Abstract
Background and Aim:
The newer trend in regional anaesthesia for ambulatory anorectal surgeries advocate use of lower dose of local anaesthetic, providing segmental block with adjuvants such as opioids and α2 agonists to prolong analgesia. The current study investigated effects of addition of 5 μg of dexmedetomidine to 6 mg of hyperbaric bupivacaine on duration of analgesia, sensory and motor block characteristics for perianal ambulatory surgeries.
Methods:
This study is a prospective randomised controlled double blind study. Forty adult patients between 18 and 55 years of age were divided into 2 groups. Group D received intrathecal 0.5% hyperbaric bupivacaine 6 mg (1.2 ml) with injection dexmedetomidine 5 μg in 0.5 ml of normal saline and Group N received intrathecal 0.5% hyperbaric bupivacaine 6 mg (1.2 ml) with 0.5 ml of normal saline. The parameters assessed were time to regression of sensory blockade, motor blockade, ambulation, time to void, first administration of analgesic. Statistical analysis was done using appropriate tests.
Results:
Time for regression of sensory level and time for first administration of analgesic were prolonged in Group D (430.05 ± 89.13 min, 459.8 ± 100.9 min, respectively) in comparison to Group N (301.10 ± 94.86 min, 321.85 ± 95.08 min, respectively). However, the duration of motor blockade, time to ambulation, and time to void were also significantly prolonged in Group D (323.05 ± 54.58 min, 329.55 ± 54.06 min, 422.30 ± 87.59 min) than in Group N (220.10 ± 63.61 min, 221.60 ± 63.84 min, 328.45 ± 113.38 min).
Conclusion:
Intrathecal dexmedetomidine 5 μg added to intrathecal bupivacaine 6 mg as adjuvant may not be suitable for ambulatory perianal surgeries due to prolongation of motor blockade.
Keywords: Adjuvant, ambulatory, dexmedetomidine, intrathecal
INTRODUCTION
Dexmedetomidine is a selective α2 -adrenergic receptor agonist (α2 -AR agonist). Dexmedetomidine has been found to prolong analgesia when used as an adjuvant to local anaesthetics for subarachnoid block.[1] Analgesic action of α2 -AR agonists is a result of depression of the release of presynaptic C-fibre transmitters and by hyperpolarisation of postsynaptic dorsal horn neurons.[2]
Smith recommended that 90% of anorectal surgeries could be carried out on ambulatory basis.[3,4] The newer trend in regional anaesthesia for ambulatory anorectal surgeries is to use lower dose of local anaesthetic providing segmental block with adjuvants such as opioids and clonidine. Clonidine has been used in low doses for outpatient anaesthesia.[5] Dexmedetomidine is an α2 -AR agonist which is 8–10 times more potent than clonidine. But studies of intrathecal dexmedetomidine for ambulatory surgeries are sparse. Hence, the authors decided to investigate the addition of 5 μg of dexmedetomidine to 6 mg of hyperbaric bupivacaine on duration of analgesia, sensory and motor block characteristics for perianal ambulatory surgeries.
METHODS
After obtaining approval from the Institutional Ethics Committee and informed written consent, 40 adult patients between 18 and 55 years of age of American Society of Anaesthesiologists physical status I and II presenting for perianal surgeries were enrolled in this prospective randomised double-blinded study done between January 2013 and September 2013. We excluded patients on α2-AR antagonists, calcium channel blockers, angiotensin-converting enzyme inhibitors or those with arrhythmias, heart block, neurological and psychiatric disorders or with any contraindication for neuraxial blockade. The various types of perianal surgeries included were fistulectomy, fissurectomy, haemorrhoidectomy, lateral internal sphincterotomy, perianal sinus, perianal abscess incision and drainage.
Before surgery, patients were given instructions to use a 10-point Verbal Rating Scale (VRS)[6] with 0 indicating no pain and 10 indicating the worst imaginable pain. Demographic data such as age, gender and weight were recorded. In the operating room, electrocardiogram, pulse oximetry and non-invasive blood pressure (BP) were monitored, and baseline values were recorded. Sedation was assessed using Ramsay Sedation Score (RSS)[7] and baseline sedation score was noted. Following infusion of 500 ml lactated Ringer's solution and with the patient in the sitting position lumbar puncture was performed at L3-L4 interspace or L4-L5 interspace. Patients were randomised using computer generated random numbers from the website www.random.org into two groups. The randomisation and loading of study drugs were done by a senior anaesthesiologist who was not involved further in the study. Just before spinal anaesthesia, syringe was handed over to the anaesthesiologist performing the subarachnoid block, who was also the observer of the study. Thus, both the observer and the patient were blinded to the study drugs.
