Abstract
Lambl's excrescences are mobile, thin, filiform structures that occur at sites of valve closure. Even though many clinicians consider them to be part of the normal aging process, evidence suggests an association between Lambl's excrescences and ischemic stroke, migrainous headaches, and acute coronary syndromes. As a thin filamentous structure, a Lambl's excrescence is better detected and characterized with transesophageal than with transthoracic echocardiography. Intracardiac oscillatory structures can also be seen as “tiger stripes” on spectral pulsed Doppler echocardiographic recordings. Herein, we present the case of a 68-year-old woman who had 3 concurrent enigmatic findings with unclear correlation: migrainous headaches, Lambl's excrescences, and the uncommon finding of “tiger stripes” on spectral Doppler echocardiography. We discuss the possible correlation between these 3 findings and review the available literature on Lambl's excrescences and tiger stripes.
Keywords: Aortic valve/pathology/ultrasonography; brain infarction/etiology; echocardiography, Doppler, pulsed; embolism/etiology; headache/diagnosis/etiology; heart valve diseases/ultrasonography; Lambl's excrescences; status migrainosus; stroke
First described in 1856,1 Lambl's excrescences (LE) are mobile, thin, filiform structures that typically occur at sites of valve closure.2 Although their clinical relevance is uncertain,2 evidence drawn from several case reports and case-control studies has associated LE with embolic events and acute coronary syndromes.3,4 We report 3 enigmatic concurrent findings (with unclear pathophysiology) in a single patient: migrainous headaches, LE, and “tiger stripes” upon transthoracic echocardiography (TTE).
Case Report
In February 2012, a 68-year-old woman presented with a 7-day history of headache, vertigo, and generalized weakness. She had experienced similar episodes in the year before the current presentation, usually once every month, but never as intense and prolonged as this last episode. The patient described the headache as pressure-like, originating in the frontal region, without triggers but associated with photophobia, phonophobia, blurred vision, and nausea.
Her heart rate was 70 beats/min, her blood pressure was 196/70 mmHg, and her temperature was 98 °F. Physical examination revealed decreased sensory reaction to light touch and nociceptive reaction in the right upper extremity.
Noncontrast computed tomography (CT) of the head showed an infarct of undetermined age in the left centrum semiovale. Magnetic resonance imaging of the brain confirmed this finding. A CT angiogram of the head and neck excluded significant stenosis of the intra- or extracranial vasculature. Transthoracic echocardiography showed a sclerotic aortic valve and a prominent LE on the right coronary cusp (Fig. 1). Spectral pulsed-Doppler echocardiography revealed high-amplitude band-like signals consistent with tiger stripes5 (Fig. 2). The diagnosis of status migrainosus was made, and treatment was begun with dihydroergotamine, divalproex sodium, hydroxyzine, and hydrocortisone. Resolution of the migrainous headache occurred within 2 days, and prophylaxis with propranolol was started. The patient was continued on aspirin for secondary prevention of stroke and, as of April 2014, she had not experienced recurrence of her symptoms or been hospitalized for status migrainosus or ischemic stroke.
Fig. 1.
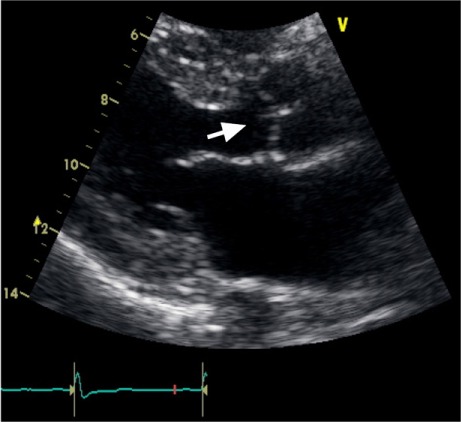
Two-dimensional transthoracic echocardiogram (parasternal long-axis view) shows a long, filiform structure in the right coronary cusp protruding into the ventricular surface of the aortic valve (arrow).
Fig. 2.
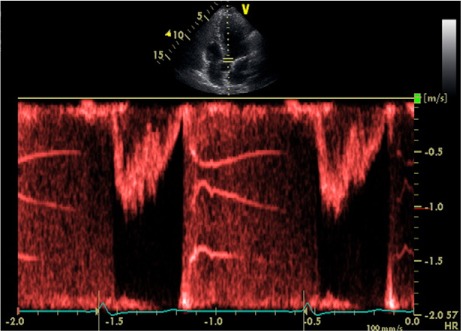
Spectral pulsed-Doppler echocardiographic reading across the aortic valve (apical 5-chamber view) shows high-amplitude band-like signals, mimicking tiger stripes.
