Abstract
Background and Aims:
Obese patients are more vulnerable to residual neuromuscular block (NMB) and its associated complications in the post-operative period. This study was carried out to compare neostigmine induced reversal of vecuronium in normal weight, overweight and obese female patients, objectively using neuromuscular (NM) monitoring.
Methods:
Twenty female patients each belonging to normal weight, overweight and obese, based on body mass index, requiring general anaesthesia were recruited for this prospective cross sectional study. NMB was induced with vecuronium (0.1 mg/kg) dose based on patient's real body weight (RBW) and monitored using acceleromyographic train of four (TOF). All patients received neostigmine 40 μg/kg and glycopyrrolate 10 μg/kg at 25% of spontaneous recovery of first twitch height (T1) of TOF (DUR 25%) and were allowed to recover to TOF ratio of 0.9. Statistical analysis was done using analysis of variance test.
Results:
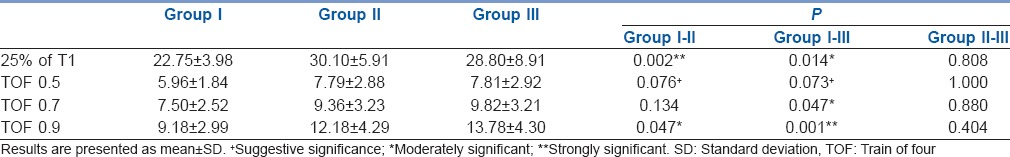
Recovery of TOF ratio to 0.5 was comparable in all three groups. Recovery of TOF ratio to 0.7 was delayed in obese (9.82 ± 3.21 min) compared with normal weight group (7.50 ± 2.52 min). Recovery of TOF to 0.9 was significantly delayed in both overweight (12.18 ± 4.29 min) and obese patients (13.78 ± 4.30 min). DUR 25% was significantly longer in overweight (mean, standard deviation [range]; 30.10 [19–40 min]) and obese (28.8 [12–45 min]) compared with normal weight patients (22.75 [16–30 min]).
Conclusion:
In overweight and obese patients, when vecuronium induction dose is based on RBW, neostigmine induced recovery of NMB is delayed in late phases (TOF 0.7-0.9), which may result in vulnerability for associated complications of incomplete recovery. Ensuring safe recovery thus requires objective NM monitoring.
Keywords: Neostigmine, neuromuscular monitoring, obesity, vecuronium bromide
INTRODUCTION
Over decades, the incidence of obesity has tripled, and number of obese patients undergoing surgery has also increased.[1] Diseases associated with obesity such as diabetes, hypertension, cardiopulmonary diseases and obstructive sleep apnoea reduce the margin of safety of anaesthetic drugs.[2] Body composition, regional blood flow and tissue affinity alter distribution of drugs in these patients posing a significant challenge to anaesthesiologist.[3]
Vecuronium is an intermediate acting neuromuscular blocker (NMB) mainly distributed in lean body mass. Hence, vecuronium dose based on the real body weight (RBW) in obese patients results in relative over dosing, with significantly increased duration of action and slower spontaneous recovery, compared to normal weight patients.[4,5] Further, the speed of facilitated recovery with anticholinesterases may also be prolonged.
The reversal of vecuronium induced NMB is not clearly understood in obese patients. Objective neuromuscular (NM) monitoring allows clinicians for accurate use of reversal agents.[6] Ensuring adequate recovery from NMB is mandatory to prevent any residual block and its associated complications such as aspiration, hypoventilation and airway obstruction secondary to impaired pharyngeal and laryngeal function, attenuation of hypoxic ventilatory response and unpleasant symptoms of muscle weakness.[7,8]
This study was designed primarily to compare the efficacy of neostigmine in reversing the NMB, induced by vecuronium dose based on the patient's RBW in normal weight, overweight and obese female patients. Time taken for recovery of NM function following administration of neostigmine to train of four (TOF) ratios of 0.5, 0.7 and 0.9 were studied. The time taken from administration of the last dose of vecuronium to spontaneous recovery of twitch height (T1) to 25% of control (DUR 25%) in each group was also noted.