Group D received intrathecal 0.5% hyperbaric bupivacaine 6 mg (1.2 ml) with injection dexmedetomidine 5 μg (0.5 ml of injection dexmedetomidine (injection Dextomid™ 100 μg/ml) was diluted with normal saline to 5 ml (10 μg/ml) and 0.5 ml (5 μg) of this solution was added to 1.2 ml bupivacaine with a 1 ml syringe).
Group N received intrathecal 0.5% hyperbaric bupivacaine 6 mg (1.2 ml) with 0.5 ml of normal saline. A trial was conducted with 0.5% hyperbaric bupivacaine in doses of 0.8, 1.0, and 1.2 ml with normal saline (0.5 ml) in 20 patients undergoing perianal surgeries, 6 in 0.8 ml group and 7 each in 1.0 and 1.2 ml groups. It was found that 2 patients (33%) in 0.8 ml group did not achieve adequate sensory blockade for the surgery, one patient (14%) in 1 ml group did not achieve adequate motor blockade and patient was uncomfortable in lithotomy position, all the patients in 1.2 ml group achieved adequate anaesthesia for the surgery and the mean duration of analgesia was 300.42 ± 38.65 min. We hypothesised that the addition of dexmedetomidine would prolong the duration of analgesia. To detect a clinically meaningful difference of 60 min for duration of analgesia, assuming similar standard deviation between two groups, minimum sample size required to attain a power of 80%, keeping alpha error at 0.05 was 8 in each group. However, for better validation of results, we included 20 patients in each group.
After injection of drug (subarachnoid), patients were made to sit for 5 min, after which patients were placed in supine position. Intraoperatively heart rate (HR), systolic BP, diastolic BP and mean arterial pressure (SBP, DBP, MAP), oxygen saturation (SpO2), respiratory rate (RR) and RSS were recorded every 2 min for first 10 min then every 5 min till end of procedure. The sensory block level was assessed using loss of temperature discrimination to cold swab along the midclavicular line bilaterally and lateral part of dorsum of foot (S1) and perianal area and motor level were checked using Breen's Modification of Bromage scale[8] (1 = Complete block, unable to move feet or knees; 2 = Almost complete block, able to move feet only; 3 = Partial block, just able to move knees; 4 = Detectable weakness of hip flexion, between scores 3 and 5;5 = No detectable weakness of hip flexion while supine, full flexion of knees; 6 = Able to perform partial knee bend in standing position). Sensory and motor block levels were noted after completion of 5 min when the patient was made supine and then every 2 min until the start of surgery. Maximum height of the block attained was recorded at 20 min from the time of subarachnoid block. None of the patients required supplemental analgesia intraoperatively. Post-operatively, HR, SBP, DBP, MAP, RR, SpO2, RSS, VRS, sensory and motor levels were noted in immediate post-operative period and then every half hourly till 3 h then at 4th, 5th, 6th, 8th and 24th h. Duration of sensory blockade was defined as the time taken from completion of 5th min after subarachnoid block till the sensory level receded to below S1 dermatome level and total duration of motor blockade was defined as time taken from the completion of 5th min after subarachnoid block till attainment of modified Bromage score 6 (partial knee bend in standing position). Time to ambulation was defined as time from the completion of 5th min after subarachnoid block till the patient was able to ambulate without support, a task that was attempted only after the patient had achieved modified Bromage score 6. Time to void and time for first administration of analgesia both recorded from completion of 5th min after subarachnoid block till the patient was able to first void urine post-operatively or when patient reported a VRS of more than 3, respectively. Analgesic was administered when VRS was more than 3 and consisted of injection diclofenac 75 mg intramuscular that could be repeated after 12 h if needed with a maximum daily dose of 150 mg. Occurrence of nausea, vomiting, hypotension, bradycardia, shivering were recorded throughout the study. Hypotension (defined as a MAP <60 mmHg) was treated with intravenous (IV) boluses of injection ephedrine 6 mg. Bradycardia, defined as a HR of <50 beats/min was treated with boluses of 0.6 mg injection atropine. Nausea/vomiting were treated with injection ondansetron 4 mg IV.
