Abstract
Acute pulmonary embolism is a substantial cause of morbidity and death. Although the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines recommend surgical pulmonary embolectomy in patients with acute pulmonary embolism associated with hypotension, there are few reports of 30-day mortality rates.
We performed a retrospective review of acute pulmonary embolectomy procedures performed in 96 consecutive patients who had severe, globally hypokinetic right ventricular dysfunction as determined by transthoracic echocardiography. Data on patients who were treated from January 2003 through December 2011 were derived from health system databases of the New York State Cardiac Surgery Reporting System and the Society of Thoracic Surgeons. The data represent procedures performed at 3 tertiary care facilities within a large health system operating in the New York City metropolitan area.
The overall 30-day mortality rate was 4.2%. Most patients (68 [73.9%]) were discharged home or to rehabilitation facilities (23 [25%]). Hemodynamically stable patients with severe, globally hypokinetic right ventricular dysfunction had a 30-day mortality rate of 1.4%, with a postoperative mean length of stay of 9.1 days. Comparable findings for hemodynamically unstable patients were 12.5% and 13.4 days, respectively.
Acute pulmonary embolectomy can be a viable procedure for patients with severe, globally hypokinetic right ventricular dysfunction, with or without hemodynamic compromise; however, caution is warranted. Our outcomes might be dependent upon institutional capability, experience, surgical ability, and careful patient selection.
Keywords: Pulmonary embolism/mortality/surgery, retrospective studies, treatment outcome
Although pulmonary embolectomies have been attempted since Trendelenburg first proposed their use in 1908, Steenburg and colleagues in 1958 were first to perform a successful traditional embolectomy in the United States.1,2 This procedure has been associated with excessive mortality rates, persistent right heart failure, intra-alveolar and interstitial pulmonary edema, and massive parenchymal and intrabronchial hemorrhage.1–3 It is not surprising, then, that the mainstay treatment in recent times has been nonsurgical therapy.
Although most studies show historically high mortality rates (32% on the basis of pooled data from 30 studies and 1,047 patients from 1961 through 1984), recent increases in the effectiveness of surgical techniques have resulted in lower mortality rates for pulmonary embolectomies.4,5 For example, a surgical approach described by Aklog and colleagues6 resulted in an 11% mortality rate among 29 patients, over a 3-year period of study. All patients had anatomically extensive embolism and moderate-to-severe right ventricular (RV) dysfunction. Other investigators have shown similar results. For example, Lehnert and associates, in 33 patients who underwent embolectomy, achieved a 30-day mortality rate of 6% and a 12-year survival rate of 80%.7 Kadner and coworkers also produced a low mortality rate of just 8% in 25 patients over a 7-year period.8 Schoepf and colleagues' study of patients with RV enlargement showed a 30-day mortality rate of 15.6%, compared with 7.7% when RV enlargement was not present.9
The American College of Chest Physicians Evidence-Based Clinical Practice Guidelines recommend surgical pulmonary embolectomy in patients with acute pulmonary embolism (PE) associated with hypotension, if 1) patients have contraindications to thrombolysis; 2) patients have undergone failure of thrombolysis or catheter-assisted embolectomy; or 3) patients are in a state of shock that is likely to result in death before thrombolysis can take effect; and 4) the surgeon possesses appropriate expertise, and obtainable or accessible resources are ready for use.10 Despite the above recommendation from the American College of Chest Physicians, surgical pulmonary embolectomy in patients with acute PE associated with hypotension is still less frequently used than is thrombolytic therapy, and there are few reports of 30-day mortality rates.
The purpose of this study is to describe our experience over a 9-year period with acute-PE patients who presented with severe (life-threatening), globally hypokinetic RV dysfunction, as determined by transthoracic echocardiography (TTE). We herein provide preliminary descriptive analysis of early pulmonary embolectomy in hemodynamically stable and unstable patients who presented with acute pulmonary embolus causative of RV compromise.
