed under standard conditions with 12 h light/12 h dark cycle. Prior to the experiment, the protocols were confirmed to be in accordance with the Guidelines of Animal Ethics Committee of the Isfahan University of Medical Sciences.
Ovariectomy surgery
One week before estradiol administration, all female rats were anesthetized, and an incision was made in the abdominal middle line above the urinary outlet. The ovaries were removed, and the skin was sutured.
Experimental protocol
The animals were randomly divided into six experimental groups;
Group 1 (n = 5, the sham-operated group): Male rats received sesame oil (intramuscularly) and after 3 days underwent the surgical procedure without RIR surgery
Group 2 (n = 8): Male rats received regimen the same as group 1 and underwent RIR surgery
Group 3 (n = 6), male rats received single dose of estradiol (500 μg/kg; intramuscularly) and 3 days later underwent RIR surgery
Groups 4, 5, and 6 (n = 5, 6, and 5): Ovariectomized female rats received treatment/regimen the same as groups 1, 2, and 3, respectively.
Renal ischemia-reperfusion surgery
To induce the RIR model, the animals were anesthetized by ketamine (75 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). Two small incisions were made on the flanks and when the kidneys were visible, the kidney arteries and veins on both sides were clamped to stop the renal blood circulation. After 45 min, the clamp was removed for recirculation of blood flow. 24 h after the RIR surgery, all rats were anesthetized again. Blood samples were obtained via heart puncture. Serum samples were removed and stored at −20°C until measurement. Finally, all animals were killed, and kidneys and testes or uterus were removed and weighed immediately. In addition, the left kidney was fixed in 10% formalin solution for pathological assessments.
Measurements
Serum levels of Cr and BUN were measured using quantitative kits (Pars Azmoon, Iran) and automatic analyzer RA-1000 (Technicon, Ireland).
Histopathological procedures
The left kidney was fixed in 10% formalin solution and embedded in paraffin. The tissue slices were stained by hematoxylin and eosin to examine the tissue damage based on the presence of tubular atrophy, hyaline cast, ischemic necrosis, vacuolization, and debris. According to the damage intensity, the samples were scored as 1–4 and 0 was assigned to normal tissue.
Statistical analysis
The data were reported as mean ± standard error of the mean. The uterus, kidney, and testis weights; as well as BUN and Cr levels in the ischemia group were compared with the values obtained for the ischemia plus estradiol group or the sham-operated group using the Student's t-test. To compare the groups with regard to the kidney tissue damage score (KTDS), the Mann–Whitney test was applied. P < 0.05 was considered as statistically significant.
RESULTS
Serum creatinine and blood urea nitrogen levels
Renal ischemia-reperfusion injury increased the serum Cr and BUN levels in both genders (P < 0.05). However, estradiol decreased these parameters toward normal values in female, but not in male rats (P < 0.05) [Figures 1 and 2].
Figure 1.
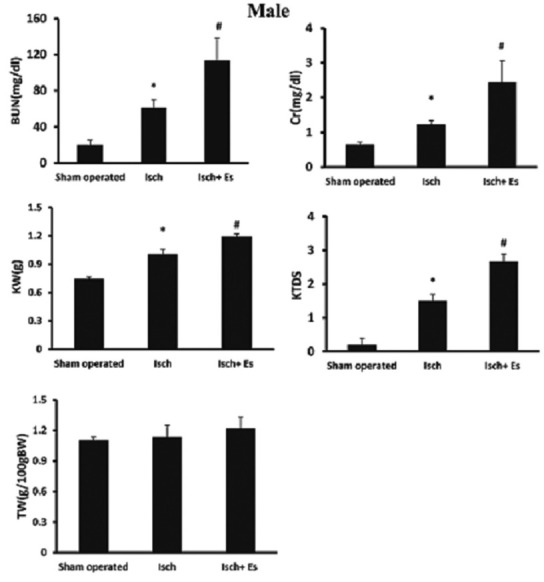
Serum creatinine (Cr) and blood urea nitrogen (BUN) levels, testis weight (TW) and kidney weight (g)/100 g bodyweight (KW), kidney tissue damage score (KTDS) in the male groups. Abbreviations of Isch and Isch + Es were applied for ischemia and ischemia + estradiol. *and# indicates significant differences between the sham-operated and Isch groups, and between the Isch and Isch + estradiol groups, respectively
Figure 2.
