Abstract
Urofacial syndrome (UFS) is an autosomal recessive congenital disease featuring grimacing and incomplete bladder emptying. Mutations of HPSE2, encoding heparanase 2, a heparanase 1 inhibitor, occur in UFS, but knowledge about the HPSE2 mutation spectrum is limited. Here, seven UFS kindreds with HPSE2 mutations are presented, including one with deleted asparagine 254, suggesting a role for this amino acid, which is conserved in vertebrate orthologs. HPSE2 mutations were absent in 23 non-neurogenic neurogenic bladder probands and, of 439 families with nonsyndromic vesicoureteric reflux, only one carried a putative pathogenic HPSE2 variant. Homozygous Hpse2 mutant mouse bladders contained urine more often than did wild-type organs, phenocopying human UFS. Pelvic ganglia neural cell bodies contained heparanase 1, heparanase 2, and leucine-rich repeats and immunoglobulin-like domains-2 (LRIG2), which is mutated in certain UFS families. In conclusion, heparanase 2 is an autonomic neural protein implicated in bladder emptying, but HPSE2 variants are uncommon in urinary diseases resembling UFS.
Keywords: genetics and development, human genetics, molecular genetics, pediatric nephrology
Urofacial, or Ochoa, syndrome (UFS) is an autosomal recessive congenital disease.1–7 Affected children have enuresis and incomplete bladder emptying associated with detrusor and bladder outlet dyssynergic contractions. ESRD, urosepsis and vesicoureteric reflux (VUR) can occur. UFS individuals have abnormal facial movements when crying and smiling. Biallelic mutations of HPSE2 cause UFS.4–7 HPSE2 encodes heparanase 2,8 which inhibits endo-β-D-glucuronidase activity of heparanase 1,9 itself implicated in releasing growth factors from heparan sulfate proteoglycans.10–12
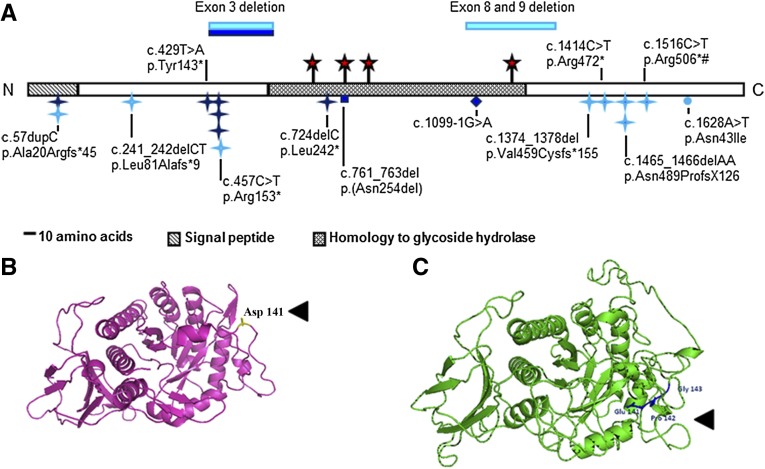
Since the first descriptions of HPSE2 mutations in UFS in 2010,4,5 only two further such families have been reported.6,7 We here describe seven new UFS kindreds with HPSE2 mutations, representing the largest series to date of genetically defined, overtly unrelated families. Their mutations and key clinical features are listed in Table 1. The Supplemental Material details their clinical histories and investigations, and Supplemental Figure 1 illustrates examples of facial grimacing and dysmorphic bladders. Considering these and previously published mutations4–7 (Figure 1A), it is clear that pathogenic HPSE2 mutations are found across the gene’s coding region. Most (i.e., nonsense or frameshift mutations) would cause loss of function, but a subset may generate abnormal proteins. In Family 2, the deleted amino acids in exon 3 correspond to the linker sequence in heparanase 1 cleaved to yield an active enzyme,8–11 and an exon 3 deletion was previously reported in a Spanish child with UFS.4 The in-frame deletion of asparagine 254 in Family 6 targets an amino acid conserved between human, mouse, and frog heparanase 2.12 It is predicted to be nitrogen-linked glycosylated13 (Figure 1A) and may direct endoplasmic reticulum processing of heparanase 2.13 Mahmood et al.7 reported a homozygous HPSE2 mutation changing asparagine 543 to isoleucine. The asparagine, located in the carboxy-terminus, is also highly conserved between vertebrate orthologs,12 but it is not predicted to be glycosylated. Its role needs further study.