Patients and Methods
This study was conducted in compliance with the human-study guidelines of the authors' institutions and in compliance with U.S. Food and Drug Administration guidelines. The protocol was approved by the institutional review board (IRB #11-342B) and was granted a waiver of informed consent. We retrospectively studied the cases of 96 consecutive patients with acute, centrally located pulmonary embolus, and severe, globally hypokinetic RV dysfunction who had undergone pulmonary embolectomy from January 2003 through December 2011. This reflected the experience of 3 tertiary care facilities within a large health system operating in the New York City metropolitan area. The experience of each surgeon performing surgical embolectomies at our institutions varies; of the 13 surgeons whose data were included, at least 8 had more than 15 years of experience. Catheter-based embolectomy is typically not performed at our institutions. Data were derived from health system databases of the New York State Cardiac Surgery Reporting System and the Society of Thoracic Surgeons.
Outcome Variables
The major outcome variable was hospital death, defined as death within 30 days of the procedure or death during the index hospitalization (beyond 30 days, if still hospitalized). Other outcome variables included postoperative length of stay (LOS), discharge location, readmission status, and postoperative complications.
Diagnostic and Therapeutic Algorithm
All patients were evaluated for RV dysfunction, by means of TTE. Indications for surgery included severe, globally hypokinetic RV dysfunction on TTE, and computed-tomographic-angiography (CTA)–documented PE, with either a large clot burden in the main pulmonary arteries (PAs) or a saddle embolism. This is consistent with the 2014 definition of intermediate-risk PE.11
No patients in this group had a history of chronic pulmonary thromboembolic disease or evidence of chronic disease on CTA. All TTEs were completed by attending echocardiologists. The following measurements were typically taken: RV size in the 4-chamber view (RV enlargement was defined as a ratio of RV diameter to left ventricular diameter of >0.9), presence of tricuspid regurgitation, systolic PA pressure, tricuspid annular plane systolic excursion, and septal shift. In addition, patients with McConnell's sign or abnormal right atrial size were evaluated.
Most of the patients were transferred from outlying hospitals or from other in-hospital services, with a completed diagnostic CTA scan of the chest accompanied by intravenous administration of contrast material during the venous phase. For the few who were transferred without such a scan, one was obtained soon after transfer. Immediately after a CTA revealed main PA or saddle PA embolism, heparin therapy was initiated and a TTE was obtained to detect and evaluate RV strain or compromise.
All patients who underwent pulmonary embolectomy had either a saddle embolus or an obstructing left or right main PA embolus with severe, globally hypokinetic RV strain. All the patients who underwent an embolectomy did so within one hour. Pulmonary embolectomy was performed through a primary sternal splitting incision or partial upper sternotomy, with use of ascending aortic cardiopulmonary bypass (CPB) and either dual-stage venous CPB or separate superior and inferior caval cannulation. All were done in patients with a beating heart at normothermia. We also have routinely used pericardial carbon dioxide insufflation to avoid pulmonary air entrapment and the release of endothelium-derived cytokines, which can contribute to “leaky” endothelium and noncardiogenic pulmonary edema.12–16 Our approach was to avoid ischemic injury to a stunned RV, keeping the right side of the heart unloaded and well-perfused, and avoiding blind instrumentation of fragile PAs. A pulmonary arteriotomy and clot extraction was performed under direct vision with the use of gallstone forceps, sponge ring forceps, or both.
On CPB with the right side of the heart decompressed, the PA was double-looped with use of silastic vascular tape proximal to the bifurcation; then traction was applied. The PA was incised longitudinally from its confluence to just above the pulmonary valve (within 1 to 2 cm of the valve). The clot was retrieved with use of a common duct basket forceps and suction tip. When all large fragments had been removed, the PAs were flushed with saline solution and the pulmonary arteriotomy was closed with use of fine Prolene suture. Temporary pacing wires and mediastinal drains were placed. The wound was closed in a normal fashion.
