Abstract
Two validated scoring systems for predicting embolic risk, CHADS2 and CHA2DS2-VASc, contribute to optimizing antithrombotic prescription practices in patients who have atrial fibrillation. However, data about anticoagulated patients are sparse. We compared CHADS2 and CHA2DS2-VASc, in terms of their predictive risk evaluation, in patients with atrial fibrillation who were and were not taking anticoagulants.
We systematically searched the Cochrane Library, PubMed, and Embase databases for studies of the comparative diagnostic performance of CHADS2 and CHA2DS2-VASc. We identified 12 cohort studies for meta-analysis. With regard to the occurrence of cardiovascular events individually, patients with CHA2DS2-VASc scores ≥2 have a greater risk of stroke (risk ratio [RR]=5.15; 95% confidence interval [CI], 3.85–6.88; P <0.00001) and thromboembolism (RR=5.96; 95% CI, 5.50–6.45; P <0.00001) (Pdiff=0.34) than do patients with CHA2DS2-VASc scores <2, independent of anticoagulation therapy (RR=5.76; 95% CI, 5.23–6.35; P <0.00001 in anticoagulated patients; and RR=6.12; 95% CI, 5.40–6.93; P <0.00001 in patients not taking anticoagulants; Pdiff=0.45). The pooled RR estimates indicate an approximate 6-fold increase in the risk of endpoint events in patients with CHA2DS2-VASc scores ≥2 (RR=5.90; 95% CI, 5.46–6.37; P <0.0001).
These results clearly indicate the discriminative capacity of the CHA2DS2-VASc score for stroke, thromboembolic events, or both, independent of optimal anticoagulation. The CHA2DS2-VASc score enables the identification of patients who are at genuinely high risk and can direct the selection of appropriate therapeutic approaches.
Keywords: Atrial fibrillation/complications/epidemiology, decision support techniques, guidelines as topic, health status indicators, population surveillance/methods, predictive value of tests, randomized controlled trials as topic, risk assessment/methods/standards, stroke/epidemiology/prevention & control, thromboembolism/epidemiology/prevention & control
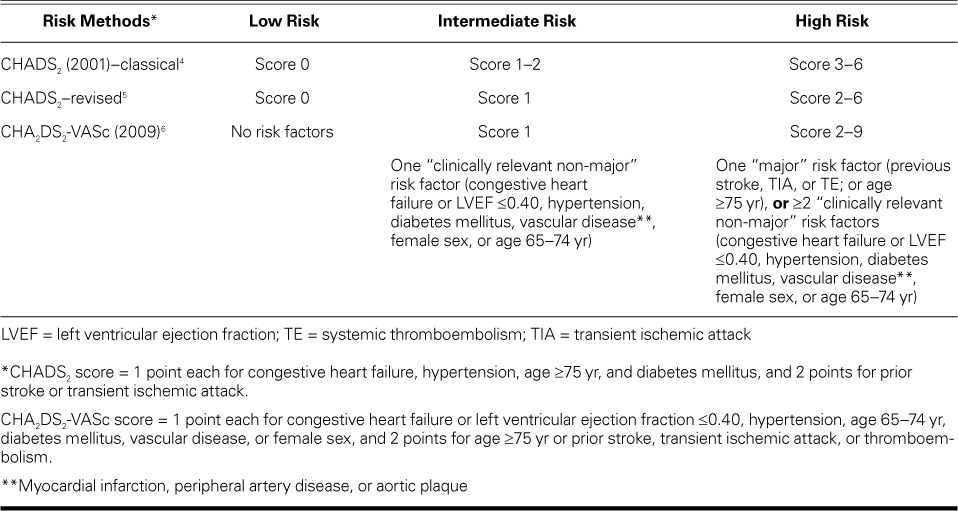
Atrial fibrillation (AF), the cardiac arrhythmia most frequently identified in clinical practice, becomes more prevalent as patients age. This condition is characterized by several devastating sequelae, including stroke and systemic thromboembolism (TE).1,2 Atrial fibrillation is a leading cause of neurologic disability and death depending on the severity of cardioembolic stroke, so oral anticoagulation is crucial for high-risk patients who have AF. Nonetheless, hemorrhagic sequelae of long-term anticoagulation are often seen during stroke prevention in patients who have AF. To date, several risk-scoring systems have shown modest predictive ability for endpoint events and have been well validated in recent studies. Two of the most widely used scores for risk prediction, CHADS2 and CHA2DS2-VASc, guide the optimization of therapy in patients who have AF, particularly if those patients are artificially categorized into low-, moderate-, and high-risk groups (Table I).3–6 The classical and revised CHADS2 score is cumulative on the basis of 6 clinical features: congestive heart failure, hypertension, diabetes mellitus, and age ≥75 years (counted as 1 point each), and a history of stroke or transient ischemic attack (2 points).4,5 In comparison, the CHA2DS2-VASc score, proposed as a complement to the CHADS2 score, ranges from 0 to 9 points; the clinical features are congestive heart failure or left ventricular ejection fraction ≤0.40, hypertension, age 65–74 years, diabetes mellitus, vascular disease, and female sex (1 point each), and age ≥75 years and prior stroke, transient ischemic attack, or thromboembolism (2 points each).6 In both systems, patient stratification into 3 risk categories—wherein a 0 score is low risk, 1 is intermediate risk, and ≥2 is high risk—has received particular attention in embolic risk evaluation and is widely included in guideline recommendations.2 The likelihood of an embolic event is closely related to the total points recorded for a given patient, and anticoagulation is advisable for patients with a score of 2 or more points.7 However, it is unclear whether anticoagulation should be recommended for intermediate-risk patients. When comparing the 2 scoring systems for stratification, investigators have reached different conclusions.8–10 In addition, current risk-stratification views are derived from analyses of patients who were not taking anticoagulants, and real-world data are sparse in terms of the value of alternative strategies for thromboprophylaxis in patients who are undergoing anticoagulation. In this meta-analysis, we sought to provide a detailed overview of previous studies in order to determine the comparative diagnostic accuracy of the CHADS2 and CHA2DS2-VASc scoring systems for risk evaluation in patients who have AF, independent of anticoagulant therapy.
