Abstract
An increasing number of young and middle-aged men are seeking treatment for symptoms related to deficient levels of androgens (hypogonadism) including depression, loss of libido, erectile dysfunction, and fatigue. The increase in prevalence of testosterone supplementation in general and anabolic steroid-induced hypogonadism specifically among younger athletes is creating a population of young men who are uniquely impacted by the testicular end-organ negative consequences of exogenous steroid use. Exogenous testosterone therapy can alter the natural regulation of the hypothalamic-pituitary-gonadal axis leading to impaired spermatogenesis with azoospermia being a serious possible result, thus rendering the individual infertile. For men of reproductive age who suffer from hypogonadal symptoms, preservation of fertility is an important aspect of their treatment paradigm. Treatment with human chorionic gonadotropin (hCG) has shown the ability not only to reverse azoospermia brought on by testosterone supplementation therapy but also to help maintain elevated intratesticular testosterone levels. In addition, selective estrogen receptor modulators, often used with hCG have been shown both to elevate total testosterone levels and to maintain spermatogenesis in hypogonadal men.
Keywords: human chorionic gonadotropin, selective estrogen receptor modulators, spermatogenesis, testosterone supplementation therapy
INTRODUCTION
Exogenous testosterone use among men has increased exponentially in the last decade with at least a three-fold growth in men 40 years old and older.1 Accumulating evidence demonstrates a significant prevalence of hypogonadism in men over the age of 45, with some estimates reaching 13.8 million hypogonadal men in the United States.2 At the same time, the age of fatherhood has been increasing.3 Many physicians are turning to exogenous testosterone supplementation therapy (TST) to help these men with hypogonadism manage symptoms of decreased libido, depression, fatigue, and erectile dysfunction. Unfortunately, use of exogenous testosterone is not limited to medical therapies alone as use of testosterone, and other anabolic steroids is quite common among young athletes. In the United States, estimates of individuals who use anabolic steroids range from 1 to 3 million people.4 A substantial proportion of these are young adults. In a study by Buckley et al. 6.6% of high school seniors reported that they used, or had a history of using, anabolic steroids.5
Exogenous testosterone use whether for therapeutic or supplementation purposes has serious consequences including diminished sperm production and possible azoospermia, which will impact a man's ability to establish a pregnancy. The purpose of this review is two-fold. First is to evaluate the potential therapies that could help restore sperm production in those men who have infertility resulting from exogenous testosterone use and a second is to look at therapies that may allow a hypogonadal man to concurrently maintain spermatogenesis as well as receive TST and thus preserve the ability to initiate a pregnancy. We will present a clinical vignette, discuss evaluation and management options, and present an algorithm for treatment of men with testosterone therapy who want to preserve fertility.
Clinical vignette
A 42-year-old man and his 30-year-old G0P0 wife are referred by their primary care physician after anti-depressant therapy has failed to improve the husband's symptoms of fatigue, decreased libido, mild depression, and progressive erectile dysfunction. They are planning to initiate a pregnancy in the next few months. His laboratory evaluation reveals a serum total testosterone (drawn before 10 a.m.) of 175 ng dl−1 (normal 250–1000), follicle-stimulating hormone (FSH) level of 4 mIU ml−1 (normal 4–10) and luteinizing hormone (LH) level of 6 mIU ml−1 (normal 4–12). Two questions arise when treatment is considered. Do we have the ability to treat this patient's low testosterone safely and effectively? How will treatment impact the patient's ability to establish a pregnancy?