Statistical Analysis
Rates were computed by means of standard methods for estimating proportions and by applying exact 95% confidence intervals. Otherwise, simple descriptive statistics were given. Comparisons of hemodynamically stable versus unstable patients were made by means of the Fisher exact test for categorical data and the Mann-Whitney test for continuous data. A P value of less than 0.05 was considered statistically significant. Data analysis was conducted with the use of SAS software version 9.2 (SAS Institute, Inc.; Cary, NC).
Results
Results for Entire Cohort
Our sample consisted of 96 patients. Seventy-two patients (75%) were stable (systolic blood pressure, >90 mmHg), and 24 patients (25%) were unstable (systolic blood pressure, <90 mmHg) and therefore placed on vasopressors. For the 3 facilities in total, 43 patients (44.8%) had contraindications to tissue plasminogen activator (tPA). All patients had severe, globally hypokinetic RV dysfunction on preoperative bedside TTE and almost all (93 patients [96.9%]) had severe RV enlargement. The average grade of tricuspid regurgitation was 1.85/4 and the average estimated pulmonary systolic pressure was 58.4 mmHg. All patients had saddle or bilateral main PA emboli on CTA and exhibited severe, globally hypokinetic RV strain and dysfunction, as determined by TTE and as confirmed by transesophageal echocardiography in the operating room. One patient experienced, in transit, emboli in the RV cavity, and 5 patients (5.2%) went on to surgery after having received tPA preoperatively, because they showed no improvement in RV strain. Seven patients (7.2%) needed repeat intervention: 2 (2.1%) for recurring PE after inferior vena cava filter placement and 5 (5.2%) for symptomatic pericardial effusion.
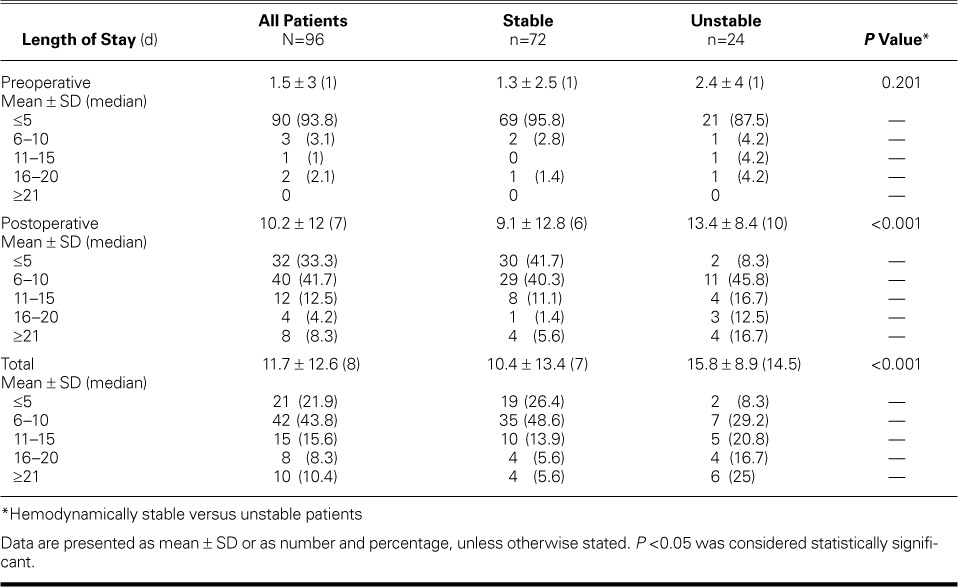
Sixty patients were male (62.5%) and 36 were female (37.5%) (Table I). The mean age at admission was 57.7 ± 14.5 years (median age, 59.3 yr); 53 (55.2%) were younger than 60 years of age. Seventy-two were white (75%) and 92 were non-Hispanic (95.8%). The total mean LOS was 11.7 ± 12.6 days (median, 8 d) (Table II). The postoperative mean LOS was 10.2 ± 12 days (median, 7 d). Although we cannot surmise that every patient with a LOS of 0 came to the emergency department, we determined on the basis of admission and surgery dates that 42 of these 96 patients (44%) had a LOS before surgery of 0 days. This is a conservative estimate of how many patients were referred from the emergency department or were transferred from another institution.