Table 1.
Key clinical features and HPSE2 mutations found in the seven families
| Country (Parental Origin) | Family/Case, Sex | Bladder Phenotype | Upper Urinary Tract | Kidney Excretory Function | HPSE2 Mutations |
|---|---|---|---|---|---|
| Saudi Arabia | 1/a, M | Trabeculation | Antenatal unilateral HN; postnatal progressive unilateral HN | Normal at 1 yr | Homozygous c.57dupC (p.Ala20Argfs*45) generating premature stop codon at position 64 |
| Portugal/Romani | 2/a, F | Dysfunctional voiding and postvoiding residual | Normal | Normal at 5 yr | Homozygous apparent in-frame deletion of exon 3 |
| 2/b, M | Dysfunctional voiding and postvoiding residual | Bilateral HN | Normal at 11 yr | ||
| 2/c, F | NR | NR | NR | Compound heterozygous deletion of exon 3 and nonsense mutation c.457C>T (p.Arg153*) | |
| Great Britain/America | 3/a, F | Antenatal megacystis; postnatal trabeculation with dyssynergia in normal-capacity bladder | Unilateral focal kidney defect; no VUR | Normal at 14 yr | Compound heterozygous mutation. c.724delC (p.Leu242*) introducing a premature stop codon after a frameshift, and c.1099–1G>A leading to loss of splice acceptor and introduction of premature stop codon |
| Turkey | 4/a, M | Trabeculation and incomplete emptying | Bilateral HN and bilateral focal kidney defects | Plasma creatinine raised (3.7 mg/dl at 12 yr) | Homozygous nonsense mutation c.457C>T (p.Arg153*) |
| 4/b, M | Pseudodiverticulae | Unilateral VUR | Normal at 11 yr | ||
| Turkey | 5/a, M | Trabeculation with postvoiding residual and high bladder pressure | Bilateral HN and bilateral focal kidney defects | Plasma creatinine raised (3.7 mg/dl at 9 yr) | Homozygous nonsense mutation c.429T>A (p.Tyr143*) |
| 5/b, M | Trabeculation and diverticulae | Normal | Normal at 13 yr | ||
| Saudi Arabia | 6/a, M | Non-neurogenic neurogenic bladder | Unilateral HN and renal parenchymal thinning | NR | Homozygous c.761_763del (p.[Asn254del]), an in-frame deletion of asparagine 254 |
| Kosovo | 7/a, M | Trabeculation and postvoiding residual. Low compliance. | Unilateral HN | Plasma creatinine raised (1.9 mg/dl at 6 yr) | Homozygous nonsense mutation c.457C>T (p.Arg153*) |
M, male; HN, hydronephrosis; F, female; NR, not reported. See Supplemental Material for further clinical details.
Figure 1.
HPSE2 mutations in UFS and the variant in primary VUR. (A) Schematic of heparanase 2 showing location of mutations. Dark blue indicates mutations described in this report. Light blue indicates mutations from previous reports. Blue stars, nonsense or frameshift mutations; circle, missense mutation; diamond, splice-site mutation; red stars, predicted N-glycosylation sites; #, founder mutation in Ochoa’s Columbian cohort. Domains were predicted by Pfam and SignalP. N and C, the proteins amino and carboxy terminals, respectively. (B and C) Wild-type full length wild-type heparanase 2 protein (B) and the c.422_423insGCCCGG-p.Asp141delinsGluProGly variant (C). The heparanase 2 sequence was aligned to 51 α-L-arabinofuranosidase with ClustalW, and the structural model was generated using PHYRE2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index).