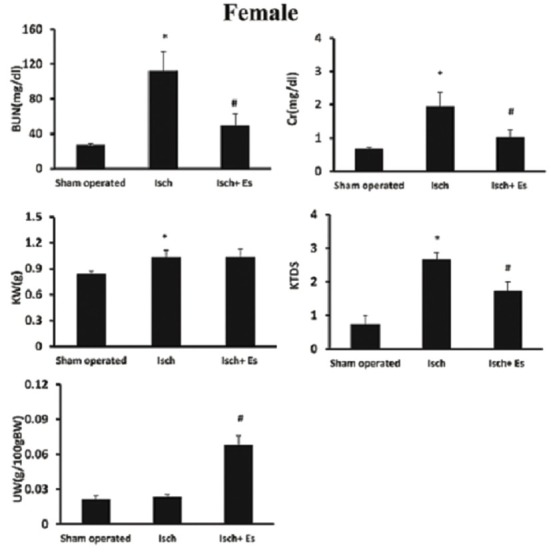
Serum levels of creatinine (Cr) and blood urea nitrogen (BUN) levels, uterus weight (UW) and kidney weight (g)/100 g body weight (KW), kidney tissue damage score (KTDS) in female groups. Abbreviations of Isch and Isch + Es were assigned to ischemia and ischemia + estradiol. *and# indicates significant differences between the sham-operated and Isch groups, and between Isch and Isch + estradiol groups, respectively
Kidney tissue damage score and kidney weight
Renal ischemia-reperfusion increased kidney weight in both genders (P < 0.05) and estradiol intensified kidney weight in male (P < 0.05), but not in female rats. In addition, estradiol ameliorated KTDS induced by RIR in female, but not in male rats (P < 0.05) [Figures 1–3].
Figure 3.
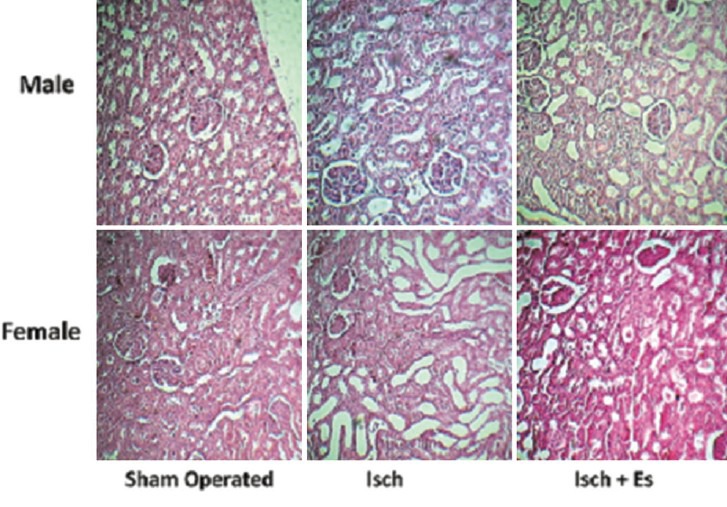
The pathology images (×100) of kidney tissue in the experimental groups. Abbreviations of Isch and Isch + Es were assigned to ischemia and ischemia + estradiol
Testis and uterus weights
Administration of estradiol increased uterus weight (P < 0.05). However, it did not alter the testis weight [Figures 1 and 2].
