Abstract
We report the successful implantation of a HeartMate II left ventricular assist device after a failed Fontan procedure in a patient with dextro-transposition of the great arteries. The patient had developed significant intrapulmonary arteriovenous shunting. Despite the theoretical risk of worsening intrapulmonary shunting due to the decrease in systemic vascular resistance after device implantation, our patient did well. He was discharged from the hospital in stable condition and had better oxygen saturation than before the device was implanted. To our knowledge, ours is the 2nd report of the use of a ventricular assist device after the failure of a Fontan procedure, and the first report concerning the effect of ventricular assist device implantation on intrapulmonary shunting.
Keywords: Arteriovenous shunt/etiology, cardiac surgical procedures/adverse effects, Fontan procedure, heart-assist devices, pulmonary circulation, recovery of function, transposition of great vessels/complications/physiopathology/surgery, treatment outcome, ventricular dysfunction/etiology
Transposition of the great arteries (TGA), which has a prevalence of 1 in 4,000 live births, is one of the most frequently diagnosed congenital cyanotic lesions.1 After birth, the discordant atrioventricular connection creates parallel pulmonary and systemic circulations, and survival is dependent on the intracardiac mixing of blood.2 In dextro-transposition, the pulmonary conus is absent and the subaortic conus does not resorb, resulting in the aorta's arising from the right ventricle, because it is positioned too anterior and rightward of the pulmonary artery. This condition is often associated with ventricular septal defect and pulmonary stenosis or atresia.
Surgical techniques that have been developed to improve outcomes in patients with TGA include pulmonary artery ligation, the Glenn and Mustard operations, and, for more complex intracardiac lesions (as in our patient), the Fontan procedure. Regardless of the surgical technique used to palliate TGA, patients can develop symptoms of congestive heart failure later in life from various causes, but mainly from failure of the systemic ventricle.3,4 The prevalence of these complications is higher for patients in whom TGA is associated with other intracardiac lesions.4 Medical therapy might be effective in managing symptoms for a period of time. In some cases, another palliative procedure may be performed. When medical and surgical therapies fail, the only options for patients are mechanical circulatory support and cardiac transplantation. We report the case of a patient who presented with decompensated dextro-TGA (D-TGA) with systemic desaturation and a previous Fontan operation. The only treatment option to support the patient safely and effectively was to implant a left ventricular assist device (LVAD).
Case Report
In 2011, a 23-year-old man was admitted to our hospital with decompensated heart failure. He had a history of D-TGA, type I inlet ventricular septal defect, atrial septal defect, a large atrioventricular canal defect, and subpulmonic stenosis. He had previously undergone placement of an aortic–pulmonary shunt as an infant and a Glenn anastomosis at about 3 years of age. At the age of 5 years, he underwent a modified nonfenestrated Fontan procedure with placement of a right atrial-to-pulmonary artery conduit.
Shortly after his hospital admission, the patient developed cardiogenic shock and had to be intubated for severe respiratory distress. Echocardiograms revealed interval worsening of the patient's systemic ventricular ejection fraction (Fig. 1). Angiograms showed acquired pulmonary arteriovenous (AV) malformations, which were a result of the cavopulmonary (Glenn and Fontan) shunt. Hemodynamic evaluation revealed equalized pressure in both ventricles (Fig. 2); the morphologic left ventricle mainly generated the systolic pressure, whereas diastolic filling occurred through the Fontan circulation back to the morphologic left atrium. The results of cardiac catheterization showed that the Fontan conduit was unobstructed, and there was no evidence of baffle obstruction or leaks.
Fig. 1.
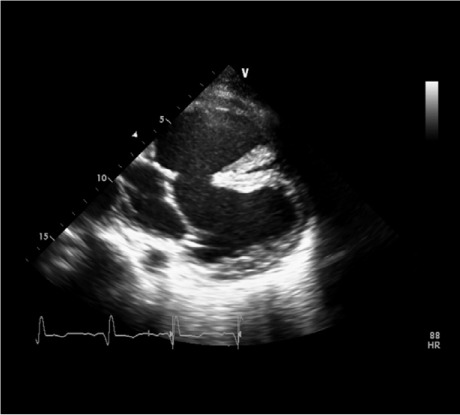
Echocardiogram shows the complex anatomy and the position of the inlet cannula after its insertion into the anterior ventricle.