TABLE I.
Three Risk-Stratification Methods Used to Predict Thromboembolism in Patients with Atrial Fibrillation
Patients and Methods
We used the following criteria for study selection: 1) studies comparing the predictive abilities of CHADS2 and CHA2DS2-VASc, preferably published in English; 2) type of study (retrospective or prospective); 3) adult participants who were eligible with an electrocardiographically documented record of AF (chronic, paroxysmal, persistent, permanent, or new-onset); 4) endpoints of stroke, TE, or both; and 5) either consistent anticoagulation or no anticoagulation at baseline. We excluded studies including subjects with mitral or aortic valve heart disease or other specific types of AF patients (for example, patients who underwent ablation, percutaneous coronary intervention, or pacemaker placement, or who had lone AF); duplicate studies and certain publication types (such as letters, case reports, and comments); and studies with insufficient data.
Literature Search. The data were systematically retrieved from the Cochrane Library, PubMed, and Embase databases with the use of search terms restricted to human beings (“CHADS2,” “CHA2DS2-VASc,” “atrial fibrillation,” “risk,” “prediction,” “thromboembolism,” and “stroke”) to identify relevant literature published in English. An electronic search was performed for articles published from 1 January 2009 through 1 April 2014, because the first paper about CHA2DS2-VASc was published in 2009.6 Further research was performed with use of reference lists, relevant journals, and conference abstracts. If necessary, we requested additional data from the authors of published studies.
Data Extraction and Quality Evaluation. The titles and abstracts of studies retrieved electronically and manually were screened independently (by Z-WG and X-QM). When the necessary information was not apparent, we comprehensively reviewed the full text. Disagreements were resolved through discussion or consultation with a 3rd reviewer (HK). Data were extracted according to the predetermined criteria, and then a quality evaluation of individual studies was performed in accordance with the methodologic standards proposed by McGinn and colleagues.11
Risk of Bias in Individual Studies. Two reviewers (Z-WG and X-QM) independently evaluated the risk of bias in accordance with the Cochrane Collaboration's “risk of publication bias” tool. Disagreements were resolved by consensus.
Consistency Test. The consistency of included studies was evaluated by means of the Cochrane Q test complemented with the I2 statistic: I2 values ≤25% indicated low heterogeneity, 25% to ≤50% indicated intermediate heterogeneity, and >50% indicated high heterogeneity. If 2 or more studies showed homogeneity or no significant heterogeneity, a fixed-effects model was chosen. Conversely, we hypothesized that the methodologic differences of individual studies, study types, treatments, follow-up durations, and patients' underlying clinical presentations would be associated with the heterogeneity. When sufficient comparable studies reported the same outcome, we performed subgroup analysis.12 We also performed random-effects model analysis.
Statistical Analysis
Review Manager 5.2 from the Cochrane Collaboration was used to perform the statistical analyses. The primary endpoints of AF patients were defined dichotomously and compared between scores <2 and ≥2 for both CHADS2 and CHA2DS2-VASc. For each trial, we calculated and pooled the risk ratios (RRs) for a comparative analysis of the occurrence of adverse events. The RRs are presented with 95% confidence intervals (CIs). Subgroup or sensitivity analyses were performed when appropriate. A P value ≤0.05 was considered statistically significant.
Results
We initially retrieved 490 articles; 432 were excluded after we read the title or abstract. Twelve of the remaining 58 studies were included in the review.6,10,13–22 Of these, 11 articles met all the inclusion criteria, and 1 article contained potentially relevant but insufficient data on the basis of the full-text article (resolved by contacting the authors) (Fig. 1). Table II shows the characteristics of the 12 studies. Seven studies6,13,17–21 reported TE outcomes, and the others10,14–16,22 included a record of stroke events. The sum of the heterogeneous populations in the included studies was 205,939. Some participants were taking anticoagulants at baseline17,19,20,22 and some were not.6,10,13–16,18,21
Fig. 1.