METHODS
This prospective cross sectional study was conducted between July 2009 and July 2011, after approval of the protocol by Institutional Ethics Committee. Three groups of 20 patients each were recruited, after their written informed consent for the study. Grouping of patients was based on body mass index (BMI = weight [kg]/height2 [m]), as Group I = Normal weight (BMI 18 to <25), Group II = Overweight (BMI 25 to <30) and Group III = Obese (BMI ≥ 30). The primary end point of the study was time taken for NM function to reach a TOF of 0.9 in all the three groups. A sample size of 20 to ensure at least a mean difference of 2 min between two groups to represent 90% power and 5% type 1 error was arrived at. This was based on previous study by Suzuki et al.[9]
Female patients belonging to American Society of Anaesthesiologists (ASA) physical status 1 and 2, aged between 18 and 60 years, posted for elective surgery under general anaesthesia were included in the study. Pregnant and lactating women, patients with NM disorders, hepatic and renal disease or on any drugs known to interact with NMB were excluded from the study.
All patients were pre-medicated with tablet ranitidine 150 mg HS and tablet alprazolam 0.25 mg HS on the previous day of surgery, with overnight fasting. In the operation theatre, electrocardiogram, non-invasive blood pressure, capnography and pulse oximetry were monitored. Acceleromyographic TOF monitor (TOF GUARD™, Organon Technika Laboratories) along with surface temperature probe was attached to the hand. Normothermia was maintained throughout the procedure.
Patients received intravenous (IV) glycopyrrolate 0.2 mg and ondansetron 4 mg. After pre-oxygenation, they were induced with IV fentanyl 2 mcg/kg, followed by IV propofol 2 mg/kg or till the loss of verbal contact. Later, ulnar nerve was stimulated at the wrist with supra-maximal stimuli of 0.2 ms duration, delivered in a TOF mode at 2 Hz every 15 s and contraction of the ipsilateral adductor pollicis muscle was measured using acceleromyography. The T1 of TOF and TOF ratio measured at the end of control stimulation was regarded as the baseline value. Vecuronium 0.1 mg/kg based on patient's RBW was given. Patients were ventilated with 40% oxygen, 60% nitrous oxide and uniform concentration of isoflurane in all groups. Airway was secured with adequate size cuffed endotracheal tube after 3 min of vecuronium administration. Repeat doses of vecuronium 1 mg was given at 45 min after first dose and then at every 30 min, based on institutional practice, till the completion of surgery.
During recovery, NM block was monitored using acceleromyographic TOF by assessing contraction of adductor pollicis muscle. At DUR 25%, IV neostigmine 40 mcg/kg and glycopyrrolate 10 mcg/kg were given to reverse the NMB. Patients were then allowed to recover to a TOF ratio of 0.9. Demographic details and variables including (a) DUR 25% (b) time taken for facilitated recovery to TOF ratios of 0.5, 0.7 and 0.9 following neostigmine administration (c) total number of doses of vecuronium (d) duration of surgery were measured.
The statistical software SPSS18.0 (PASW Statistics, 18.0, SPSS Inc., Chicago, IL, USA) was used for the analysis of the data. Results on continuous measurements were presented on mean ± standard deviation (SD) and results on categorical measurements were presented in a number (%). To measure the significance between the groups, inferential statistics was analysed by analysis of variance test. P < 5% was considered as significant.
RESULTS
Overweight and obese patients were found to be more in older age groups (age in years in Group I [33.80 ± 12.73], Group II [42.65 ± 12.11] and Group III [45.30 ± 9.97]) with statistical significance (P = 0.008). ASA grading, baseline vital parameters (heart rate, blood pressure and oxygen saturation) and skin temperature were comparable in all three groups. Statistically significant difference in BMI was observed among the groups, which validate the grouping [Table 1].
Table 1.
Comparison of BMI (kg/m2) among three groups

DUR 25% was significantly longer in overweight (30.10 ± 5.91 min) and obese patients (28.80 ± 8.91 min) when compared with normal weight (22.75 ± 3.98 min) patients (P = 0.002 and 0.014 respectively). Time taken for recovery of TOF to 0.5 following reversal was comparable in all three groups. Recovery of TOF ratio to 0.7 was delayed in obese (9.82 ± 3.21 min) as compared to normal weight group (7.50 ± 2.52 min) with P = 0.047. Furthermore, recovery of TOF to 0.9 was delayed in both overweight (12.18 ± 4.29 min) and obese patients (13.78 ± 4.30 min), which was statistically significant [Table 2] with P = 0.047 and 0.001 respectively.
Table 2.