Epidemiology of hypogonadism in men of reproductive age
A national, population-based study using “Clinformatics Data Mart” looked at 10 739 815 men aged 40 and older over a period of 10 years (2001–2011). The study found that the percentage of men receiving TST quadrupled in men aged 40–49 and that it had tripled in men aged 50–69.1 In another retrospective analysis of 410 019 men who initiated testosterone treatment, 12% were men aged 18–39 that is, men of reproductive age.6
The reasons for this trend are multifactorial and include an increasing population of hypogonadal men, advancing ages of men attempting to establish pregnancy, and the decreases in testosterone levels seen in these men as they age. Harman et al.7 in their Baltimore Longitudinal Study of Aging looked at the prevalence of hypogonadism as defined by a low testosterone level or free testosterone index, and found that men 20–45 years of age have a 3%–8% incidence of hypogonadism. The age of fathers per live birth has risen over the last 30 years; more live births are occurring with fathers aged 35–44 than were occurring in 1980.3 Data emerging from the hypogonadism in males study hints at the fact that a sizeable percentage of men over age 45 have decreased testosterone levels. In the study, which included 13.8 million men over the age of 45, 38.7% had testosterone levels <300 ng dl−1.2 The use of exogenous testosterone is not limited to men with low testosterone levels; it also is found among adolescents, collegiate athletes, and professional athletes.5,8
Testosterone supplementation impairs fertility
In healthy adult males, testosterone production is regulated by the hypothalamic-pituitary-gonadal axis. Higher cortical centers in the brain signal the hypothalamus, which in turn modulates the anterior pituitary via pulsatile secretion of gonadotropin-releasing hormone (GnRH). GnRH leads to the release of LH from the anterior pituitary, which then stimulates the Leydig cells in the testicles to produce testosterone. As testosterone levels increase, negative feedback occurs on both the hypothalamus and the anterior pituitary. Exogenous testosterone use, therefore, results in both impaired endocrine regulation of GnRH and LH release and subsequent decrease of endogenous testosterone.
The use of testosterone supplementation in men of reproductive age could be a form of male contraception, albeit not consistently effective. While such phenomena are not experienced by every patient, exogenous testosterone can lead to the atrophy of the germinal epithelium in normal men and suppresses spermatogenesis, leading to azoospermia after 10 weeks of use.9 Testicular atrophy is not uncommon, and it usually is reflective of loss of both spermatogenesis and Leydig cell function. Normal men can be expected to rebound after a period of 6–18 months;9 however, patients with impaired spermatogenesis at the initiation of androgen supplementation may remain azoospermic (4%–10%), with significant negative consequences if the patient desires children in the future.10
The majority of prescriptions for testosterone do not come from practicing urologists. Prescribing patterns of testosterone reveal that urologists are the third leading prescribers of testosterone at 15.25%, ranking behind endocrinologists (23.73%) and general practitioners (16.95%).11 Surprisingly, 25% of urologists in one American Urological Association survey reported using testosterone therapy for the treatment of infertility12 even though testosterone is known to have a contraceptive effect. Before initiating testosterone therapy in men who desire to maintain fertility, it is imperative that a semen analysis be evaluated in order to rule out idiopathic male factor infertility or undiagnosed Klinefelter syndrome that can be uncovered before TST is initiated.
Preventing infertility in hypogonadal males receiving testosterone supplementation therapy
So what options exist for those patients who are hypogonadal and require TST for symptomatic relief? Can we protect these patients from becoming sterile as a result of the negative impact of exogenous testosterone on the hypothalamic-pituitary-gonadal axis of these patients?
Human chorionic gonadotropin therapy
A known critical element in the development of healthy spermatogenesis is high intratesticular testosterone.13 In men using exogenous testosterone, these levels can be greatly diminished. Intramuscular human chorionic gonadotropin (hCG) therapy is an option shown to protect against, or at least to diminish, the impact that exogenous testosterone has on intratesticular testosterone levels. In a randomized, controlled trial of 29 healthy men randomly assigned to four groups, testosterone enanthate was given 200 mg per week plus either intramuscular saline, 125, 250, or 500 IU hCG every other day. Sperm, intratesticular testosterone levels, and gonadotropins were measured at day 0 and day 21. Intratesticular testosterone levels were suppressed by 94% in the placebo group, 25% in the 125 IU hCG treatment group, and 7% in the 250 IU hCG treatment group, and they were increased 26% from baseline in the 500 IU hCG treatment group.13 Thus, even with supraphysiologic doses of testosterone replacement, healthy levels of intratesticular testosterone were maintained by low-dose hCG therapy.
The benefits of hCG therapy are not limited to maintaining healthy levels of intratesticular testosterone levels alone. These benefits also include maintenance of spermatogenesis in males receiving testosterone supplementation. We have previously demonstrated the ability of hCG therapy to maintain spermatogenesis in men receiving TST. When 26 hypogonadal men receiving TST via transdermal patches or intramuscular injections and concomitant low-dose hCG were studied retrospectively, factors such as serum and free testosterone, estradiol, serum parameters, and pregnancy rates were evaluated. Results showed no differences in semen parameters during 1 year of follow-up, and none of the men became azoospermic during the treatment.14
These studies indicate that low-dose hCG may be beneficial for men requiring testosterone supplementation therapy during their reproductive years and that intramuscular or transdermal TST does not necessarily significantly impact spermatogenesis. Further studies are needed to determine whether this benefit is sustained both qualitatively and quantitatively.