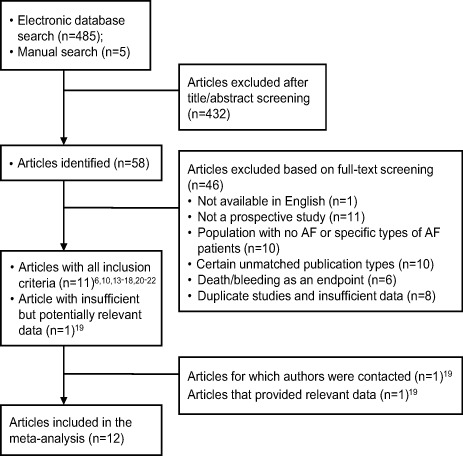
Flow-chart shows the number of articles considered during each stage of the systematic review process.
AF = atrial fibrillation
TABLE II.
Basic Characteristics of All Included Studies
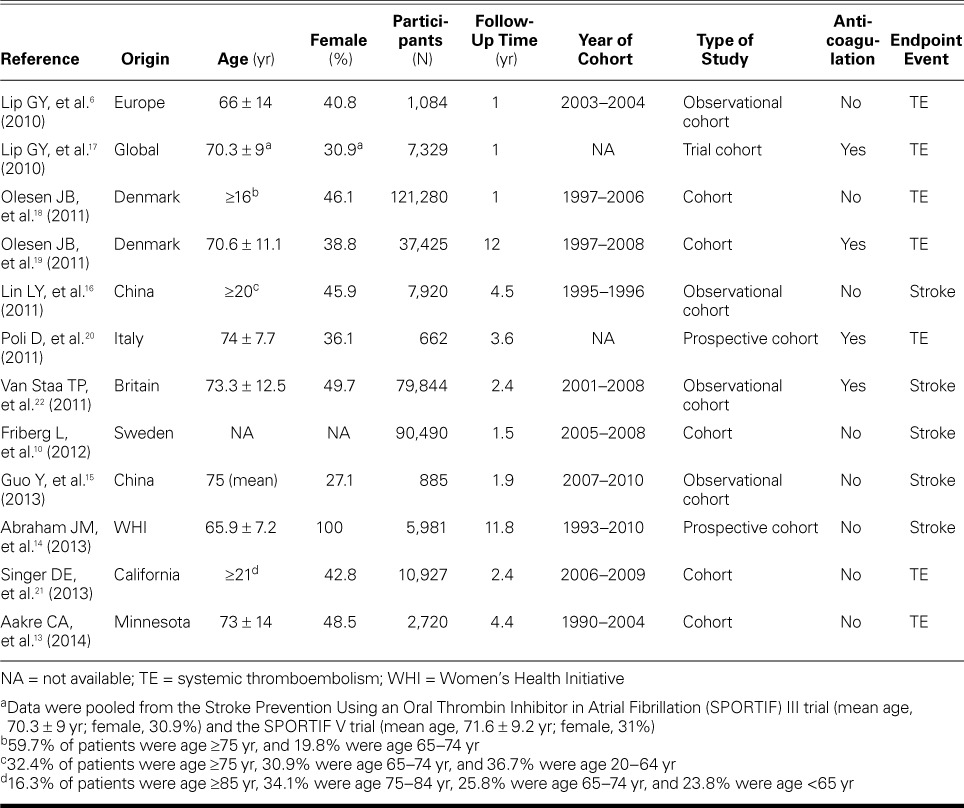
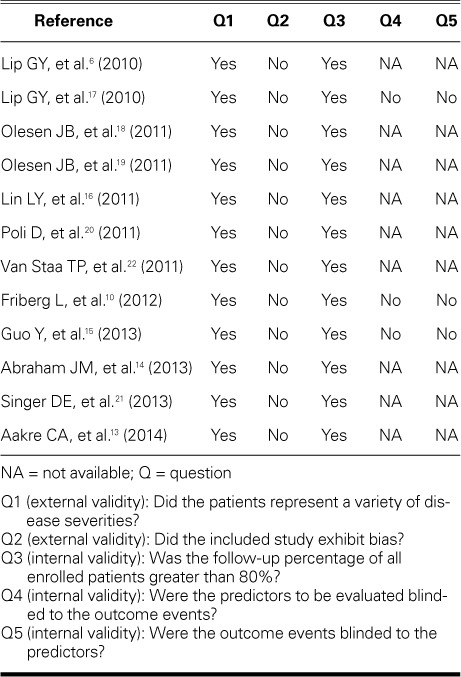
Quality Evaluation. Table III shows that the included studies generally represented a variety of disease severities and that the patients were generally selected in an unbiased fashion. Therefore, external validity was adequate. However, mixed results were reported in terms of internal validity. The follow-up durations of the patients were acceptable; however, issues relating to blinding were largely unreported.
TABLE III.