Comparison of spontaneous recovery of T1 and TOF ratio (min) among three groups
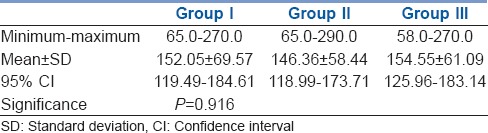
Mean number of repeat doses of vecuronium received by Groups I, II and III were 3.75 ± 2.57, 3.80 ± 2.22 and 3.90 ± 2.25 respectively. They were statistically similar in all three groups with P = 0.979 [Figure 1]. Duration of surgery was comparable among all three groups with P = 0.916 [Table 3].
Figure 1.
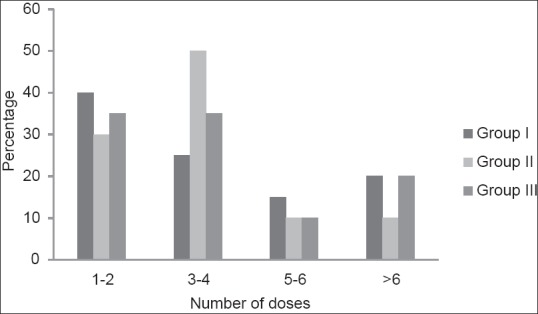
Comparison of number of repeat doses of vecuronium
Table 3.
Comparison of total duration (min) of surgery among three groups
DISCUSSION
Obese patients pose a significant challenge to anesthetic management. The physiological and anthropometric changes associated with obesity alter the pharmacokinetic properties of most drugs.[2,10] Obese individuals are often excluded from clinical trials despite the growing recognition of the impact of obesity on pharmacokinetic and pharmacodynamic properties of the drugs. Dosing information in the package inserts is usually based on the kilogram of RBW, which can result in incorrect doses when applied to the obese patients.[11]
Previous studies have demonstrated that obese patients have prolonged duration of action when vecuronium is given based on RBW.[4,5] Weinstein et al. demonstrated that vecuronium had prolonged duration of action in obese patients probably due to overdose and impaired clearance.[4] Our study was undertaken to evaluate whether these factors are likely to affect the neostigmine induced recovery of NM block also.
We studied recovery indices in 60 patients when vecuronium and neostigmine were given based on RBW. ASA grading and vital parameters were comparable in all three groups. Standard anaesthetic technique included the uniform concentration of isoflurane in all groups. In the study by Ingrande and Lemmens obese subjects given 0.6 minimum alveolar concentration of isoflurane for surgery lasting 2-4 h showed similar recovery profiles as non-obese subjects. In addition, the time constant for isoflurane to reach equilibrium with adipose tissue is approximately 2110 min, much longer than most surgical cases. This property, coupled with low adipose tissue blood flow, diminish the effect of excess adiposity on isoflurane distribution and recovery.[12] Relatively older patients in obese (45.3 ± 9.97 years) and overweight (42.65 ± 12.11 years) groups in our study were considered clinically not significant, as all patients enrolled were <60 years of age. McCarthy et al. noted duration of action of vecuronium and recovery indices are prolonged only in elderly patients (80 ± 4.6 years) and not in younger (34 ± 11.1 years) and middle aged (60 ± 5.8 years) group.[13]
Vecuronium has weak to moderate lipophilic property due to its steroidal structure and is mainly distributed in lean tissues.[1,14] Obese patients have larger lean body mass and fat mass than lean patients.[3] Therefore, when vecuronium is given based on RBW, it results in over dosing and prolonged duration of action.[3] In our study, DUR 25% of control was significantly longer in the overweight (mean, SD [range]; 30.10 [19–40 min]) and obese (28.8 [12–45 min]) as compared to normal weight patients (22.75 [16–30 min]). Our findings are comparable to study done by Suzuki et al.[9] They found that DUR 25% was increased with increase in BMI. Overweight and obese patients took longer time (mean [49.3 min] and [68.4 min] respectively) to recover as compared to normal weight patients (41 min).[9] The relatively longer recovery time in their study could be because of single dose administration of vecuronium with short duration of surgery. However, their study did not address the impact of multiple doses required in long duration surgeries. In our study, induction dose of vecuronium (0.1 mg/kg) used was based on RBW. Literature is limited on specific recommendations for repeat doses of vecuronium in obese and overweight patients. It may be ideal to use NM monitor in such a scenario. However, in view of longer duration of surgery, repeat doses of vecuronium 1 mg were used uniformly in all patients as per our routine institutional protocol, with adequate clinical NM blockade. The resultant dose was much lower than the dose calculated based on RBW in obese and overweight patients. Fisher and Rosen's pharmacokinetic and pharmacodynamic modelling attributes the large initial dose of vecuronium to slow recovery.[15] Weinstein et al. reported in their study that DUR 25% was longer in obese (14.6 ± 6.7 min) as compared to the control group (6.9 ± 1.9 min).[4] Schwartz et al. in their study with vecuronium noted an increase in time to recover to 5-25% of T1 in obese patients (14.9 ± 4.0 min) as compared to control group (10.0 ± 1.7 min) and attributed this to a large total dose of vecuronium.[5]
Reversal time is determined by two processes which include direct antagonism by anticholinesterase and spontaneous recovery from the elimination of the drug from the plasma.[16] The optimum time to administer neostigmine for antagonizing vecuronium induced NM blockade is found to be during spontaneous recovery of T1 between 1% and 10%.[17] Baurain et al. noted that highest NM transmission recovery occurs when neostigmine is administered at 25% to 50% recovery of twitch height.[18] In our study, reversal was administered at 25% recovery of T1 .