Data were analysed using computer statistical software system SPSS® version 15 (Statistical Packages for the Social Sciences, Chicago, IL, USA), Stata 10.1 (StataCorp. 2007. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP), MedCalc for Windows, version 9.0.1 (MedCalc software, Ostend, Belgium). (Results on continuous measurements are presented on Mean ± standard deviation (Min-Max) and results on categorical measurements are presented in a number. Student's t-test (two-tailed, independent) and Mann-Whitney U-test were used to compare the parametric data between the groups. Chi-square/Fisher Exact test were used to compare nominal data as and when required. P < 0.05 was considered statistically significant.
RESULTS
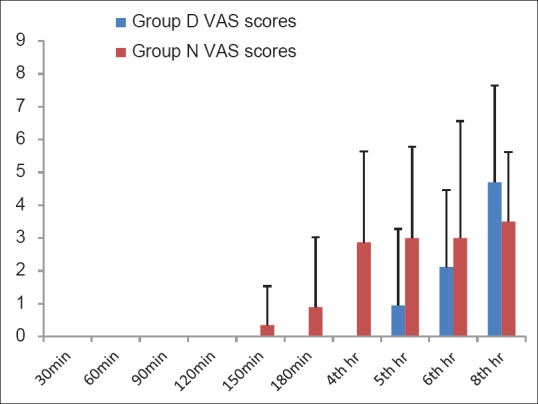
The groups were comparable with respect to age, weight, height, sex distribution and operative time [Table 1]. All the patients achieved sensory level of at least S1 dermatome block and motor blockade of at least modified Bromage score 4, that is, detectable weakness of hip when they were made supine after completion of 5 min after subarachnoid block. There was no difference between Group D and N in the maximum level of blocks achieved (T10). In all the patients, maximum sensory level recorded at 20 min was similar to or higher than the sensory level recorded immediately post-operatively. Time for regression of sensory level to S1 (301.10 ± 94.86 min and 430.05 ± 89.13 min in Group N and Group D respectively, P < 0.001) and time for first administration of analgesic (321.85 ± 95.08 min, 459.8 ± 100.9 min in Group N and Group D, respectively, P < 0.001) were clinically and statistically prolonged in Group D. The duration of motor blockade (220.10 ± 63.61 min, 323.05 ± 54.58 min in Group N and Group D, respectively, P < 0.001), time to ambulation (221.60 ± 63.84, 329.55 ± 54.06 min in Group N and Group D, respectively, P < 0.001) and time to void (328.45 ± 113.38, 422.30 ± 87.59 min in Group N and Group D, respectively, P < 0.007) were significantly delayed in Group D [Table 2]. The post-operative VRS scores were higher in Group N than in Group D after 180 min in the post-operative period [Figure 1]. Intraoperative HR and BP were comparable between the two groups [Figures 2 and 3]. All patients in both the groups were calm and cooperative and no undue sedation (sedation score > 3) was observed intraoperatively (Group D 2.09 ± 0.38, Group N 1.96 ± 0.24, P < 0.203). The post-operative mean sedation scores were also comparable (Group D 2.14 ± 0.50, Group N 2.02 ± 0.21, P < 0.331). The incidence of side effects was not statistically significant in both the groups [Table 3].
Table 1.
Demographics
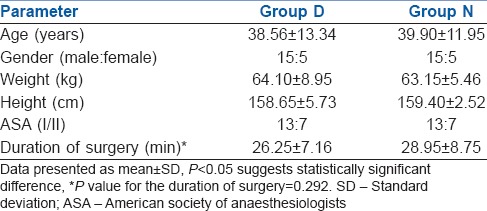
Table 2.
Sensory and motor parameters
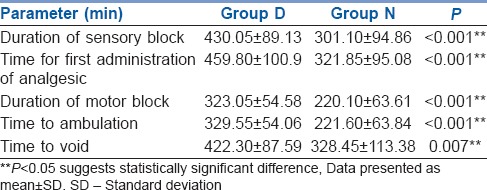
Figure 1.
Post-operative Verbal Rating Scale scores. Data presented as mean ± standard deviation
Figure 2.