Quality Evaluation of the Individual Studies
Bias Analysis and Consistency Test. We used funnel plots to determine publication bias (Fig. 2). In the present study, the consistency test showed a different heterogeneity for the global effect of the samples (I2 value, 0–88%). To explore the sources of the heterogeneity, we performed a subgroup analysis and a sensitivity analysis. One study in the meta-analysis did not provide the basic characteristics of age and sex,10 and the female population was 100% in another study.14 Of note, these 2 factors were important determinants of stroke and TE events. We excluded these 2 studies and performed the meta-analysis again. The RRs were not significantly changed; however, the corresponding I2 value of the heterogeneity test markedly fell. Therefore, we concluded that these 2 studies were the main source of the heterogeneity.
Fig. 2.
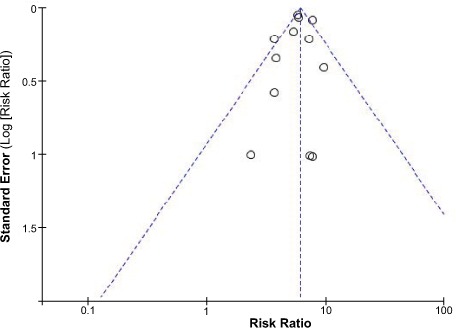
Funnel plot shows all studies included in the bias analysis.
Compared Capacity of CHADS2 Scores <2 versus ≥2
CHADS2 and Endpoint Events. Heterogeneity was obvious in the global effect of the samples (I2=53% for the TE subgroup and I2=88% for the stroke subgroup). After we excluded the 2 studies mentioned above, the I2 value of the stroke subgroup fell to 0. However, heterogeneity of the TE subgroup was still high (I2=53%). This was problematic for diagnostic test accuracy and for validating the CHADS2 score, so we performed a Mantel-Haenszel dichotomous-weighted random-effects model analysis. In this regard, the results should be interpreted cautiously. The occurrence of endpoint events is shown as a forest plot (Fig. 3). The pooled RRs indicated good calibration between CHADS2 scores <2 and ≥2. The incidence of TE in patients with CHADS2 scores ≥2 was significantly higher than in patients with scores <2 (RR=3.37; 95% CI, 3.11–3.65; P <0.00001). Results were similar for stroke (RR=3.36; 95% CI, 2.93–3.85; P <0.00001). There was no statistically significant difference between the 2 distinct groups (χ2=0, Pdiff=0.97). The pooled RRs showed an important stroke or TE risk in AF patients with scores ≥2, indicating an approximately 3-fold greater risk (RR=3.39; 95% CI, 3.18–3.61; P <0.0001). Despite the heterogeneity among studies, all had effects in the same direction, individually indicating an association between a score ≥2 and a greater risk of major cardiovascular events. Given the heterogeneity, the results should be interpreted cautiously.
Fig. 3.
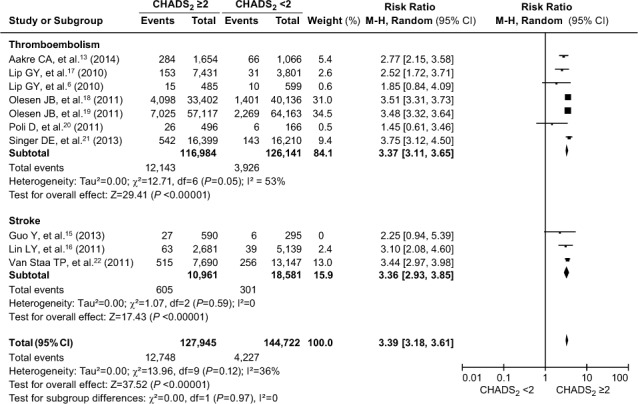
Forest plot shows a comparative analysis of the occurrence of thromboembolism and stroke in patients with atrial fibrillation when evaluated by means of the CHADS2 score. P ≤0.05 was considered statistically significant. The occurrence of thromboembolism and stroke when evaluated by means of the CHADS2 score was not significantly different (Pdiff=0.97).
AF = atrial fibrillation; CHADS2 = congestive heart failure, hypertension, age ≥75 yr, diabetes mellitus, and prior stroke or transient ischemic attack; CI = confidence interval; M-H = Mantel-Haenszel
CHADS2 and Anticoagulation. The incidence of cardiovascular events in AF patients was described in 2 subgroups: participants who were taking anticoagulants and those who were not. After exclusion of the 2 above-mentioned studies,10,14 a random-effects model analysis revealed that the increased risk of a cardiovascular event was higher in AF patients with CHADS2 scores ≥2, independent of anticoagulation (Fig. 4). In the subgroup of patients taking anticoagulants, the risk of cardiovascular events was significantly higher in those with scores ≥2 (RR=3.28; 95% CI, 2.85–3.77; P <0.00001; I2=54%) than in the subgroup not taking anticoagulants (RR=3.33; 95% CI, 2.96–3.75; P <0.00001; I2=33%). The contrast between the 2 subgroups was not statistically significant (χ2=0.03, Pdiff =0.86). These results indicated that the risk of cardiovascular events was more than 3-fold greater in individuals with a CHADS2 score ≥2, independent of anticoagulation.