We noted that time taken for recovery of TOF to 0.5 following reversal was comparable in all three groups. However, recovery to TOF ratio of 0.7 was delayed in obese (9.82 ± 3.21 min) as compared to normal weight group (7.50 ± 2.52 min). Furthermore, recovery of TOF to 0.9 was delayed in both overweight (12.18 ± 4.29 min) and obese patients (13.78 ± 4.30 min) which was statistically significant. Suzuki et al. found that time required to recover to TOF ratio of 0.5 and 0.7 were comparable between the groups and the late phase of recovery to 0.9 was delayed in overweight (3.3–28.5 min) and obese groups (13.5–41.0 min). This was attributed to over dose of vecuronium in obese patients.[9]
Reversal effect of neostigmine usually appears within about 1–2 min and the maximum effect occurs in about 6–10 min.[19,20] It is therefore considered that the early facilitated recovery to a TOF ratio of 0.5 observed in overweight and obese patients may be attributed to competitive antagonism to vecuronium at the NM junction (NMJ) because of increased acetylcholine concentration. However, once the true cholinesterase at the NMJ is totally inactivated, additional neostigmine produces no further increase in available acetylcholine.[19] Recovery to TOF ratio of 0.7 and 0.9 was delayed in overweight and obese, which may represent a balance between spontaneous recovery (elimination of drug from plasma) from overdosed vecuronium induced block and the waning reversal effect of neostigmine. Residual paralysis causes increased airway collapsibility in obese individuals.[8] Clinical parameters used for NM recovery such as patient's ability to maintain a 5 s head lift and hand grip maybe present at TOF ratio of 0.7 and does not ensure complete recovery.[21] Clinical evidence clearly indicates that low levels of residual paralysis corresponding to TOF ratio 0.7–0.9 may be harmful.[22] Therefore, the delay in recovery between TOF ratio of 0.7-0.9 in overweight and obese patients in our study has important clinical relevance for patient safety. This emphasises the need for objective NM monitoring in overweight and obese patients.
Baurain et al. studied the conditions to optimize the reversal action of neostigmine upon vecuronium induced NMB and concluded that in order to obtain the highest NM transmission recovery (TOF of 0.9), 40 mcg/kg dose of neostigmine has to be given at 25–50% recovery of twitch height. Increasing the dose of neostigmine to 80 mcg/kg did not accelerate the recovery.[18] According to Donati et al. higher doses of neostigmine (50 μg/kg) can antagonize the block more rapidly than smaller doses (5, 10, 20 μg/kg).[23] To reverse the profound block, maximum dose of neostigmine (70–80 μg/kg) may be used for a better recovery profile.[23,24] Anticholinesterases exhibit a ceiling effect when used in large doses and recovery will not be accelerated with further increase in dose of neostigmine.[20,25] Kopman and Eikermann used low dose of neostigmine (15–20 μg/kg for TOF count of four and 40–50 μg/kg for TOF count of two or three) to minimise potential cardiovascular and respiratory side effects when NMB was not intense.[20] The recommended dose of neostigmine is 40–80 μg/kg, not exceeding a total of 5 mg.[3] In obese subjects, recovery of NM function after reversal with neostigmine is found to be incomplete as compared to normal weight subjects.[12] Further studies are recommended to assess the dose of neostigmine in overweight and obese patients in order to obtain safe anaesthetic outcome.