Selective estrogen receptor modulators
The ideal testosterone therapy for male patients suffering from hypogonadism not only would correct signs and symptoms of androgen deficiency, such as decreased libido, erectile dysfunction, and depression, but also would have predictable pharmacokinetics and would not interfere with the patient's sperm production. In order to make treatment and compliance more realistic and sustainable, testosterone-enhancing therapy would also come in a form that allowed for a convenient and acceptable route of administration, preferably in pill form.
One therapy that may help to alleviate azoospermia and avoid infertility associated with TST is use of selective estrogen receptor modulators (SERMs). SERMs are a class of compounds that act on the estrogen receptor. However, they are unique in that they are not pure receptor agonists and antagonists but have variable effects depending upon the tissue type. In the brain SERMs are antagonists for the estrogen receptors, and act to antagonize the effects of estrogen on the hypothalamus and anterior pituitary.
Clomiphene citrate (Clomid) is a SERM composed of a trans-isomer and longer acting zu-isomer. It was first used clinically in the 1960's to enhance ovulation, but has been used off-label to treat secondary hypogonadism and male infertility. Through modulation of the estrogen receptors in the hypothalamus and anterior pituitary, clomiphene citrate increases production of LH and FSH, and thus leads to increased production of intra-testicular testosterone.
Katz et al. conducted a prospective study of the efficacy and safety of prolonged clomiphene citrate therapy (average duration of therapy was 19 months) in 86 men with clinically confirmed hypogonadism. Average age of the participants was 29 years. Treatment was initiated with administration of 25 mg of clomiphene citrate every other day with a target total serum testosterone level of 550 ± 50 ng dl−1. For those patients not meeting the target level, doses were titrated up to 50 mg. Results demonstrated an increase in testosterone and gonadotropin levels, improvement in symptoms of hypogonadism as indicated by the androgen deficiency in aging males (ADAM) questionnaire, and importantly, no major side effects associated with the therapy.15 Another study of 46 hypogonadal men (mean age 44 years) with a baseline testosterone level of 228 ng dl−1 showed clinical improvement when given clomiphene citrate therapy (≥12 months) as ADAM scores improved from 7 before therapy to 3 after 1 year. Testosterone levels at 1, 2, and 3 years of follow-up were 612 ng dl−1, 562 ng dl−1, and 582 ng dl−1, respectively.16 Furthermore, we previously demonstrated a similar degree of satisfaction and hypogonadal symptoms among patients who used clomiphene citrate compared with patients receiving testosterone gel or testosterone injection therapy.17
Enclomiphene citrate is the more potent, but shorter acting trans-isomer of clomiphene citrate that inhibits the negative feedback loop of estrogen on higher cortical centers. A randomized, open-label, fixed dose, active control, phase IIB proof-of-concept study was designed to evaluate fertility in men with secondary hypogonadism who previously had been treated with 1% testosterone gel for at least 6 months. After the 1% testosterone gel was discontinued, the treatment group was given 25 mg enclomiphene citrate, while the control group was given 1% testosterone gel, and results were compared at 3 and 6 months. Enclomiphene citrate restored both testosterone and sperm counts, while also elevating LH and FSH levels in the treatment group.18 In a multicenter, double-blind, placebo-controlled phase III study of enclomiphene citrate, 120 hypogonadal males were assigned to one of four treatment groups receiving 12.5 mg or 25 mg enclomiphene citrate therapy or 1% testosterone gel or were randomly assigned to the placebo group, and primary and secondary endpoints were measured. The primary endpoint was changes in baseline testosterone, and secondary endpoints included changes in FSH and LH levels, ophthalmic safety, and reproductive safety, as assessed by changes in semen quality. The study found that enclomiphene citrate was as effective as 1% testosterone gel in elevating total testosterone levels and maintained sperm concentrations at levels near those of the placebo group and much higher than those of the 1% testosterone gel treatment group.18,19
Enclomiphene citrate may represent an oral option for treatment of hypogonadism in younger patients who desire to raise testosterone levels, while also maintaining semen quality.20 Future phase IIIB studies are planned to verify the efficacy of enclomiphene citrate in maintaining semen quality.