Fig. 4.
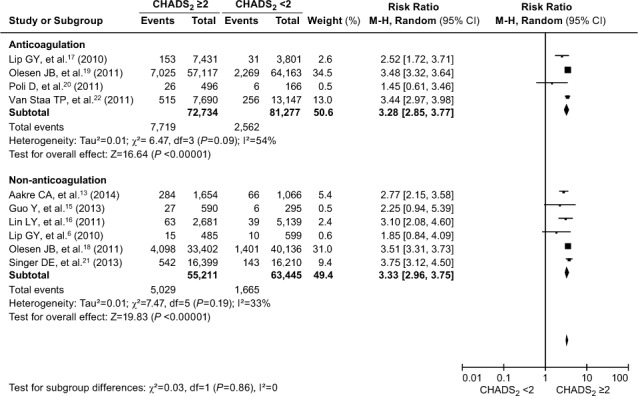
Forest plot shows a comparative analysis of the occurrence of cardiovascular events in atrial fibrillation patients, whether taking or not taking anticoagulants, when evaluated by means of the CHADS2 score. P ≥0.05 was considered statistically significant. The occurrence of cardiovascular events was approximately 3-fold greater in atrial fibrillation patients with CHADS2 scores ≥2, independent of anticoagulation (Pdiff=0.86).
AF = atrial fibrillation; CHADS2 = congestive heart failure, hypertension, age ≥75 yr, diabetes mellitus, and prior stroke or transient ischemic attack; CI = confidence interval; M-H = Mantel-Haenszel
Compared Capacity of CHA2DS2-VASc Scores <2 versus ≥2
CHA2DS2-VASc and Endpoint Events. All the included studies were divided into TE and stroke subgroups. The result of the consistency test showed high heterogeneity of the stroke subgroup (I2=72%). When we excluded the above-mentioned 2 articles10,14 and again performed the meta-analysis, the I2 value of both the TE and stroke subgroups fell to 0. We therefore used a fixed-effects model in the meta-analysis to compare the predictive ability of CHA2DS2-VASc scores <2 and ≥2. Similar to the results for the CHADS2 scores, the incidence of TE (RR=5.96; 95% CI, 5.50–6.45; P <0.00001) and stroke (RR=5.15; 95% CI, 3.85–6.88; P <0.00001) was significantly greater in patients who had CHA2DS2-VASc scores ≥2 (Fig. 5). There was no significant difference between the TE subgroup and the stroke subgroup (χ2 =0.90, Pdiff=0.34). According to the pooled RR estimates, there was an approximate 6-fold increase in the risk of endpoint events in patients who had CHA2DS2-VASc scores ≥2 (RR=5.90; 95% CI, 5.46–6.37; P <0.0001).
Fig. 5.
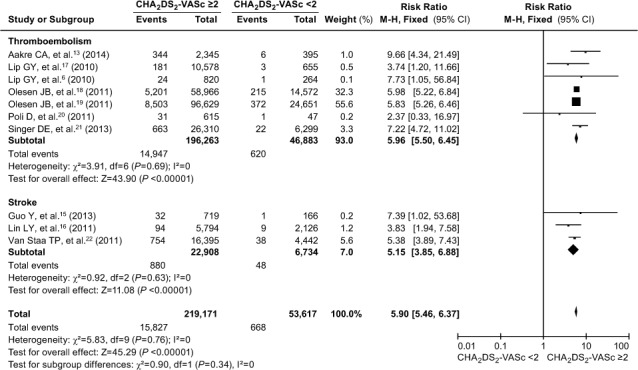
Forest plot shows a comparative analysis of the occurrence of thromboembolism and stroke in patients with atrial fibrillation when evaluated by means of the CHA2DS2-VASc score. P ≥0.05 was considered statistically significant. The occurrence of thromboembolism or stroke when evaluated by means of the CHA2DS2-VASc score was not significantly different (Pdiff=0.34).
AF = atrial fibrillation; CHA2DS2-VASc = congestive heart failure or left ventricular ejection fraction ≥0.40, hypertension, age ≥75 or 65–74 yr, diabetes mellitus, vascular disease, age, female sex, and prior stroke, transient ischemic attack, or thromboembolic event; CI = confidence interval; M-H = Mantel-Haenszel
CHA2DS2-VASc and Anticoagulation. The I2 value of the heterogeneity test fell to 0 after the adjustment noted above. Similar to the results for CHADS2, this meta-analysis showed that the increased risk of a cardiovascular event was higher in AF patients who had CHA2DS2-VASc scores ≥2, independent of anticoagu lation (Fig. 6). In the subgroup of patients who were taking anticoagulants, the risk of cardiovascular events was significantly higher in those with a CHA2DS2-VASc score ≥2 (RR=5.76; 95% CI, 5.23–6.35; P <0.00001) than in the subgroup not taking anticoagulants (RR=6.12; 95% CI, 5.40–6.93; P <0.00001). The difference between the 2 subgroups was not statistically significant (χ2 =0.56, Pdiff=0.45). The results indicated that the risk of cardiovascular events was approximately 6-fold greater in patients who had CHA2DS2-VASc scores ≥2, independent of anticoagulation.