Sugammadex is a specifically designed gamma cyclodextrin and selective relaxant binding drug that rapidly reverses the effects of rocuronium and vecuronium induced block.[26] However, cost and non-availability may still be considered as major limiting factors for its widespread use.[27] Therefore, use of neostigmine as a reversal agent continues to be relevant. Decisions with regard to dosage and timing of neostigmine in overweight and obese patients require clinical and NM monitoring for a safe recovery.[21] Further studies are required for optimising the dosing of neostigmine in obese and overweight patients.
CONCLUSION
When vecuronium is administered based on RBW, the total reversal time by neostigmine 40 mcg/kg, is prolonged in overweight and obese patients compared to normal weight patients. Early recovery of TOF to 0.5 was comparable in all three groups, recovery of TOF to 0.7 was delayed in obese and TOF to 0.9 was delayed in both obese and overweight patients. This finding of incomplete NM recovery has important clinical significance in view of associated complications. Vecuronium induction dose, based on RBW in obese and overweight groups has longer duration of action with slower spontaneous recovery (DUR 25%). Clinical evaluation of adequate recovery should be supplemented with objective monitoring of NM function for patient safety.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Donohoe CL, Feeney C, Carey MF, Reynolds JV. Perioperative evaluation of the obese patients. J Clin Anesth. 2011;23:575–86. doi: 10.1016/j.jclinane.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Lemmens HJ. Perioperative pharmacology in morbid obesity. Curr Opin Anaesthesiol. 2010;23:485–91. doi: 10.1097/ACO.0b013e32833b0a8c. [DOI] [PubMed] [Google Scholar]
- 3.Lemmens HJ, Ingrande J. Pharmacology and obesity. Int Anesthesiol Clin. 2013;51:52–66. doi: 10.1097/AIA.0b013e31829a4d56. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein JA, Matteo RS, Ornstein E, Schwartz AE, Goldstoff M, Thal G. Pharmacodynamics of vecuronium and atracurium in the obese surgical patient. Anesth Analg. 1988;67:1149–53. [PubMed] [Google Scholar]
- 5.Schwartz AE, Matteo RS, Ornstein E, Halevy JD, Diaz J. Pharmacokinetics and pharmacodynamics of vecuronium in the obese surgical patient. Anesth Analg. 1992;74:515–8. doi: 10.1213/00000539-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Brull SJ, Murphy GS. Residual neuromuscular block: Lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth Analg. 2010;111:129–40. doi: 10.1213/ANE.0b013e3181da8312. [DOI] [PubMed] [Google Scholar]
- 7.Murphy GS, Brull SJ. Residual neuromuscular block: Lessons unlearned. Part I: Definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111:120–8. doi: 10.1213/ANE.0b013e3181da832d. [DOI] [PubMed] [Google Scholar]
- 8.Herbstreit F, Peters J, Eikermann M. Impaired upper airway integrity by residual neuromuscular blockade: Increased airway collapsibility and blunted genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology. 2009;110:1253–60. doi: 10.1097/ALN.0b013e31819faa71. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Masaki G, Ogawa S. Neostigmine-induced reversal of vecuronium in normal weight, overweight and obese female patients. Br J Anaesth. 2006;97:160–3. doi: 10.1093/bja/ael142. [DOI] [PubMed] [Google Scholar]
- 10.Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000;85:91–108. doi: 10.1093/bja/85.1.91. [DOI] [PubMed] [Google Scholar]
- 11.Pai MP, Paloucek FP. The origin of the “ideal” body weight equations. Ann Pharmacother. 2000;34:1066–9. doi: 10.1345/aph.19381. [DOI] [PubMed] [Google Scholar]