TABLE I.
Characteristics of the 96 Patients
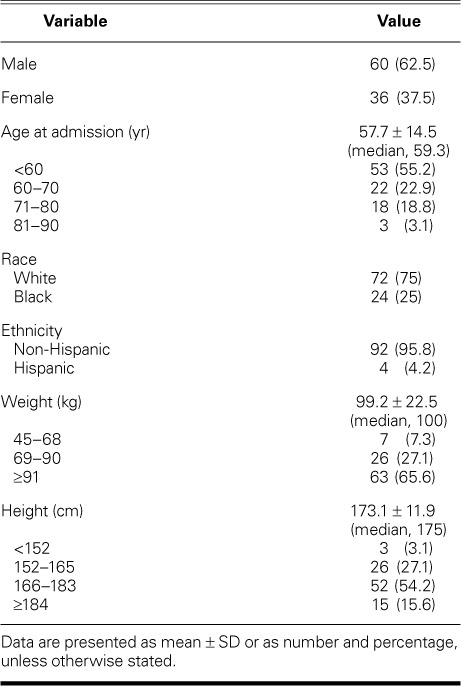
TABLE II.
Lengths of Stay for All Patients and for Hemodynamically Stable versus Unstable Patients
The 30-day mortality rate was 4.2% (95% confidence interval, 1.1%–10.3%); 4 of the 96 patients died (none in facility 1, 3 in facility 2, and 1 in facility 3). Sixty-eight patients were discharged home (73.9%) and 23 to rehabilitation facilities (25%) (Table III). The total mean time on the ventilator, postoperatively, was 32.9 ± 81 hours, and the total mean CPB time was 38.5 ± 23.8 minutes.
TABLE III.
Outcomes for All Patients and for Preoperatively Hemodynamically Stable versus Unstable Patients
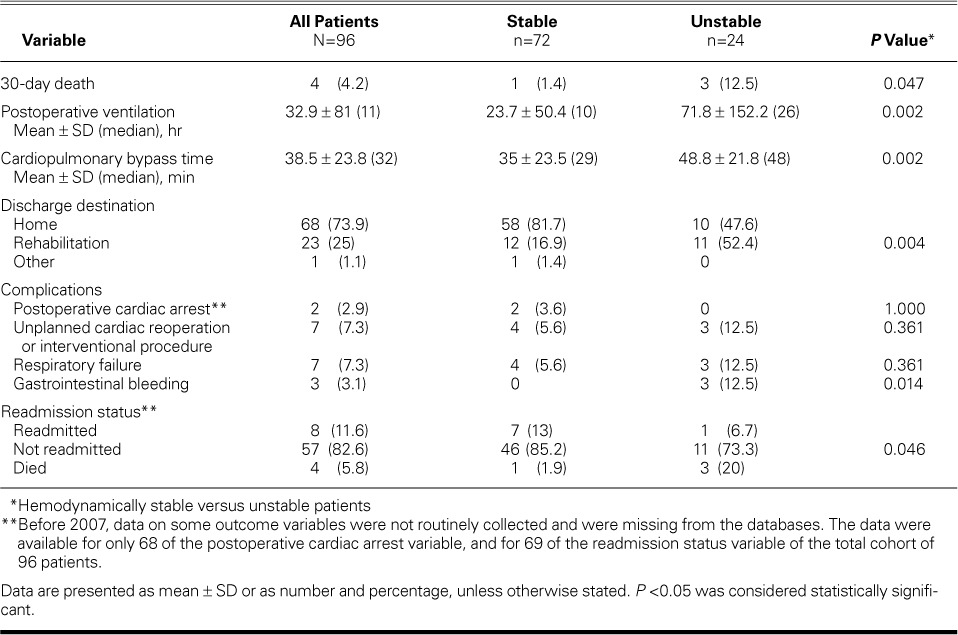
Overall, there were few complications: 2 patients (2.9%) had a postoperative cardiac arrest, 7 (7.3%) had to undergo an unplanned cardiac reoperation or interventional procedure, 7 (7.3%) had respiratory failure as defined by mechanical ventilation time >48 hours, and 3 (3.1%) experienced gastrointestinal bleeding. With regard to readmission, 8 patients (11.6%) were readmitted; 4 (50%) of those readmissions were secondary to pericardial effusions and took the form of hemopericardium—most likely caused by aggressive early oral anticoagulation or by systemic intravenous anticoagulation begun early in the perioperative period.