Discussion
Histologically, LE consists of an acellular central elastic connective-tissue core that is continuous with the connective tissue of the valve and covered by a single layer of endothelium. This last characteristic differentiates it from a fibroelastoma.6 Lambl's excrescences have a papillary appearance; their number and locations are variable, but typically they occur at valvular closure lines, on the atrial surface of the mitral valve, or on the ventricular surface of the aortic valve.6,7 They can present as a single strand, in rows or clusters, and are usually 1 mm thick and 1 to 10 mm long.2 The most accepted theory regarding the pathogenesis of LE proposes that they are organized thrombi. The continual striking upon the valves causes small tears in the endothelium, which leads to fibrin deposition over the lesions. In high-pressure environments such as the left ventricle, thrombi are converted into flat, fibrous scars. This is followed by growth of an endothelial layer over the fibrous surface and condensation of the enclosed fibrin.6
Although Lambl first described LE on aortic valves,1 subsequent pathologic and echocardiographic series have shown that LE more often appears on the mitral valve. Magarey8 found a prevalence of 85% in mitral valves and 2% in aortic valves on autopsy. In a clinical setting, both Freedberg9 and Roldan10 found LE more often in the mitral valve (70% and 68%–76%, respectively).
Both TTE and transesophageal echocardiography (TEE) have been used for LE diagnosis, but TEE is preferred. The sensitivity and specificity of TEE for mobile echogenic structures are 68% and 85%, respectively. These low values are due mainly to the lack of standardized definitions for the identification of mobile echoes.6 Despite these drawbacks, echocardiography remains a valuable tool for LE diagnosis.
On spectral pulsed-Doppler recordings, high-amplitude band-like signals (known as tiger stripes) are indicative of an intracardiac vibratory and oscillating structure.5 In addition, the differential diagnosis of tiger stripes includes substantial valve regurgitation and flail prosthetic valve.5 Given that both of those conditions were absent in our patient, we hypothesize that the structure giving origin to the stripes was an aortic LE. This hypothesis could be proved only by surgical resection of the LE, which was not indicated consequent to the resolution of our patient's symptoms upon follow-up investigation (and to the lack of evidence of benefits to be gained, in this case, from resection). To our knowledge, this is the first time that LE have been reported concurrently with tiger stripes on spectral Doppler echocardiography.5
There is conflicting evidence regarding the clinical relevance of LE. Magarey8 considered them normal to the aging process of the cardiac valves, because all the individuals in his autopsy study who were older than 60 years had LE. Others, using case-control studies, argue that LE significantly increase the risk of embolic events—mainly strokes, especially in young patients and in those with prosthetic valves.11 However, one longitudinal follow-up investigation on patients with prior stroke3 did not find an increased risk of recurrence in patients with strands, which argues against a causal relationship. In that study, the effects of therapy could not explain the absence of difference, since the incidence of recurrent stroke was lowest in patients not treated with antithrombotic therapy. Moreover, Menzel and colleagues7 found no significant difference, by TEE, between the prevalence of strands in 129 patients with a history of embolic events and the prevalence of strands in 89 patients without such a history. In addition, Roldan and colleagues10 found no differences in the prevalence of stroke in a prospective study of patients with and without LE, or in the frequency of LE in patients with a history of unexplained ischemic stroke. However, because most of the clinical studies failed to provide an accurate pathologic diagnosis, there is some uncertainty that all the strands were truly LE.
Lambl's excrescences have also been related to valvular stenosis6 and to the obstruction of coronary ostia in the manifest form of myocardial infarction.4 In summary, the clinical significance of LE remains unclear. Multiple opinions have arisen, mostly from small studies that were not prospective in nature.
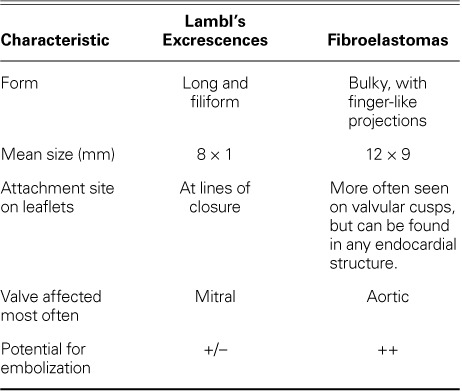
The main differential diagnosis in suspected cases of LE is fibroelastoma. Although the gold standard for diagnosis is histopathologic examination of the affected valve (a single layer of endothelial cells in diagnosing LE1,8 vs multiple layers in fibroelastoma), certain echocardiographic characteristics can help set the 2 conditions apart (Table I).2,6
TABLE I.