Certain HPSE2 mutations found in apparently unrelated UFS families might be explained by founder effects (e.g., c.1465_1466del AA [p.Asn489Profs*126] in Irish kindreds).5 In this study, the c.457C>T (p.Arg153*) mutation was found in Families 2, 4, and 7 from Portugal, Turkey, and Kosovo, respectively, and was reported in Turkish UFS twins.4 Despite sharing a mutation (c.1516C>T, p. Arg506*),5 disease severity varied in Ochoa’s Columbian UFS cohort2 and some individuals were recognized as having UFS only after the syndrome was diagnosed in a relative. In this report, probands in Families 4 and 5 were part of the pediatric cohort 4C Study, all with renal insufficiency and a subset with urinary tract anomalies. In both families, the UFS diagnosis had not been considered before our genetic screen and, moreover, UFS in siblings was recognized only after diagnosis of the syndrome in probands. Thus, UFS is probably underrecognized, particularly when urinary tract features are mild. Such phenotypic variability may be due to environmental influences (e.g., superimposed urosepsis) or genetic modifiers. Mutations of LRIG2, encoding leucine-rich repeats and immunoglobulin-like domains 2, exist in some UFS families lacking HPSE2 mutations.14 We excluded the possibility (data not shown) that probands in Families 4 and 5, who had severe kidney disease, also carried mutations of LRIG2.
Compound heterozygous missense LRIG2 variants have been reported14 in 1 of 23 individuals with non-neurogenic neurogenic bladder, which phenocopies15 the UFS bladder. Moreover, primary nonsyndromic VUR is heritable in humans,16,17 so perhaps primary VUR is associated with rare variants in genes such as HPSE2. HPSE2 was sequenced and multiplex ligation-dependent probe amplification undertaken in 23 non-neurogenic neurogenic bladder individuals.14 No mutations were detected. HPSE2 was sequenced in probands from 193 United Kingdom and 246 Irish families with nonsyndromic VUR.16,17 In one, a heterozygous novel variant (c.422_423insGCCCGG-p.Asp141delinsGluProGly [NM_021828.4]) was detected; he presented neonatally with urosepsis and unilateral VUR. A sister with unilateral VUR carried one copy of the variant, an in-frame deletion of Asp141 with insertion of three residues, GluProGly (Figure 1, B and C). The insertion lies between the equivalent sequence, which links the two heparanase 1 subunits,8,9 and thus, by analogy, may functionally disrupt heparanase 2. Heterozygous missense variants with a minor allele frequency <1% (Supplemental Table 1) were not over-represented in patients (5 of 439 probands) versus European ancestry controls (38 of 6503 cases; P=0.2).