Comparison between Hemodynamically Stable and Unstable Patients
We define unstable patients as those requiring vasopressors to compensate for systolic blood pressures of <90 mmHg. Given that unstable patients have usually had high morbidity and mortality rates, whether with surgery or with tPA administration, we looked specifically at outcomes among this high-risk population. As seen in Table II, hemodynamically stable patients had significantly lower postoperative and total lengths of stay (both P <0.001) than did unstable patients. Unstable patients had significantly longer CPB time and postoperative total ventilator hours (both P=0.002) than did stable patients (Table III). There was a significant relationship between the stability of patients and the discharge destination, with stable patients more likely to be discharged home (P=0.004).
Discussion
Overall, our findings suggest that pulmonary embolectomy is a viable approach to consider as part of the treatment repertoire. Meyer and colleagues' recent randomized double-blinded study11 of fibrinolytic therapy in intermediate-risk PE patients who were evaluated by means of echocardiography or computed tomography found that although fibrinolysis prevents hemodynamic decompensation, it increases the risk of major hemorrhage and stroke. It is our hypothesis that patients with contraindications to thrombolytic use in this intermediate-risk group can benefit from surgical embolectomy. Our experience suggests that the appropriateness of surgery for acute PE revolves around 3 important principles: the significance of RV dysfunction on TTE or CTA, the presence of contraindications for thrombolytic therapy, and the safety of the surgical procedure being offered.
As we say above, the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines10 recommend surgical pulmonary embolectomy in patients with acute PE that is associated with hypotension; however, this procedure is still not frequently used and there are few reports of the 30-day mortality rate.
The results of our study support Aklog and colleagues' previous findings that pulmonary embolectomy can be performed with low mortality rates.6 Schoepf and associates showed a 30-day mortality rate of 15.6% for patients with RV enlargement; patients without RV enlargement had a mortality rate of 7.7%.9 Our overall mortality rate of 4 (4.2%) is lower than those of previous studies, in which patients were treated with embolectomy or thrombolytic agents.
Limitations of the Study
Our study is not without limitations. The retrospective design used in this study relies on the accuracy of written records, and missing or unavailable data could have affected the results. In addition, there was no comparison group of medically treated patients. For these reasons, we cannot definitively state, on the basis of our evidence, that an early pulmonary embolectomy is a safe and effective procedure. It is also important to take into account the extent to which our results are dependent on the capability of our institutions, the extensive experience and ability of our surgeons, and careful patient selection.
Conclusion
Early pulmonary embolectomy in patients presenting with acute PE—manifested by severe, globally hypokinetic RV dysfunction, as determined initially by means of TTE—might be a viable alternative to thrombolytic therapy, but caution is warranted. We must continue, through large-scale studies, to identify factors that can influence the outcome of this potentially life-saving procedure.