DISCUSSION
The present study was performed to compare the protective effect of estradiol against RIR-induced renal injury in male and female rats. The results obtained show that RIR induces renal dysfunction in both male and female rats; characterized by increasing serum BUN and Cr levels. These results were in agreement with the findings of other studies.[2,3,26] RIR-induced renal failure increases oxidative stress, inflammation, and apoptosis by increasing the malondialdehyde, interleukines, and tumor necrosis factor-alpha levels, respectively.[27] Estrogen may protect renal and cardiovascular systems by affecting glomerular mesangial cells in the kidney and smooth muscle cells in the vasculature[28] and improve renal and vascular damage induced by renal ischemia in atherosclerotic female mice through antiinflammatory cytokine, interleukine-6.[29] Furthermore, administration of estradiol decreased albuminuria, glomerulosclerosis and tubulointerstitial in spontaneously hypertensive stroke-prone female rats.[30] In addition, the protective effects of estrogen were exhibited via nitric oxide synthase system,[16] antioxidant,[31] and inflammatory[32] properties. It is possible that the estrogen ameliorates renal blood flow diminished by ischemia due to activation of endothelial nitric oxide synthase and nitric oxide production.[16] Estrogen protects kidney against RIR through suppression of endothelin 1 overproduction.[17] Also, it is possible that the presence of progesterone in the female gender plays a role in ameliorating RIR injury. It has been reported that progesterone has neuroprotective effects[33] and in combination with estradiol improves the brain ischemia-induced injury[34,35] and ameliorates bone loss in female rats.[36] In the present study, administration of estradiol not only did not ameliorate kidney dysfunction in male gender, but also intensified it. It is demonstrated that administration of estradiol (10 μg/kg) attenuated renal damage induced by bilateral RIR, while this was not the case at the doses of 20 and 100 μg/kg.[16] In another study, high mortality rate in male pretreated with estradiol was reported, while the course of ischemia-induced renal failure improved.[7] It seems that estradiol administration led to some unusual results[37] with an unknown mechanism. Administration of estradiol also attenuates vascular responses against acetylcholine in women but not men,[38] and estrogen supplementation improves endothelium-related dilation through increasing level of nitrite/nitrate in women, but not in men.[39] Our study showed that normalized kidney weight was increased by RIR injury in both genders. This observation was consistent with the findings of other studies,[2,3,40] which is probably due to edema.[40] Administration of estradiol intensified normalized kidney weight in male gender; confirmed by renal dysfunction and pathological findings. Estradiol also increased normalized uterus weight, confirmed by our recent studies.[23,25] It has been demonstrated that estrogen increases uterus weight through increasing the glucose metabolism, activity of RNA polymerase, DNA content, and finally cell proliferation.[41]
CONCLUSIONS
It was concluded that administration of estradiol could attenuate renal injury induced by RIR in female rats; however, as an antioxidant agent, it was not efficient in preventing renal dysfunction induced by RIR in male rats.
ACKNOWLEDGEMENTS
We appreciate Isfahan University of Medical Sciences for supporting this project.
Footnotes
Source of Support: This research was supported by Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004;66:1613–21. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 2.Azarkish F, Nematbakhsh M, Fazilati M, Talebi A, Pilehvarian AA, Pezeshki Z, et al. N-acetylcysteine prevents kidney and lung disturbances in renal ischemia/reperfusion injury in rat. Int J Prev Med. 2013;4:1139–46. [PMC free article] [PubMed] [Google Scholar]
- 3.Moeini M, Nematbakhsh M, Fazilati M, Talebi A, Pilehvarian AA, Azarkish F, et al. Protective role of recombinant human erythropoietin in kidney and lung injury following renal bilateral ischemia-reperfusion in rat model. Int J Prev Med. 2013;4:648–55. [PMC free article] [PubMed] [Google Scholar]