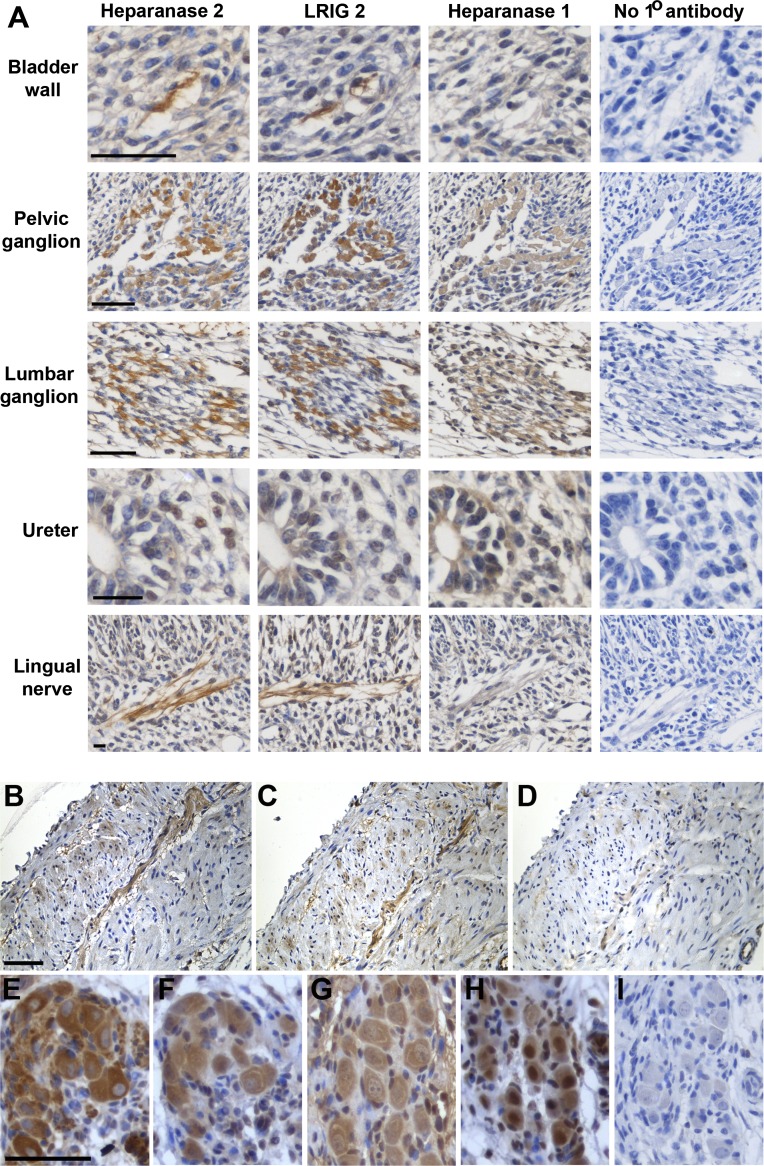
It has been speculated that UFS bladder defects are neurogenic because similar dyssynergia can occur after spinal cord damage.2,18 Moreover, nerves invading human fetal bladders contain heparanase 2 and LRIG2.14 At mouse embryonic days (E) 14 and 17 (Supplemental Figure 2 and data not shown), Hpse2 transcripts were detected by RT-PCR in the bladder, metanephros, hindgut, neural tube, and eye. Lrig2 RNA was detected in these locations and in the brain, heart, and liver. Pelvic ganglia give rise to postganglionic parasympathetic axons mediating detrusor contraction, while lumbar ganglia emit postganglionic sympathetic axons mediating detrusor relaxation and sphincter contraction.19,20 Immunohistochemistry at E14 (Figure 2A) showed heparanase 2 in pelvic and lumbar ganglia; nerves running in bladder wall mesenchyme, beginning to form smooth muscle at this stage21; mesenchymal ureteric cells; and peripheral nerves within the head. The protein data accord with Hpse2 transcripts being detectable in embryonic pelvic ganglia,22 where Hpse2+ cells coexpress SRY-related HMG-box 10, which regulates neural crest development.19 LRIG2 and heparanase 1 were also immunodetected in E14 pelvic and lumbar ganglia. Postnatally, bladder wall nerves and pelvic ganglia contained heparanase 1 and 2 and LRIG2 (Figure 2, B–I). Heparanase 1 elicits neuritogenesis in cell culture,23 and heparanase 2 inhibits its enzymatic activity.13 Thus, the HPSE2 gene product may modify heparanase 1 activity within ganglia. Our observation that LRIG2 was also present in pelvic ganglia, combined with a report that LRIG2 modifies growth factor signaling in glioma cells,24 suggests that LRIG2 may operate in similar biologic pathways as heparanase 2. If autonomic nerve function is perturbed, then the cyclic low-pressure storage of urine and complete bladder emptying will be impaired.20 We speculate that this abnormal scenario operates in UFS. Here, there is an analogy with another congenital bladder disease in which M3, the cholinergic receptor mediating detrusor contraction, is mutated.25
Figure 2.