Footnotes
From: Hofstra North Shore-LIJ School of Medicine (Drs. Glassman, Graver, Hall, Hartman, Kalimi, Lessen, Manetta, Palazzo, Pekmezaris, Pogo, Scheinerman, Singh, Vatsia, and Yu, and Ms Jahn), Hempstead, New York 11550; Department of Medicine (Drs. Kozikowski and Pekmezaris), North Shore-LIJ Health System, Great Neck, New York 11021; and the Feinstein Institute for Medical Research, Biostatistics Unit (Drs. Lesser and Pekmezaris, and Ms Akerman), Manhasset, New York 11030
References
- 1.Steenburg RW, Warren R, Wilson RE, Rudolf LE. A new look at pulmonary embolectomy. Surg Gynecol Obstet. 1958;107(2):214–20. [PubMed] [Google Scholar]
- 2.Doerge H, Schoendube FA, Voss M, Seipelt R, Messmer BJ. Surgical therapy of fulminant pulmonary embolism: early and late results. Thorac Cardiovasc Surg. 1999;47(1):9–13. doi: 10.1055/s-2007-1013100. [DOI] [PubMed] [Google Scholar]
- 3.Meyer G, Tamisier D, Sors H, Stern M, Vouhe P, Makowski S et al. Pulmonary embolectomy: a 20-year experience at one center. Ann Thorac Surg. 1991;51(2):232–6. doi: 10.1016/0003-4975(91)90792-o. [DOI] [PubMed] [Google Scholar]
- 4.Anyanwu AC, Aklog L. Surgical pulmonary embolectomy. In: Konstantinides SV, editor. Management of acute pulmonary embolism. Totowa (NJ): Humana Press, Inc.; 2007. pp. 147–60. editor. p. [Google Scholar]
- 5.Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol. 2007;99(3):421–3. doi: 10.1016/j.amjcard.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Aklog L, Williams CS, Byrne JG, Goldhaber SZ. Acute pulmonary embolectomy: a contemporary approach. Circulation. 2002;105(12):1416–9. doi: 10.1161/01.cir.0000012526.21603.25. [DOI] [PubMed] [Google Scholar]
- 7.Lehnert P, Moller CH, Carlsen J, Grande P, Steinbruchel DA. Surgical treatment of acute pulmonary embolism--a 12-year retrospective analysis. Scand Cardiovasc J. 2012;46(3):172–6. doi: 10.3109/14017431.2011.642811. [DOI] [PubMed] [Google Scholar]
- 8.Kadner A, Schmidli J, Schonhoff F, Krahenbuhl E, Immer F, Carrel T, Eckstein F. Excellent outcome after surgical treatment of massive pulmonary embolism in critically ill patients. J Thorac Cardiovasc Surg. 2008;136(2):448–51. doi: 10.1016/j.jtcvs.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276–80. doi: 10.1161/01.CIR.0000147612.59751.4C. [DOI] [PubMed] [Google Scholar]
- 10.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines [published erratum appears in Chest 2012;142(6):1698–704] Chest. 2012;141(2 Suppl):e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–11. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 12.Huang KL, Lin YC. Activation of complement and neutrophils increases vascular permeability during air embolism. Aviat Space Environ Med. 1997;68(4):300–5. [PubMed] [Google Scholar]
- 13.Kuhn M, Fitting JW, Leuenberger P. Acute pulmonary edema caused by venous air embolism after removal of a subclavian catheter. Chest. 1987;92(2):364–5. doi: 10.1378/chest.92.2.364. [DOI] [PubMed] [Google Scholar]
- 14.Fitchet A, Fitzpatrick AP. Central venous air embolism causing pulmonary oedema mimicking left ventricular failure. BMJ. 1998;316(7131):604–6. doi: 10.1136/bmj.316.7131.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boer WH, Hene RJ. Lethal air embolism following removal of a double lumen jugular vein catheter. Nephrol Dial Transplant. 1999;14(8):1850–2. doi: 10.1093/ndt/14.8.1850. [DOI] [PubMed] [Google Scholar]
- 16.Spagnolo S, Grasso MA, Tesler UF. Retrograde pulmonary perfusion improves results in pulmonary embolectomy for massive pulmonary embolism. Tex Heart Inst J. 2006;33(4):473–6. [PMC free article] [PubMed] [Google Scholar]