Fig. 6.
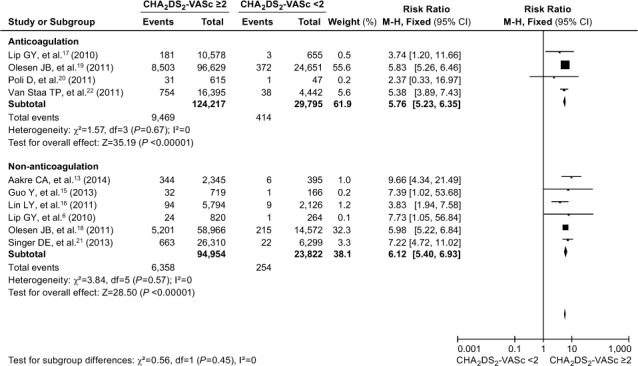
Forest plot shows a comparative analysis of the occurrence of cardiovascular events in atrial fibrillation patients, whether taking or not taking anticoagulants, when evaluated by means of the CHA2DS2-VASc score. P ≥0.05 was considered statistically significant. The occurrence of cardiovascular events was approximately 6-fold greater in atrial fibrillation patients with CHA2DS2-VASc scores ≥2, independent of anticoagulation (Pdiff=0.45).
AF = atrial fibrillation; CHA2DS2-VASc = congestive heart failure or left ventricular ejection fraction ≥0.40, hypertension, age ≥75 or 65–74 yr, diabetes mellitus, vascular disease, female sex, and prior stroke, transient ischemic attack, or thromboembolism; CI = confidence interval; M-H = Mantel-Haenszel
Discussion
Even when AF is not immediately life-threatening, it substantially raises morbidity and mortality rates. Antithrombotic therapy has severe bleeding sequelae and necessitates intensive monitoring. One reason why patients with AF have been artificially assigned into low-, moderate-, and high-risk groups is the inconvenience and disadvantage of anticoagulation with oral vitamin K antagonists. It is thought that genuinely low-risk patients should not be prescribed antithrombotic therapy, whereas all other patients might need an anticoagulant. The CHA2DS2-VASc classification method, which extends the validity of CHADS2 by incorporating additional stroke-risk factors, is likely to improve the prediction of stroke and improve therapeutic decision-making in high-risk patients, but not as much in patients who are classified at low or moderate risk.
An earlier meta-analysis23 showed the association between a higher CHADS2 score and the risk of stroke in AF patients. However, CHA2DS2-VASc had not yet been similarly evaluated. The CHA2DS2-VASc score is a powerful predictor of stroke and a predictor of the occurrence of TE. Our study shows that patients with a CHADS2 score ≥2 and chronic AF have a 3-fold greater risk of stroke, TE, or both. Of note, our analysis reveals that the more-than-3-fold increase in TE risk predicted by means of the CHADS2 score is statistically different from the 6-fold increase predicted by means of the CHA2DS2-VASc score (χ2=65.54, P <0.00001). Our data thus extend the available evidence base of the CHA2DS2-VASc method in predicting stroke, TE, or both in patients with AF, and show that the CHA2DS2-VASc score enables the identification of a larger number of AF patients for whom oral anticoagulation would be recommended.24
Our meta-analysis also reveals a powerful predictive value of both the CHADS2 and CHA2DS2-VASc for the highest-risk stratum of patients, independent of anticoagulation. Our results indicate that cardiovascular events might also occur in anticoagulated AF patients and reveal a stepwise increase in stroke or TE events upon increasing scores across risk strata in anticoagulated patients. This discovery is important because of the existing uncertainty about whether aggressive anticoagulation of AF patients might improve clinical outcomes. Oral anticoagulation is undoubtedly highly effective in reducing the risk of adverse events in AF patients. Nonetheless, cardiovascular events still occur in anticoagulated AF patients, so it is important to identify risk factors and the performance of current stroke-risk stratification methods in these patients. The published risk-stratification schemes arose from studies of patients who were not taking anticoagulants, and real-world data have been sparse with regard to the predictive abilities in anticoagulated patients. Current stroke-risk stratification methods contribute to identifying which kinds of patients benefit from anticoagulation; however, it is unclear which method helps to detect those who remain at higher risk despite anticoagulant therapy at baseline. Our data show that the CHADS2 and CHA2DS2-VASc methods have value in stroke-risk evaluation in patients who have a substantial risk of stroke despite optimal anticoagulation. Previously, Jover and co-authors25 reported that CHA2DS2-VASc meaningfully predicted cardiovascular events and death among real-world anticoagulated patients with AF.