Immunohistochemistry in wild-type mice detects UFS proteins. Sections were counterstained (blue) with hematoxylin. Positive immunodetection signals are brown. (A) Embryonic day 14. Note the similar patterns for heparanase 2 and LRIG2 in a bladder wall nerve, a nascent pelvic ganglion, a nascent lumber ganglion, mesenchymal-like cells in the wall of the proximal (i.e., top of the) ureter, and the lingual nerve. Heparanase 1 was also detected in the ganglia cell bodies but not in the nerve trunks themselves. (B–D) Serial sections of 1-week postnatal bladder immunostained for heparanase 2 (B) or LRIG2 (C). (D) The primary antibody was omitted. Note signals for both proteins in the nerve (running from top right to bottom left). (E–I) Immunohistochemistry of pelvic ganglia flanking the bladder outflow tract 2 weeks after birth: β3-tubulin, a neuronal marker (E); heparanase 2 (F); heparanase 1 (G); LRIG2 (H); negative control with no primary antibody (I). Scale bars are 50 μm.
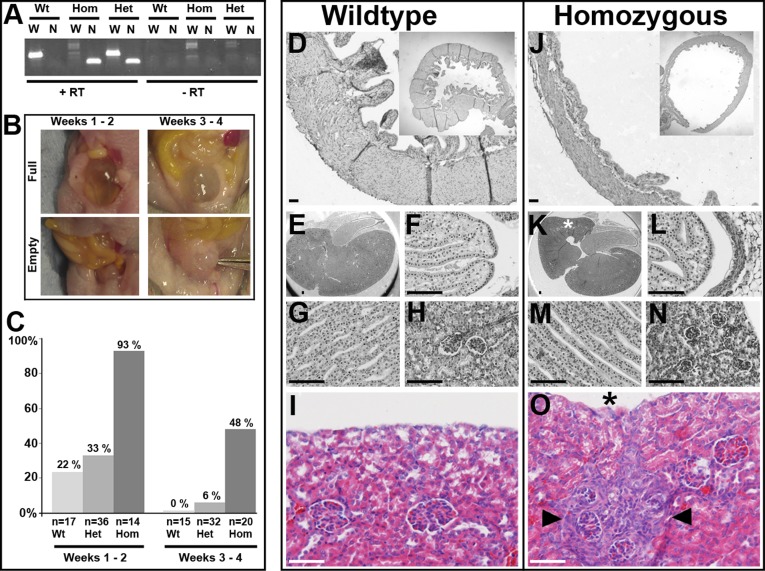
Mating heterozygous mice carrying a gene trap in intron 6 of Hpse2 led to birth of wild-type, heterozygous, and homozygous offspring in Mendelian ratios. Homozygous mutant tissue contained a truncated Hpse2 transcript but lacked wild-type Hpse2 transcripts extending beyond the trap (Figure 3A). Given that UFS is a congenital disease characterized by incomplete bladder emptying, we studied bladders in the first month of life when neural circuits normally become established.26 At autopsy, we recorded whether bladders appeared as “full” translucent globes or “empty” opaque beads (Figure 3B). In the first fortnight after birth (Figure 3C), of 17 wild-type bladders, 4 were full and 13 empty; of 14 homozygous bladders, 13 were full and 1 empty (wild-type versus mutant; P<0.001, Fisher exact test, two tailed). In the second fortnight, all 15 wild-type bladders were empty; of 29 homozygous bladders, 14 were full and 15 were empty (P=0.001). Heterozygous bladders did not differ significantly from wild-types, and there was no influence of sex. Injecting India ink into homozygous bladders demonstrated urethral patency. In humans with UFS, large bladders have been reported antenatally and neonatally,3 whereas bladder capacity may be normal later in childhood (e.g., Family 3). Perhaps the change in bladder phenotype between the first and second postnatal fortnights in Hpse2 mutant mice (93% versus 48% “full”) reflects an analogous evolution of dysfunction in this species.
Figure 3.