- 4.Williams P, Lopez H, Britt D, Chan C, Ezrin A, Hottendorf R. Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods. 1997;37:1–7. doi: 10.1016/s1056-8719(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 5.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol. 2000;11:319–29. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 6.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 1995;25:515–33. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 7.Müller V, Losonczy G, Heemann U, Vannay A, Fekete A, Reusz G, et al. Sexual dimorphism in renal ischemia-reperfusion injury in rats: Possible role of endothelin. Kidney Int. 2002;62:1364–71. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- 8.Antus B, Yao Y, Song E, Liu S, Lutz J, Heemann U. Opposite effects of testosterone and estrogens on chronic allograft nephropathy. Transpl Int. 2002;15:494–501. doi: 10.1007/s00147-002-0449-2. [DOI] [PubMed] [Google Scholar]
- 9.Baltatu O, Cayla C, Iliescu R, Andreev D, Jordan C, Bader M. Abolition of hypertension-induced end-organ damage by androgen receptor blockade in transgenic rats harboring the mouse ren-2 gene. J Am Soc Nephrol. 2002;13:2681–7. doi: 10.1097/01.asn.0000033327.65390.ca. [DOI] [PubMed] [Google Scholar]
- 10.Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, et al. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50:1173–9. doi: 10.1038/ki.1996.425. [DOI] [PubMed] [Google Scholar]
- 11.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol. 2004;15:1546–56. doi: 10.1097/01.asn.0000128219.65330.ea. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen CB, Flyvbjerg A, Bruun JM, Forman A, Wogensen L, Thomsen K. Decreases in renal functional reserve and proximal tubular fluid output in conscious oophorectomized rats: Normalization with sex hormone substitution. J Am Soc Nephrol. 2003;14:3102–10. doi: 10.1097/01.asn.0000096262.18679.25. [DOI] [PubMed] [Google Scholar]
- 13.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279:52282–92. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 14.Hutchens MP, Nakano T, Kosaka Y, Dunlap J, Zhang W, Herson PS, et al. Estrogen is renoprotective via a nonreceptor-dependent mechanism after cardiac arrest in vivo. Anesthesiology. 2010;112:395–405. doi: 10.1097/ALN.0b013e3181c98da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park KM, Cho HJ, Bonventre JV. Orchiectomy reduces susceptibility to renal ischemic injury: A role for heat shock proteins. Biochem Biophys Res Commun. 2005;328:312–7. doi: 10.1016/j.bbrc.2004.12.177. [DOI] [PubMed] [Google Scholar]
- 16.Satake A, Takaoka M, Nishikawa M, Yuba M, Shibata Y, Okumura K, et al. Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int. 2008;73:308–17. doi: 10.1038/sj.ki.5002690. [DOI] [PubMed] [Google Scholar]
- 17.Takaoka M, Yuba M, Fujii T, Ohkita M, Matsumura Y. Oestrogen protects against ischaemic acute renal failure in rats by suppressing renal endothelin-1 overproduction. Clin Sci (Lond) 2002;103(Suppl 48):434S–7. doi: 10.1042/CS103S434S. [DOI] [PubMed] [Google Scholar]
- 18.Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exp Pharmacol Physiol. 1999;26:127–31. doi: 10.1046/j.1440-1681.1999.02996.x. [DOI] [PubMed] [Google Scholar]
- 19.Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F399–405. doi: 10.1152/ajprenal.00195.2004. [DOI] [PubMed] [Google Scholar]
- 20.Wise PM, Dubal DB. Estradiol protects against ischemic brain injury in middle-aged rats. Biol Reprod. 2000;63:982–5. doi: 10.1095/biolreprod63.4.982. [DOI] [PubMed] [Google Scholar]
- 21.Raval AP, Borges-Garcia R, Javier Moreno W, Perez-Pinzon MA, Bramlett H. Periodic 17ß-estradiol pretreatment protects rat brain from cerebral ischemic damage via estrogen receptor-ß. PLoS One. 2013;8:e60716. doi: 10.1371/journal.pone.0060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamden K, Carreau S, Ellouz F, Masmoudi H, El FA. Protective effect of 17beta-estradiol on oxidative stress and liver dysfunction in aged male rats. J Physiol Biochem. 2007;63:195–201. doi: 10.1007/BF03165782. [DOI] [PubMed] [Google Scholar]