In addition, our data show the increasing trend toward a risk of endpoint events in AF patients who are given anticoagulants, supported by the average incidence rates of events across the 3 risk categories (1.66%, 3.88%, and 10.61% for CHADS2 and 0.75%, 1.81%, and 7.62% for CHA2DS2-VASc; both Ptrend <0.001). We have shown a clear relationship between increasing CHADS2 or CHA2DS2-VASc scores and higher rates of endpoint events, even in patients who are given anticoagulants. In the comparison of the rates of endpoint events among low-risk patients (1.67% vs 0.75%; P <0.001), the findings imply that some CHADS2low-risk patients might still benefit from anticoagulation. The findings further support the superior diagnostic for performance of CHA2DS2-VASc over CHADS2 identifying genuinely low-risk patients with AF. The CHA2DS2-VASc method has the more important advantage of identifying extremely low-risk patients with AF and classifying a lower proportion of patients in the moderate-risk category.26,27 The results of several studies have indicated that CHADS2 stratifies some non-low-risk patients as low- or intermediate-risk.28–30 Thus, the predictive value of CHADS2 has been limited to patients at low and intermediate risk, whereas CHA2DS2-VASc has been recommended to identify genuinely low-risk patients who might not need antithrombotic therapy.
Implications for Clinical Practice
Our study extends the available evidence base by examining a broader spectrum of patients with AF who were diagnosed across all healthcare venues and were included in different studies. The results of diagnostic accuracy suggest that the CHA2DS2-VASc score is helpful in terms of clinical decision-making. A CHA2DS2-VASc score ≥2 was better at predicting stroke and TE events, and this might help clinicians to direct the most intensive antithrombotic regimens toward patients who are genuinely at high risk. In particular, more patients in the high-risk stratum would receive a clear recommendation for anticoagulation, and fewer patients in the moderate-risk stratum would be given an ambiguous recommendation for either antiplatelet or anticoagulant therapy. In addition, the CHA2DS2-VASc score is a good indicator of stroke risk in anticoagulated patients who have AF. It is important to emphasize that the predictive ability of the CHA2DS2-VASc score is independent of anticoagulant therapy—this extends the scope of the score's usefulness and appropriately deals with the matter of anticoagulation.
Limitations of the Meta-Analysis
In the present study, high heterogeneity between 12 studies was observed in both of the calibration analyses. High heterogeneity across studies was problematic for the diagnostic test accuracy, as well as in the validation of the CHA2DS2-VASc score; the multiple sources of heterogeneity might have included different interventions, different follow-up times, and different anticoagulation intensity. To identify the sources of heterogeneity, we continued with subgroup and sensitivity analyses, which showed that age and sex were important determinants of stroke and TE. We excluded 2 studies that possibly affected these and again performed the meta-analysis. The I2 value of the heterogeneity test fell to 0 (Figs. 5 and 6), so the main source of the heterogeneity was probably those 2 studies.10,14
Several limitations of this meta-analysis are as follows. First, there was a wide variability across different validation studies secondary to methodologic differences. The external validity of the included studies was adequate; however, results were mixed in terms of internal validity, mostly because of unreported issues relating to blinding. Second, some studies did not use consistent definitions for stroke or TE events, which complicated the synthesis of their findings. Because of the combination of stroke and TE as primary endpoints, the incidence of event rates in different risk stratifications was not true despite consistent reporting. Third, there was not a clear restriction of follow-up time across the individual studies during collection of the samples of endpoint events. A linear correlation analysis between the average follow-up time and the statistics of both scoring systems should be performed. Finally, the variability in the intensity of anticoagulation across studies might be a further source of heterogeneity. Future meta-analyses should include studies that evaluated the predictive role of the international standard ratio and its association with stroke risk in AF patients.
Conclusion
The CHADS2score is simple and easy to use; however, the CHA2DS2-VASc score enables a substantially more comprehensive risk evaluation and improves the ability to identify genuinely low-risk patients who have AF. This latter method concomitantly places a lower proportion of patients into the intermediate-risk category. Moreover, the CHA2DS2-VASc score distributes more patients and a higher incidence of endpoint events into the high-risk stratum and can identify patients with AF who are at substantial risk of endpoint events despite optimal anticoagulation. This approach should therefore be used to direct stroke and TE risk stratification and to guide decision-making for thromboprophylaxis in patients who have AF.
Acknowledgments
We thank Dr. H.-Q. Yu (School of Public Health, Nanchang University, PRC) for assistance with the bias analysis and consistency test.
Footnotes
From: Cardiology Department (Drs. Hong and Xiong, and Mr. Zhu), the Second Affiliated Hospital of Nanchang University; and Jiangxi Key Laboratory of Molecular Medicine (Dr. Hong); Jiangxi 330006, People's Republic of China
The authors acknowledge support from the Ministry of Chinese Education Innovation Team Development Plan (IRT1141, HK), National Basic Research Program of China (973 Program: 2013CB531103), the National Natural Science Foundation of China (81160023, 81370288), and Jiangxi Science Foundation of China (20121BBG70030).