Fig. 2.
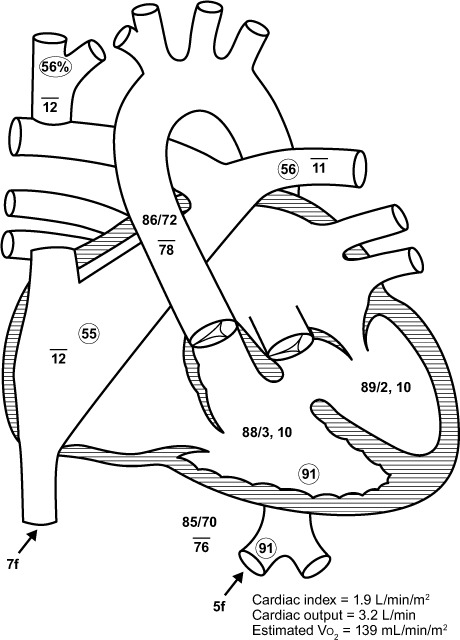
Diagram of the heart with hemodynamic data shows equalized pressure in both ventricles.
Vo2 = oxygen consumption
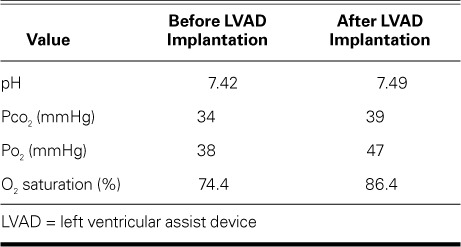
The AV malformations played a substantial role in the patient's systemic desaturation. His hospital stay was complicated by multisystem organ failure. Because the main outlet for both ventricles was the aorta arising from the right ventricle, the LVAD was implanted into the main systemic ventricle—the morphologic left ventricle in our patient. (Because of his many comorbidities and the large size of the AV canal defect, biventricular repair was not attempted.) In July 2011, the patient underwent HeartMate II® implantation (Thoratec Corporation; Pleasanton, Calif), even though there was concern that his pulmonary AV shunting might worsen after device implantation. He uneventfully recovered, was discharged from the hospital in stable condition, and had better oxygen saturation than before the device was implanted (Table I). Thereafter, he underwent LVAD support as an outpatient and was monitored regularly in our clinic.
TABLE I.
Arterial Blood Sampling Before and After LVAD Implantation
In September 2014, the patient underwent orthotopic heart transplantation, immediately after sustaining a stroke while on extracorporeal membrane oxygenation support. As of January 2015, he was living at home and was physically capable but had some residual neurologic effects from the stroke.
Discussion
Multiple cases of successful HeartMate II implantation have been reported in patients with D-TGA, a history of a Mustard procedure, or congestive heart failure from systemic right ventricular dysfunction.5–7 Here, we report the successful implantation of a HeartMate II LVAD in a patient with D-TGA after a Fontan operation who had developed significant intrapulmonary AV shunting. Despite the theoretical risk of worsening the shunting because of the decrease in systemic vascular resistance after HeartMate II LVAD implantation, our patient did well.
Survival rates after surgical repair for D-TGA are generally good, and severe complications, such as supra-ventricular tachycardia or failing Fontan circulation, are more likely to occur late—up to 20 years after correction.8 Diffuse pulmonary AV fistulas, another potentially fatal complication, can develop after cavopulmonary anastomosis or the Fontan procedure.9 In all of these cases, heart failure is typically the ultimate result.
Recently, LVAD support has taken on a role in the treatment of patients with corrected congenital cardiac defects who have developed advanced heart failure. These devices have been shown to provide satisfactory, safe circulatory support as a bridge to transplantation or recovery in pediatric and adult patients.5,10–13
Keeping pulmonary vascular resistance low is a factor in the success of ventricular assist device (VAD) support, including in those patients with complex congenital heart disease.7 In our patient, who had developed multiple AV malformations after a Fontan procedure, our concern that shunting would worsen after VAD implantation was not realized, and his oxygen saturations actually improved.