Hpse2 mutant mouse bladder and kidney phenotypes. (A) RT-PCR of RNA from postnatal bladders. Note the abnormal Hpse2 transcript (N, 158 base pairs) in gene trap homozygous (Hom) and heterozygous (Het) tissues. The wild-type Hpse2 transcript (W, 364 base pairs) was the only one present in wild-type (Wt) mice but was not detected in homozygous (Hom) tissue. The right side of the gel shows the experiment when RT was not used. (B) Examples of “full” and “empty” bladder phenotypes in autopsies of mice in the first and second postnatal fortnights. (C) Frequencies of “full” bladder phenotypes in the three genotypes, in the first and second postnatal fortnights (total numbers analyzed are also shown). (D–O) Histology of mutant mouse bladders and kidneys; D–I are wild-type organs and J–O are homozygous mutant organs. All sections were stained with hematoxylin; I and O were also stained with eosin. Wild-type (D) and homozygous Hpse2 mutant (J) bladders at 2 weeks. Note muscle in walls of both organs. When bladders are harvested, urine can escape and organs tend to deflate. Nevertheless, an impression of the difference between homozygous and wild-type bladders is shown in the low power insets (upper right of each frame). Wild-type kidneys at 2 weeks showing sagittal section overview (E) and higher powers of papilla (F), medullary tubules (G), and glomeruli (H). Counterpart zones in littermate homozygous kidney (K–N) are grossly similar to those in wild-type organ. An area of glomerular crowding in a mutant kidney is shown in N (from area marked by asterisk in K). High-power views of outer cortex in 3-week-old kidneys from wild-type (I) and mutant (O) littermate mice. Note the small area (demarcated by arrowheads in O) of glomerular crowding and tubulo-interstitial changes next to a concavity (asterisk) in the organ’s surface. Scale bars are 50 µm.
Homozygous mutant bladders contained muscle and urothelium (Figure 3, D and J). Quantitative PCR showed similar levels of epithelial (uroplakin 3A) and smooth muscle (α-smooth muscle actin and myosin heavy chain 11) transcripts in homozygous and wild-type littermate bladders harvested at 1 and 14 days after birth (Supplemental Figure 3). These results argue against a primary myogenic defect, as is present in the previously reported megabladder mutant mouse.27 In turn, this supports the conclusion that the HPSE2 gene product is necessary for peripheral neural control of bladder function. Heparanase 2 deficiency during Xenopus embryogenesis increases phospho-ERK (pERK),12 so we immunoprobed mouse pelvic ganglia for pERK (Supplemental Figure 4). Although a subset of neural cell bodies immunostained for pERK, the patterns were similar in mutants and wild-type littermates. So, if heparanase 2 acts to modulate intracellular signaling in pelvic ganglia, it may do so by other means, and we note that the heparanase 1, itself antagonized by heparanase 2,13 regulates AKT and SRC as well as ERK signaling.10
Homozygous mice showed poor weight gain in the third week, and subsequently some died. We performed autopsies in postnatal weeks 2–3. No pathologic abnormality was noted in the lungs. Homozygous kidneys were grossly normal, with a well defined cortex, outer medulla. and papilla (compare Figure 3, E–H, with Figure 3, K–N). These findings accord with the observation12 that knockdown of heparanase 2 in embryonic frogs does not lead to overtly malformed pronephric kidneys. Although Hpse2 mutant mouse kidneys lacked major aplastic/hypodysplastic malformations, three harbored focal cortical defects manifest as glomerular crowding (compare Figure 3, H and N) and/or tubular atrophy (compare Figure 3, I and O). Whether these are analogous to isotope scanning defects detected in some UFS kidneys (e.g., Families 3–5), and which may result from ascending infection, remains to be established.
Concise Methods
Human Genetic Studies
Since discovering mutations of HPSE24 and LRIG214 mutations in UFS, our research laboratory has sought mutations of these genes in other UFS families. Ethical approval was granted, and informed consent was obtained from all patients and/or their parents. Parental consent was obtained to show images of faces. Human studies were approved by institutional ethical review and approval (University of Manchester [06138] and National Health Service ethics committees [06/Q1406/52 and 11/NW/0021]). The VUR DNA samples from the United Kingdom were collected under National Research Ethics Service no. MREC/01/6/15. Irish VUR DNA samples were collected at Our Lady’s Children’s Hospital Crumlin and the National Children’s Hospital, Tallaght, Dublin, Ireland. HPSE24 and LRIG214 were sequenced as previously described. Multiplex ligation-dependent probe amplification was carried out using MRC-Holland reagents and standard protocol with custom made probes (conditions available on request).