In 2012, Liu and associates12 described an association between migraine-like headache (that did not completely meet criteria for a specific primary headache) and ischemic stroke in 2 patients with LE, a presentation similar to that of our patient. These authors hypothesize that microemboli from an LE cause cerebral ischemia, which presents as a migraine-like headache.12
The therapeutic approach to LE should be carefully individualized. In asymptomatic patients, observation is reasonable, but some physicians prefer antiplatelet therapy. If no other embolic sources are found after the first stroke, some authors recommend anticoagulation or antiplatelet therapy. Surgical intervention should be performed in patients who experience recurrent stroke in the absence of an obvious cause and in patients whose LE causes a functional abnormality, such as coronary occlusion that leads to myocardial ischemia or valve obstruction.6,12
Although our patient presented with migrainous headaches, imaging evidence of a previous ischemic stroke, and “tiger stripes” on spectral pulsed-Doppler echocardiography, there is to date no clear proof of a causal link between these. Conversely, there is no clear evidence to confute a correlation. The purpose of this report is to bring these circumstances to the attention of the medical community, both to promote further study of LE's significance and to help cardiologists recognize the characteristics of LE on echocardiography.
Footnotes
From: Department of Internal Medicine (Dr. Davogustto) and Division of Cardiology, Department of Internal Medicine (Drs. Fernando and Loghin), University of Texas Health Science Center at Houston, Houston, Texas 77030
References
- 1.Lambl VD. Papillare excrescenzen an der semilunar-klappe der aorta [in German] Wien Med Wochenschr. 1856;6:244–7. [Google Scholar]
- 2.Jaffe W, Figueredo VM. An example of Lambl's excrescences by transesophageal echocardiogram: a commonly misinterpreted lesion. Echocardiography. 2007;24(10):1086–9. doi: 10.1111/j.1540-8175.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 3.Cohen A, Tzourio C, Amarenco P. Lambl's excrescences and stroke. Echocardiography. 2000;17(1):101–3. doi: 10.1111/j.1540-8175.2000.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 4.Dangas G, Dailey-Sterling FG, Sharma SK, Chockalingham S, Albanese JR, Reich DL et al. Non-Q-wave infarction and ostial left coronary obstruction due to giant Lambl's excrescences of the aortic valve. Circulation. 1999;99(14):1919–21. doi: 10.1161/01.cir.99.14.1919. [DOI] [PubMed] [Google Scholar]
- 5.Kerut EK. Tiger stripes. Echocardiography. 2007;24(5):558–9. doi: 10.1111/j.1540-8175.2007.00431.x. [DOI] [PubMed] [Google Scholar]
- 6.Voros S, Nanda NC, Thakur AC, Winokur TS, Samal AK. Lambl's excrescences (valvular strands) Echocardiography. 1999;16(4):399–414. doi: 10.1111/j.1540-8175.1999.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 7.Menzel T, Mohr-Kahaly S, Arnold KJ, Kolsch B, Kopp H, Spiecker M et al. Detection of strands in native aortic valves by transesophageal echocardiography. Am J Cardiol. 1997;79(11):1549–52. doi: 10.1016/s0002-9149(97)00193-8. [DOI] [PubMed] [Google Scholar]
- 8.Magarey FR. On the mode of formation of Lambl's excrescences and their relation to chronic thickening of the mitral valve. J Pathol Bacterial. 1949;61(2):203–8. doi: 10.1002/path.1700610207. [DOI] [PubMed] [Google Scholar]
- 9.Freedberg RS, Goodkin GM, Perez JL, Tunick PA, Kronzon I. Valve strands are strongly associated with systemic embolization: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;26(7):1709–12. doi: 10.1016/0735-1097(95)00394-0. [DOI] [PubMed] [Google Scholar]
- 10.Roldan CA, Shively BK, Crawford MH. Valve excrescences: prevalence, evolution and risk for cardioembolism. J Am Coll Cardiol. 1997;30(5):1308–14. doi: 10.1016/s0735-1097(97)00315-x. [DOI] [PubMed] [Google Scholar]
- 11.Orsinelli DA. Prosthetic valve strands: clinically significant or irrelevant to management? J Am Soc Echocardiogr. 2009;22(8):895–8. doi: 10.1016/j.echo.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Liu RZ, Yu SY, Li Y. Migraine-like headache and ischemic strokes in two patients with Lambl's excrescences. Chin Med J (Engl) 2012;125(18):3346–8. [PubMed] [Google Scholar]