Pulmonary blood flow distribution is not necessarily even between the right and left lung in patients with TGA. The pattern of preferential blood flow to either lung can also change after surgical correction.14,15 This change plays an important role in the development of shunts and pulmonary venous hypertension. In a single cohort of TGA patients with cavopulmonary shunts, two thirds developed subclinical pulmonary AV fistulas. However, only half of them presented with symptomatic hypoxia.16 This clinical problem was of interest to investigators, who described the usefulness of contrast echocardiography and perfusion scans in locating and defining the extent of these pulmonary shunts.16,17 In selected cases involving isolated shunts, investigators have suggested coiling, embolization, or both as a therapeutic option.17 In more diffuse cases, this approach has resulted in short-term improvement but subsequent deterioration of oxygen saturation and recurrent shunting.9 In the report of these cases, the authors speculated that their patients' pulmonary AV shunts had worsened because of nonpulsatile flow in the cavopulmonary conduits. Our case does not support this hypothesis, because our patient's oxygen saturation improved remarkably with the use of a continuous-flow VAD. Multiple factors are implicated in the origin of AV fistulas; however, the exact mechanism is not yet well defined.
Morales and co-authors18 were the first to report the use of an intracorporeal systemic VAD in the long-term support of a patient who had a failing Fontan circulation. We think that ours is the 2nd report of the use of a VAD after the failure of a Fontan procedure, and the first report concerning the effect of VAD implantation on intrapulmonary shunting.
We are encouraged by the postimplantation result in this patient, especially because many more patients with TGA are reaching the 20-year follow-up mark. In those who have had a Fontan procedure and need VAD implantation, extreme caution should be used with regard to the anatomy of the right ventricle and baffle. It might also be necessary to resect right ventricular trabeculations to facilitate adequate placement of the inflow cannula.
Acknowledgments
We thank Marianne Mallia, ELS, of the Department of Scientific Publications at the Texas Heart Institute, for her editorial assistance in the preparation of the manuscript.
Footnotes
From: Department of Cardiology (Drs. Abdul Jabbar, Civitello, Delgado, and Simpson), and Center for Cardiac Support (Dr. Frazier), Texas Heart Institute; and Department of Medicine, Section of Cardiology (Drs. Abdul Jabbar, Civitello, Franklin, and Simpson), Baylor College of Medicine; Houston, Texas 77030
Dr. Abdul Jabbar is now with the Cardiovascular Disease Fellowship Program, Boonshoft School of Medicine, Wright State University, Dayton, Ohio.
References
- 1.Brown DW, Fulton DR. Congenital heart disease in children and adolescents. In: Fuster V, Walsh RA, Harrington RA, editors. Hurst's the heart. 13th ed. Vol. 2. New York: The McGraw-Hill Companies, Inc.; 2011. pp. 1827–83. p. [Google Scholar]
- 2.Karamlou TB, Welke KF, Ungerleider RM. Congenital heart disease. In: Schwartz SI, Brunicardi FC, editors. Schwartz's principles of surgery. 9th ed. New York: McGraw-Hill, Medical Pub. Division; 2010. p. xxi. p. [Google Scholar]
- 3.Beauchesne LM, Warnes CA, Connolly HM, Ammash NM, Tajik AJ, Danielson GK. Outcome of the unoperated adult who presents with congenitally corrected transposition of the great arteries. J Am Coll Cardiol. 2002;40(2):285–90. doi: 10.1016/s0735-1097(02)01952-6. [DOI] [PubMed] [Google Scholar]
- 4.Graham TP, Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F et al. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol. 2000;36(1):255–61. doi: 10.1016/s0735-1097(00)00682-3. [DOI] [PubMed] [Google Scholar]