Recovery of spermatogenesis in steroid suppressed patients
For healthy patients who use exogenous testosterone and are unable to establish a pregnancy because of the deficient spermatogenesis, there are now solutions to reverse the negative impact of testosterone supplementation. In our experience treatment involves discontinuation of exogenous testosterone and administration of 3000 units of hCG (either with the aromatase inhibitor anastrozole or the selective estrogen receptor modulator tamoxifen or clomiphene citrate) intramuscularly every other day for 3 or more months. As higher doses of hCG are known to suppress FSH levels, simultaneous administration of clomiphene citrate not only preserves, but enhances the secretion of FSH and LH from the anterior pituitary. With such treatments, testosterone-induced azoospermia was successfully reversed with hCG therapy in nearly all men receiving treatment. While further studies need to be carried out, every-other-day intramuscular hCG therapy is a viable option in the treatment of men who suffer suppressed spermatogenesis due to testosterone replacement. However, recovery is not immediate; patient spermatogenesis returned in 4–6 months.
Algorithm for simultaneous treatment of hypogonadism and preservation of fertility
An algorithm based on historical evidence may be followed in determining the appropriate course of therapy for men who desire to maintain fertility yet wish to correct their significant symptoms of hypogonadism with TST (Figure 1). In men seeking for hypogonadal symptoms and low testosterone, the first question addressed must be whether fertility is desired. If it is not, the patient may maintain testicular size by adding 1500 IU hCG weekly. If the patient desires to maintain some degree of testicular size, he may cycle off of TST every 6 months, with a 4 week treatment cycle of 3000 IU hCG every other day. If a man does wish to maintain fertility, a baseline semen analysis should be performed and the timeframe for which the patient desires to establish a pregnancy discussed. For those patients desiring to establish a pregnancy within 6 months, testosterone therapy should be discontinued, and treatment begun with 3000 IU hCG ± clomiphene citrate (25 mg daily) and a semen analysis performed every 2 months. If the semen analysis remains suboptimal and FSH continues to be suppressed, adding Gonal f (FSH) 75 IU to an hCG regimen can be considered. In those patients desiring to establish a pregnancy within 6–12 months, testosterone therapy can be continued with 500 IU hCG every other day ± clomiphene citrate. Those patients desiring to establish a pregnancy after more than 12 months should cycle off testosterone every 6 months with a 4 week cycle of 3000 IU hCG every other day.
Figure 1.
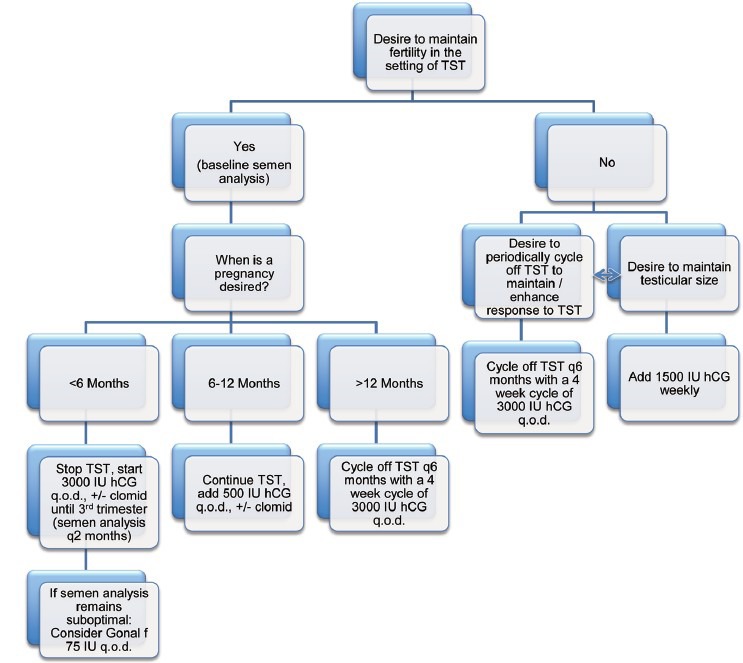
Algorithm for management of hypogonadal men who wish to preserve fertility. hCG: human chorionic gonadotropin; q.o.d.: every other day; TST: testosterone supplementation therapy.
Going back to the clinical vignette that introduced this discussion, on the basis of this algorithm, the man with a serum testosterone level of 175 ng dl−1, but desiring to establish a pregnancy within 6 months could be started on 3000 IU of hCG plus clomiphene citrate. This would effectively increase his serum testosterone levels alleviating most hypogonadal symptoms, while maintaining or even enhancing his ability to establish a pregnancy with his wife.