- 12.Ingrande J, Lemmens HJ. Anesthetic Pharmacology and the morbidly obese patient. Curr Anesthesiol Rep. 2013;3:10–17. doi: 10.1007/s40140-012-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy G, Elliott P, Mirakhur RK, Cooper R, Sharpe TD, Clarke RS. Onset and duration of action of vecuronium in the elderly: Comparison with adults. Acta Anaesthesiol Scand. 1992;36:383–6. doi: 10.1111/j.1399-6576.1992.tb03485.x. [DOI] [PubMed] [Google Scholar]
- 14.Weindlamayr-Goettel M, Gilley H, Sipos E, Steinbereithner K. Lipid solubility of pancuronium and vecuronium determined by n-octanol/water partitioning. Br J Anaesth. 1993;70:579–80. doi: 10.1093/bja/70.5.579. [DOI] [PubMed] [Google Scholar]
- 15.Fisher DM, Rosen JI. A pharmacokinetic explanation for increasing recovery time following larger or repeated doses of nondepolarizing muscle relaxants. Anesthesiology. 1986;65:286–91. [PubMed] [Google Scholar]
- 16.Beemer GH, Bjorksten AR, Dawson PJ, Dawson RJ, Heenan PJ, Robertson BA. Determinants of the reversal time of competitive neuromuscular block by anticholinesterases. Br J Anaesth. 1991;66:469–75. doi: 10.1093/bja/66.4.469. [DOI] [PubMed] [Google Scholar]
- 17.Kirkegaard-Nielsen H, Toft P, Severinsen IK, May O. Optimum time for neostigmine administration to antagonize vecuronium-induced neuromuscular blockade. Eur J Anaesthesiol. 1995;12:585–9. [PubMed] [Google Scholar]
- 18.Baurain MJ, Dernovoi BS, D'Hollander AA, Hennart DA, Cantraine FR. Conditions to optimise the reversal action of neostigmine upon a vecuronium-induced neuromuscular block. Acta Anaesthesiol Scand. 1996;40:574–8. doi: 10.1111/j.1399-6576.1996.tb04490.x. [DOI] [PubMed] [Google Scholar]
- 19.Kirkegaard-Nielsen H, Helbo-Hansen HS, Lindholm P, Severinsen IK, Pedersen HS, Jensen EW. Optimum time for neostigmine reversal of atracurium-induced neuromuscular blockade. Can J Anaesth. 1996;43:932–8. doi: 10.1007/BF03011807. [DOI] [PubMed] [Google Scholar]
- 20.Kopman AF, Eikermann M. Antagonism of non-depolarising neuromuscular block: Current practice. Anaesthesia. 2009;64(Suppl 1):22–30. doi: 10.1111/j.1365-2044.2008.05867.x. [DOI] [PubMed] [Google Scholar]
- 21.Claudius C, Garvey LH, Viby-Mogensen J. The undesirable effects of neuromuscular blocking drugs. Anaesthesia. 2009;64(Suppl 1):10–21. doi: 10.1111/j.1365-2044.2008.05866.x. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs-Buder T, Schreiber JU, Meistelman C. Monitoring neuromuscular block: An update. Anaesthesia. 2009;64(Suppl 1):82–9. doi: 10.1111/j.1365-2044.2008.05874.x. [DOI] [PubMed] [Google Scholar]
- 23.Donati F, McCarroll SM, Antzaka C, McCready D, Bevan DR. Dose-response curves for edrophonium, neostigmine, and pyridostigmine after pancuronium and d-tubocurarine. Anesthesiology. 1987;66:471–6. doi: 10.1097/00000542-198704000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Magorian TT, Lynam DP, Caldwell JE, Miller RD. Can early administration of neostigmine, in single or repeated doses, alter the course of neuromuscular recovery from a vecuronium-induced neuromuscular blockade? Anesthesiology. 1990;73:410–4. doi: 10.1097/00000542-199009000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava A, Hunter JM. Reversal of neuromuscular block. Br J Anaesth. 2009;103:115–29. doi: 10.1093/bja/aep093. [DOI] [PubMed] [Google Scholar]
- 26.Khuenl-Brady KS, Wattwil M, Vanacker BF, Lora-Tamayo JI, Reitbagen H, Alvarez Gomez JA. Sugammadex provides faster reversal of vecuronium induced neuromuscular blockade compared with neostigmine: A multicentric, randomized, controlled trial. Anesth Analg. 2010;110:64–73. doi: 10.1213/ane.0b013e3181ac53c3. [DOI] [PubMed] [Google Scholar]
- 27.Geldner G, Niskanen M, Laurila P, Mizikov V, Hübler M, Beck G, et al. A randomised controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia. 2012;67:991–8. doi: 10.1111/j.1365-2044.2012.07197.x. [DOI] [PubMed] [Google Scholar]