Intraoperative heart rate (bpm). Data presented as mean ± standard deviation
Figure 3.
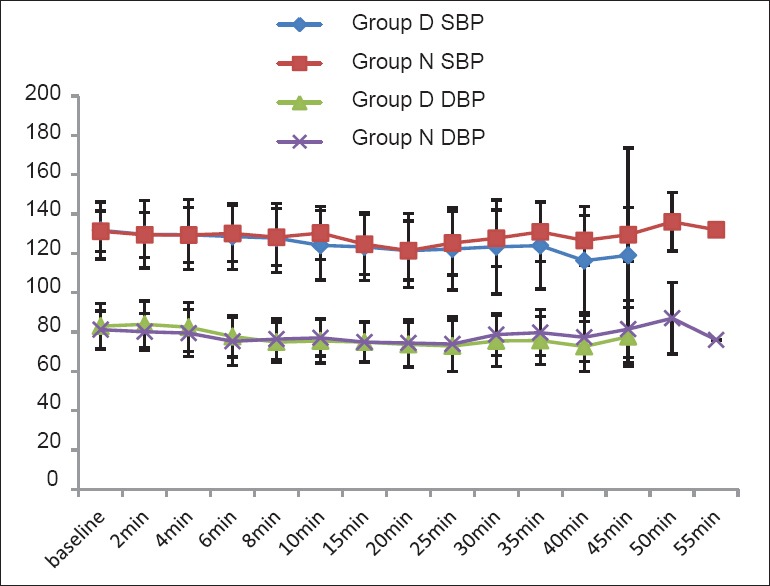
Intraoperative blood pressure (mmHg). Data presented as mean ± standard deviation
Table 3.
Side-effects
DISCUSSION
The recommended dose for subarachnoid block for anorectal surgery is 1–1.5 ml of hyperbaric 0.5% bupivacaine or 5% lignocaine.[5] Initially, we conducted a trial study to determine dose of bupivacaine to be used for the study and it was found that intrathecal bupivacaine 0.5% heavy, 6 mg (1.2 ml) produced better quality of anaesthesia compared to lower doses for anorectal surgery. Dexmedetomidine was available as hospital supply. In our study, we found comparable onset times and maximum height of the blockade achieved in both the groups. The times to administration of analgesic, regression of sensory block to S1 and regression of motor block were prolonged in the dexmedetomidine group.
Dexmedetomidine has been used intrathecally in varying doses ranging from 3 μg to 15 μg.[9,10,11,12,13] The optimal dose of intrathecal dexmedetomidine has not been established. Sullivan et al.[14] have found in their study that ED50 of dexmedetomidine for inhibition of C fibre responses of dorsal horn neurones was 2.5 μg and Aβ-evoked responses were inhibited to a lesser degree with a maximum inhibition seen above 10 μg dose. Hence, in this study, a low dose of 5 μg (more than ED50) was used in order to provide adequate post-operative analgesia, limit the motor blockade and facilitate early recovery and ambulation. Further studies with 3 μg dexmedetomidine need to be done to decide optimal dose for ambulatory surgeries and the use of 5 μg dexmedetomidine which prolonged the motor blockade could be a limitation of our study.
In the current study, the maximum height of the block achieved was comparable between the two groups but the duration of sensory block and post-operative analgesia were prolonged. This was comparable to the results of the study conducted by Kim et al., Kanazi et al. and Abdelhamid et al.[10,11,12]
Kazak et al. in their study with 1.5 mg hyperbaric levobupivacaine for anal surgeries kept the patients in the sitting position at least 20 min in order to confine the small bolus of levobupivacaine to the lower end of the dural sac. Their patients did not have any motor blockade.[15] In our study, 6 mg of hyperbaric bupivacaine was used and the patients were made to sit only for 5 min due to constraints related to operation theatre time management which led to a prolonged motor blockade. Furthermore, the time to ambulation and time to micturition were prolonged in the dexmedetomidine group. Further studies should be done aiming to reduce the motor blockade by decreasing dose of bupivacaine or dexmedetomidine or keeping the patient in sitting position for a longer time to allow fixation of drug and prevent cephalad spread of drug for perianal surgeries.