CONCLUSIONS
The incidence of testosterone supplementation among men during their reproductive years is increasing. Various options exist to re-initiate sperm production and to mitigate the suppression of spermatogenesis following TST including hCG therapy. These therapies are successful but in a time-dependent manner. For those men who seek TST because of symptoms of androgen deficiency, concurrent low-doses of hCG may be a viable option to avoid the azoospermia induced by exogenous testosterone. However, in order to establish hCG treatment as reliable and efficacious a longer study or a larger population is required than that already reported. Encomiphene citrate, a potent isoform of a SERM, may provide a viable future alternative option for testosterone supplementation that may pose a less negative impact on sperm production while still affording the benefits of increased total testosterone levels.
COMPETING INTERESTS
Larry I Lipshultz is a clinical trials participant, consultant and speaker for Auxilium and Endo.
ACKNOWLEDGMENTS
RR is an NIH K12 Scholar supported by a Male Reproductive Health Research Career Development Physician-Scientist Award (HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Program. The authors would like to thank James M Dupree, MD and Gavin M Langille, MD for their assistance with the figure.
REFERENCES
- 1.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465–6. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–9. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovac JR, Addai J, Smith RP, Coward RM, Lamb DJ, et al. The effects of advanced paternal age on fertility. Asian J Androl. 2013;15:723–8. doi: 10.1038/aja.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjöqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371:1872–82. doi: 10.1016/S0140-6736(08)60801-6. [DOI] [PubMed] [Google Scholar]
- 5.Buckley WE, Yesalis CE, 3rd, Friedl KE, Anderson WA, Streit AL, et al. Estimated prevalence of anabolic steroid use among male high school seniors. JAMA. 1988;260:3441–5. [PubMed] [Google Scholar]
- 6.Layton JB, Li D, Meier CR, Sharpless JL, Stürmer T, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835–42. doi: 10.1210/jc.2013-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 8.McCabe SE, Brower KJ, West BT, Nelson TF, Wechsler H. Trends in non-medical use of anabolic steroids by U.S. college students: results from four national surveys. Drug Alcohol Depend. 2007;90:243–51. doi: 10.1016/j.drugalcdep.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet. 1990;336:955–9. [PubMed] [Google Scholar]
- 10.Gu Y, Liang X, Wu W, Liu M, Song S, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910–5. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- 11.Samplaski MK, Loai Y, Wong K, Lo KC, Grober ED, et al. Testosterone use in the male infertility population: prescribing patterns and effects on semen and hormonal parameters. Fertil Steril. 2014;101:64–9. doi: 10.1016/j.fertnstert.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES., Jr Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187:973–8. doi: 10.1016/j.juro.2011.10.137. [DOI] [PubMed] [Google Scholar]
- 13.Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005;90:2595–602. doi: 10.1210/jc.2004-0802. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013;189:647–50. doi: 10.1016/j.juro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 15.Katz DJ, Nabulsi O, Tal R, Mulhall JP. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 2012;110:573–8. doi: 10.1111/j.1464-410X.2011.10702.x. [DOI] [PubMed] [Google Scholar]
- 16.Moskovic DJ, Katz DJ, Akhavan A, Park K, Mulhall JP. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012;110:1524–8. doi: 10.1111/j.1464-410X.2012.10968.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramasamy R, Scovell JM, Kovac JR, Lipshultz LI. Testosterone supplementation versus clomiphene citrate for hypogonadism: an age matched comparison of satisfaction and efficacy. J Urol. 2014;192:875–9. doi: 10.1016/j.juro.2014.03.089. [DOI] [PubMed] [Google Scholar]
- 18.Kaminetsky J, Werner M, Fontenot G, Wiehle RD. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med. 2013;10:1628–35. doi: 10.1111/jsm.12116. [DOI] [PubMed] [Google Scholar]
- 19.Wiehle R, Cunningham GR, Pitteloud N, Wike J, Hsu K, et al. Testosterone restoration by enclomiphene citrate in men with secondary hypogonadism: pharmacodynamics and pharmacokinetics. BJU Int. 2013 doi: 10.1111/bju.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiehle RD, Fontenot GK, Wike J, Hsu K, Nydell J, et al. Enclomiphene citrate stimulates testosterone production while preventing oligospermia: a randomized phase II clinical trial comparing topical testosterone. Fertil Steril. 2014;102:720–7. doi: 10.1016/j.fertnstert.2014.06.004. [DOI] [PubMed] [Google Scholar]