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association [published erratum appears in Circulation 2011;124 (16):e425] Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. Writing Group Members. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385–413. doi: 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- 3.Lip GY, Halperin JL. Improving stroke risk stratification in atrial fibrillation. Am J Med. 2010;123(6):484–8. doi: 10.1016/j.amjmed.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 5.Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156(1):57–64. doi: 10.1016/j.ahj.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 7.Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57(7):831–8. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopes RD, Crowley MJ, Shah BR, Melloni C, Wood KA, Chatterjee R Stroke prevention in atrial fibrillation. U.S. Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 9.Olesen JB, Lip GY, Lane DA, Kober L, Hansen ML, Karasoy D et al. Vascular disease and stroke risk in atrial fibrillation: a nationwide cohort study. Am J Med. 2012;125(8):813–26. doi: 10.1016/j.amjmed.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500–10. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 11.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users' guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 13.Aakre CA, McLeod CJ, Cha SS, Tsang TS, Lip GY, Gersh BJ. Comparison of clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation. Stroke. 2014;45(2):426–31. doi: 10.1161/STROKEAHA.113.002585. [DOI] [PubMed] [Google Scholar]
- 14.Abraham JM, Larson J, Chung MK, Curtis AB, Lakshminarayan K, Newman JD et al. Does CHA2DS2-VASc improve stroke risk stratification in postmenopausal women with atrial fibrillation? Am J Med. 2013;126(12):1141–3. doi: 10.1016/j.amjmed.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y, Apostolakis S, Blann AD, Wang H, Zhao X, Zhang Y et al. Validation of contemporary stroke and bleeding risk stratification scores in non-anticoagulated Chinese patients with atrial fibrillation. Int J Cardiol. 2013;168(2):904–9. doi: 10.1016/j.ijcard.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Lin LY, Lee CH, Yu CC, Tsai CT, Lai LP, Hwang JJ et al. Risk factors and incidence of ischemic stroke in Taiwanese with nonvalvular atrial fibrillation--a nationwide database analysis. Atherosclerosis. 2011;217(1):292–5. doi: 10.1016/j.atherosclerosis.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41(12):2731–8. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- 18.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olesen JB, Lip GY, Lindhardsen J, Lane DA, Ahlehoff O, Hansen ML et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106(4):739–49. doi: 10.1160/TH11-05-0364. [DOI] [PubMed] [Google Scholar]
- 20.Poli D, Lip GY, Antonucci E, Grifoni E, Lane D. Stroke risk stratification in a “real-world” elderly anticoagulated atrial fibrillation population. J Cardiovasc Electrophysiol. 2011;22(1):25–30. doi: 10.1111/j.1540-8167.2010.01858.x. [DOI] [PubMed] [Google Scholar]
- 21.Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2:e250. doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Staa TP, Setakis E, Di Tanna GL, Lane DA, Lip GY. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost. 2011;9(1):39–48. doi: 10.1111/j.1538-7836.2010.04085.x. [DOI] [PubMed] [Google Scholar]
- 23.Santos C, Pereira T, Conde J. CHADS2 score in predicting cerebrovascular events: a meta-analysis [in Portuguese] Arq Bras Cardiol. 2013;100(3):294–301. doi: 10.5935/abc.20130068. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Nodar JM, Marin F, Roldan V, Valencia J, Manzano-Fernandez S, Caballero L et al. Should we recommend oral anticoagulation therapy in patients with atrial fibrillation undergoing coronary artery stenting with a high HAS-BLED bleeding risk score? Circ Cardiovasc Interv. 2012;5(4):459–66. doi: 10.1161/CIRCINTERVENTIONS.112.968792. [DOI] [PubMed] [Google Scholar]
- 25.Jover E, Roldan V, Gallego P, Hernandez-Romero D, Valdes M, Vicente V et al. Predictive value of the CHA2DS2-VASc score in atrial fibrillation patients at high risk for stroke despite oral anticoagulation. Rev Esp Cardiol (Engl Ed) 2012;65(7):627–33. doi: 10.1016/j.recesp.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Boriani G, Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M et al. Improving stroke risk stratification using the CHADS2 and CHA2DS2-VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke. 2011;42(6):1768–70. doi: 10.1161/STROKEAHA.110.609297. [DOI] [PubMed] [Google Scholar]
- 27.Chen JY, Zhang AD, Lu HY, Guo J, Wang FF, Li ZC. CHADS2 versus CHA2DS2-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta-analysis. J Geriatr Cardiol. 2013;10(3):258–66. doi: 10.3969/j.issn.1671-5411.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppens M, Eikelboom JW, Hart RG, Yusuf S, Lip GY, Dorian P et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34(3):170–6. doi: 10.1093/eurheartj/ehs314. [DOI] [PubMed] [Google Scholar]
- 29.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107(6):1172–9. doi: 10.1160/TH12-03-0175. [DOI] [PubMed] [Google Scholar]
- 30.Piyaskulkaew C, Singh T, Szpunar S, Saravolatz L, 2nd, Rosman H. CHA(2)DS(2)-VASc versus CHADS(2) for stroke risk assessment in low-risk patients with atrial fibrillation: a pilot study from a single center of the NCDR-PINNACLE registry. J Thromb Thrombolysis. 2014;37(4):400–3. doi: 10.1007/s11239-013-0983-z. [DOI] [PubMed] [Google Scholar]