Dexmedetomidine, an imidazole compound, is the pharmacologically active dextroisomer of medetomidine that displays specific and selective α2-adrenoceptor agonism. Activation of the receptors in the brain and spinal cord inhibits neuronal firing and results in sympatholytic effect, causing hypotension, bradycardia and sedation.[9] The sedation score was low (<3) in all the patients in this study, as in other studies.[16,17,18] There was only one patient with hypotension in the dexmedetomidine group which was corrected with a single dose of vasopressor. The incidence of nausea and vomiting was lower in the dexmedetomidine group, even though it was not statistically significant in concurrence with all the previous published studies.[9,10,11,12,13,14,15,16,17,18]
CONCLUSION
Intrathecal dexmedetomidine 5 μg added to intrathecal bupivacaine 6 mg as adjuvant, administered in sitting position with patients made supine after 5 min of the subarachnoid block provides prolonged post-operative analgesia and it also prolongs the duration of motor blockade, time for ambulation and time to void which can be a hindrance to its routine use in ambulatory care.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sudheesh K, Harsoor SS. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth. 2011;55:323–4. doi: 10.4103/0019-5049.84824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenach JC, De Kock M, Klimscha W. alpha (2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Gupta PJ. Ambulatory proctology surgery - An Indian experience. Eur Rev Med Pharmacol Sci. 2006;10:257–62. [PubMed] [Google Scholar]
- 4.Smith LE. Ambulatory surgery for anorectal diseases: An update. South Med J. 1986;79:163–6. doi: 10.1097/00007611-198602000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Gudaityte J, Marchertiene I, Pavalkis D. Anesthesia for ambulatory anorectal surgery. Medicina (Kaunas) 2004;40:101–11. [PubMed] [Google Scholar]
- 6.Brunelli C, Zecca E, Martini C, Campa T, Fagnoni E, Bagnasco M, et al. Comparison of numerical and verbal rating scales to measure pain exacerbations in patients with chronic cancer pain. Health Qual Life Outcomes. 2010;8:42. doi: 10.1186/1477-7525-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breen TW, Shapiro T, Glass B, Foster-Payne D, Oriol NE. Epidural anesthesia for labor in an ambulatory patient. Anesth Analg. 1993;77:919–24. doi: 10.1213/00000539-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed AA, Fares KM, Mohamed SA. Efficacy of intrathecally administered dexmedetomidine versus dexmedetomidine with fentanyl in patients undergoing major abdominal cancer surgery. Pain Physician. 2012;15:339–48. [PubMed] [Google Scholar]
- 10.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim JE, Kim NY, Lee HS, Kil HK. Effects of intrathecal dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients undergoing transurethral prostatectomy. Biol Pharm Bull. 2013;36:959–65. doi: 10.1248/bpb.b12-01067. [DOI] [PubMed] [Google Scholar]
- 12.Abdelhamid SA, El-Lakany MH. Intrathecal dexmedetomidine: Useful or not? J Anesth Clin Res. 2013;4:351. [Google Scholar]
- 13.Hala EA, Mohamed SA, Hend Y. Dose-related prolongation of hyperbaric bupivacaine spinal anesthesia by dexmedetomidine. Ain Shams J Anesthesiol. 2011;4:83–95. [Google Scholar]
- 14.Sullivan AF, Kalso EA, McQuay HJ, Dickenson AH. The antinociceptive actions of dexmedetomidine on dorsal horn neuronal responses in the anaesthetized rat. Eur J Pharmacol. 1992;215:127–33. doi: 10.1016/0014-2999(92)90617-d. [DOI] [PubMed] [Google Scholar]
- 15.Kazak Z, Ekmekci P, Kazbek Z. Hyperbaric levobupivacaine in anal surgery. Anesthetist. 2010;59:709–13. doi: 10.1007/s00101-010-1755-1. [DOI] [PubMed] [Google Scholar]
- 16.Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl intrathecal bupivacaine on spinal block characteristics in gynaecological procedures: A double blind controlled study. Am J Appl Sci. 2009;6:882–7. [Google Scholar]
- 17.Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J. 2009;30:365–70. [PubMed] [Google Scholar]
- 18.Gupta R, Bogra J, Verma R, Kohli M, Kushwaha JK, Kumar S. Dexmedetomidine as an intrathecal adjuvant for postoperative analgesia. Indian J Anaesth. 2011;55:347–51. doi: 10.4103/0019-5049.84841. [DOI] [PMC free article] [PubMed] [Google Scholar]