- 5.Agusala K, Bogaev R, Frazier OH, Franklin WJ. Ventricular assist device placement in an adult with D-transposition of the great arteries with prior Mustard operation. Congenit Heart Dis. 2010;5(6):635–7. doi: 10.1111/j.1747-0803.2010.00408.x. [DOI] [PubMed] [Google Scholar]
- 6.Wiklund L, Svensson S, Berggren H. Implantation of a left ventricular assist device, back-to-front, in an adolescent with a failing Mustard procedure. J Thorac Cardiovasc Surg. 1999;118(4):755–6. doi: 10.1016/S0022-5223(99)70027-9. [DOI] [PubMed] [Google Scholar]
- 7.Frazier OH, Gregoric ID, Messner GN. Total circulatory support with an LVAD in an adolescent with a previous Fontan procedure. Tex Heart Inst J. 2005;32(3):402–4. [PMC free article] [PubMed] [Google Scholar]
- 8.Karl TR. The role of the Fontan operation in the treatment of congenitally corrected transposition of the great arteries. Ann Pediatr Cardiol. 2011;4(2):103–10. doi: 10.4103/0974-2069.84634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon BS, Bae EJ, Kim GB, Noh CI, Choi JY, Yun YS. Development of bilateral diffuse pulmonary arteriovenous fistula after Fontan procedure: is there nonhepatic factor? Ann Thorac Surg. 2009;88(2):677–80. doi: 10.1016/j.athoracsur.2008.12.065. [DOI] [PubMed] [Google Scholar]
- 10.Fan Y, Weng YG, Xiao YB, Huebler M, Franz N, Potapov E, Hetzer R. Outcomes of ventricular assist device support in young patients with small body surface area. Eur J Cardiothorac Surg. 2011;39(5):699–704. doi: 10.1016/j.ejcts.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Ihnat CL, Zimmerman H, Copeland JG, Meaney FJ, Sobonya RE, Larsen BT et al. Left ventricular assist device support as a bridge to recovery in young children. Congenit Heart Dis. 2011;6(3):234–40. doi: 10.1111/j.1747-0803.2011.00494.x. [DOI] [PubMed] [Google Scholar]
- 12.Morales DL, Almond CS, Jaquiss RD, Rosenthal DN, Naftel DC, Massicotte MP et al. Bridging children of all sizes to cardiac transplantation: the initial multicenter North American experience with the Berlin Heart EXCOR ventricular assist device. J Heart Lung Transplant. 2011;30(1):1–8. doi: 10.1016/j.healun.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Joyce DL, Crow SS, John R, St Louis JD, Braunlin EA, Pyles LA et al. Mechanical circulatory support in patients with heart failure secondary to transposition of the great arteries. J Heart Lung Transplant. 2010;29(11):1302–5. doi: 10.1016/j.healun.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Muster AJ, Paul MH, Van Grondelle A, Conway JJ. Asymmetric distribution of the pulmonary blood flow between the right and left lungs in d-transposition of the great arteries. Am J Cardiol. 1976;38(3):352–61. doi: 10.1016/0002-9149(76)90178-8. [DOI] [PubMed] [Google Scholar]
- 15.Vidne BA, Duszynski D, Subramanian S. Pulmonary blood flow distribution in transposition of the great arteries. Am J Cardiol. 1976;38(1):62–6. doi: 10.1016/0002-9149(76)90063-1. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Bae EJ, Cho DJ, Park IS, Kim YM, Kim WH, Kim SH. Development of pulmonary arteriovenous fistulas after bidirectional cavopulmonary shunt. Ann Thorac Surg. 2000;70(6):1918–22. doi: 10.1016/s0003-4975(00)02164-0. [DOI] [PubMed] [Google Scholar]
- 17.Cloutier A, Ash JM, Smallhorn JF, Williams WG, Trusler GA, Rowe RD, Rabinovitch M. Abnormal distribution of pulmonary blood flow after the Glenn shunt or Fontan procedure: risk of development of arteriovenous fistulae. Circulation. 1985;72(3):471–9. doi: 10.1161/01.cir.72.3.471. [DOI] [PubMed] [Google Scholar]
- 18.Morales DL, Adachi I, Heinle JS, Fraser CD., Jr. A new era: use of an intracorporeal systemic ventricular assist device to support a patient with a failing Fontan circulation. J Thorac Cardiovasc Surg. 2011;142:e138–40. doi: 10.1016/j.jtcvs.2011.05.018. [DOI] [PubMed] [Google Scholar]