Splicing Experiment
In silico prediction performed using the Human Splice Finder (www.umd.be/HSF) predicted that c.1099–1G>A mutates the splice acceptor of exon 8 and that potential alternative splice acceptor sites exist at nucleotides c.1100 and c.1121 within exon 8. HPSE2 is not expressed by lymphocytes, so we were unable to prove the alternate splicing by sequencing of constitutional cDNA. To assess effects of the missense mutation on splicing, we therefore performed a splicing assay using the pTB vector28 to express mini-gene constructs of the mutated and wild-type HSPE2 sequence in HEK293 cells. Briefly, exon 8 and approximately 500 bp of flanking sequence were amplified with the oligonucleotide primers: forward 5′AAACATATGGGCTTTTAGGGAGTACGTGTGTCA-3′ and reverse 5′AACCATATGGCACTTCAGACAGCGGTAAGCAC3′ containing appended NdeI restriction sites. Amplicons derived from patient and control DNA were cloned into TOPO pCR2.1 vector and, following verification of correct sequence (BigDye Terminator v3.1Cycle Sequencing Kit, analyzed on the Applied Biosystems 3730xl DNA analyzer), were subsequently subcloned as NdeI digested fragments into the pTB vector using standard procedures. DNA of the mutant and wild-type expression constructs was prepared from bacterial cultures using the Qiagen mini-prep kit. For analysis of splicing, HEK293 cells cultured in low glucose DMEM (Invitrogen) supplemented with 10% FBS and 1% penicillin were transfected with the pTB mini-gene constructs using Effectene transfection reagents (Qiagen) according to the manufacturer’s protocol. RNA was extracted 24 hours after transfection using the RNeasy kit (Qiagen) according to the manufacturer’s protocol. cDNA was synthesized using sequence specific oligo primers (5′TGTGCCTCCAGCTGAGGTGGT3′, internal exon 8 sequence and 5′GGTCACCAGGAAGTTGGTTAAATCA3′, pTB vector specific sequence) with Promega reverse transcription reagents according to a standard protocol. PCR amplification was performed using forward primer 5′CAACTTCAAGCTCCTAAGCCACTGC3′with reverse primers 5′TGTGCCTCCAGCTGAGGTGGT3′ and 5′GGTCACCAGGAAGTTGGTTAAATCA3′.
PCR products were purified by gel extraction (EMD Millipore Montage DNA gel extraction kit) and sequenced as previously described. Two predominant variants were produced by expression of the mutant allele. The 5′ pTB exon was spliced into two alternate splice acceptors within exon 8, NM_021828.4 c.1100 and c.1121 (as predicted by Human Splice Finder). These both result in a frameshift that would be predicted to result in a premature stop mutation and nonsense mediated decay (p.Val367Glyfs*2 or p.Val367Lysfs*6).
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Sections of 5 µm were mounted on polylysine-coated glass slides. Endogenous peroxidase was quenched by incubation with hydrogen peroxide (30% solution in PBS). Antigen exposure was undertaken by heating at 95°C for 5 minutes in sodium citrate (pH, 6). Antibodies were used at optimized concentrations in PBS Triton-X (0.1%). Primary antibodies used were against β3-tubulin (AB9354; EMD Millipore), heparanase 1 (OBT1975G; AbD Serotec), heparanase 2 generated against NH2-QLDPSIIHDGWLDC-CONH2 (Generon) as validated by Roberts et al.,17 LRIG2 (AP13821b; Abgent), and pERK (Sigma-Aldrich). Each was mixed with 3% serum specific to the species of the secondary antibody and incubated overnight at 4°C. Secondary antibodies were added for 2 hours at 4°C, followed by streptavidin-horseradish peroxidase (Vector) for 1 hour. 3, 3'-diaminobenzidine (Vector) was added for 2 minutes, and sections were counterstained with hematoxylin and/or eosin. For immunofluorescence, Alexa Fluor secondary antibodies (Life Technologies) were applied for 1 hour at room temperature, washed in PBS, and incubated with DAPI (Vector) for 10 minutes, washed in PBS, and mounted with Mowiol; a coverslip was then applied and sealed with nail varnish.