- 23.Pezeshki Z, Nematbakhsh M, Nasri H, Talebi A, Pilehvarian AA, Safari T, et al. Evidence against protective role of sex hormone estrogen in Cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol Int. 2013;20:43–7. doi: 10.4103/0971-6580.111568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pezeshki Z, Nematbakhsh M, Mazaheri S, Eshraghi-Jazi F, Talebi A, Nasri H, et al. Estrogen abolishes protective effect of erythropoietin against cisplatin-induced nephrotoxicity in ovariectomized rats. ISRN Oncol 2012. 2012 doi: 10.5402/2012/890310. 890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nematbakhsh M, Pezeshki Z, Eshraghi-Jazi F, Ashrafi F, Nasri H, Talebi A, et al. Vitamin E, Vitamin C, or Losartan is not nephroprotectant against cisplatin-induced nephrotoxicity in presence of estrogen in ovariectomized rat model. Int J Nephrol 2012. 2012 doi: 10.1155/2012/284896. 284896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prókai A, Fekete A, Bánki NF, Müller V, Vér A, Degrell P, et al. Renoprotective effect of erythropoietin in rats subjected to ischemia/reperfusion injury: Gender differences. Surgery. 2011;150:39–47. doi: 10.1016/j.surg.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Kucuk A, Kabadere S, Tosun M, Koken T, Kinaci MK, Isikli B, et al. Protective effects of doxycycline in ischemia/reperfusion injury on kidney. J Physiol Biochem. 2009;65:183–91. doi: 10.1007/BF03179069. [DOI] [PubMed] [Google Scholar]
- 28.Dubey RK, Jackson EK. Estrogen-induced cardiorenal protection: Potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol. 2001;280:F365–88. doi: 10.1152/ajprenal.2001.280.3.F365. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Ji H, Holloway C, Sandberg K, Ecelbarger C. Effects of 17 β-estradiol replacement in a model of renal ischemia in the ovariectomized female apolipoprotein E knockout mouse (1135.2) FASEB J. 2014;28(1 Suppl):1135–2. [Google Scholar]
- 30.Gross ML, Adamczak M, Rabe T, Harbi NA, Krtil J, Koch A, et al. Beneficial effects of estrogens on indices of renal damage in uninephrectomized shrsp rats. J Am Soc Nephrol. 2004;15:348–58. doi: 10.1097/01.asn.0000105993.63023.d8. [DOI] [PubMed] [Google Scholar]
- 31.Mooradian AD. Antioxidant properties of steroids. J Steroid Biochem Mol Biol. 1993;45:509–11. doi: 10.1016/0960-0760(93)90166-t. [DOI] [PubMed] [Google Scholar]
- 32.Josefsson E, Tarkowski A, Carlsten H. Anti-inflammatory properties of estrogen. I. In vivo suppression of leukocyte production in bone marrow and redistribution of peripheral blood neutrophils. Cell Immunol. 1992;142:67–78. doi: 10.1016/0008-8749(92)90269-u. [DOI] [PubMed] [Google Scholar]
- 33.Moralí G, Letechipía-Vallejo G, López-Loeza E, Montes P, Hernández-Morales L, Cervantes M. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci Lett. 2005;382:286–90. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 34.Ozacmak VH, Sayan H. The effects of 17beta estradiol, 17alpha estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol Res. 2009;58:909–12. doi: 10.33549/physiolres.931647. [DOI] [PubMed] [Google Scholar]
- 35.Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24:1160–6. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 36.Kalu DN, Salerno E, Liu CC, Echon R, Ray M, Garza-Zapata M, et al. A comparative study of the actions of tamoxifen, estrogen and progesterone in the ovariectomized rat. Bone Miner. 1991;15:109–23. doi: 10.1016/0169-6009(91)90002-h. [DOI] [PubMed] [Google Scholar]
- 37.Oestreicher EM, Guo C, Seely EW, Kikuchi T, Martinez-Vasquez D, Jonasson L, et al. Estradiol increases proteinuria and angiotensin II type 1 receptor in kidneys of rats receiving L-NAME and angiotensin II. Kidney Int. 2006;70:1759–68. doi: 10.1038/sj.ki.5001897. [DOI] [PubMed] [Google Scholar]
- 38.Collins P, Rosano GM, Sarrel PM, Ulrich L, Adamopoulos S, Beale CM, et al. 17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995;92:24–30. doi: 10.1161/01.cir.92.1.24. [DOI] [PubMed] [Google Scholar]
- 39.Kawano H, Motoyama T, Kugiyama K, Hirashima O, Ohgushi M, Fujii H, et al. Gender difference in improvement of endothelium-dependent vasodilation after estrogen supplementation. J Am Coll Cardiol. 1997;30:914–9. doi: 10.1016/s0735-1097(97)00234-9. [DOI] [PubMed] [Google Scholar]
- 40.Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute renal failure: Long-term histology of cell and matrix changes in the rat. Kidney Int. 2000;57:2375–85. doi: 10.1046/j.1523-1755.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 41.Murphy LJ, Ghahary A. Uterine insulin-like growth factor-1: Regulation of expression and its role in estrogen-induced uterine proliferation. Endocr Rev. 1990;11:443–53. doi: 10.1210/edrv-11-3-443. [DOI] [PubMed] [Google Scholar]