Detection of Hpse2 and Lrig2 Transcripts in Wild-Type Mice
Total RNA was extracted and following Dnase1 treatment, cDNA was generated by standard techniques. PCR was performed using Reddy Mix (Thermo Fisher Scientific) and the following primers: Hpse2 forward 5′CTTAAGCTCCAAGCGTCTGG3′ and reverse 5′TGAATCCATCTAGGAGAGCAATG’3′; Lrig2 forward 5′GTTATCGGCAGCAGGATGGA3′ and reverse 5′ACGAGGGTGTCTCTAACACTG3′; and Gapdh forward 5′GGGTTCCTATAAATACGGACTGC3′ and reverse 5′ CCATTTTGTCTACGGGACGA3′.
Mutant Mice
Mouse experiments were ethically approved by the University of Manchester Biologic Services Facility committee and the UK Home Office (PPL 40/3550, Modeling human UT malformations). Hpse2 gene trap mice were initially studied on a mixed (129SvEvBrd×C57BL6N) background; subsequent experiments showed a similar bladder phenotype on a C3H background. Mutant founders were generated at the Texas A&M Institute of Genetic Medicine from embryonic stem cells infected with a replication-defective retrovirus (http://www.tigm.org/). The gene trap vector VICTR4829 was found to have inserted into the sixth intron of Hpse2, which is located on chromosome 19 (Mouse accession NM_001081257 and Omnibank clone OST441123). The gene trap is predicted to cause splicing of exon 6 of Hpse2 with inclusion of a Neo cassette and premature stop codon. Genotyping was carried out using a common forward primer (5′CCAGCCCTAATGCAATTACC3′) and wild-type (5′TGAGCACTCACTTAAAAGGAC3′) or gene trap (5′ATAAACCCTCTTGCAGTTGCA3′) reverse primers. RT-PCRs were undertaken to detect wild-type and gene trap insertion transcripts in postnatal bladders using the primers: Hpse2 exon 5 forward 5′CGGACGGCCCAGGAAGAACG3′; Hpse2 exon 9 reverse 5′TCCGTGGTCGAAAAATGAATGTC3′; and Neo reverse r5′TGTGCCCAGTCATAGCCGAATAGC3′. Samples were run on a 1% agarose gel with SafeView nucleic acid stain (NBS Biologic). After weaning, homozygous mutant mice fail to gain weight and die when maintained for more than 1 month of age. The cause of their death is unknown. By contrast, heterozygous mice appear healthy; they are fertile and live to over 15 months of age. For quantitative PCR on mutant bladders, reactions were performed on a StepOnePlus platform (Applied Biosystems) and data analyzed using the accompanying software. The following Taqman probes (Applied Biosystems) were used: Hprt1, used as a reference housekeeping transcript (Mm00446968_m1); Acta2 (Mm00656102_m1); Myh11 (Mm00443013_m1); and Upk3a: (Mm00452321_m1).
Disclosures
None.
Supplementary Material
Acknowledgments
The UK VUR Study Group consists of Beattie J, Bradbuty M, Coad N, Coulthard M, Cuckow P, Dossetor J, Dudley J, Hughes D, Feather S, Fitzpatrick M, Goodship JA, Goodship TH, Griffin N, Gullett AM, Haycock G, Hodes D, Houtman P, Hughes A, Hulton S, Hunter E, Iqbal J, Inward C, Jackson J, Jadresic L, Jaswon M, Jones C, Jones R, Judd B, Kier M, Kilby A, Lambert H, Lewis M, Malcolm S, Marks S, Maxwell H, McGraw M, Milford D, Moghal N, O’Connor M, O'Donoghue DJ, Ognanovic M, Plant N, Postlethwaite R, Rees L, Reid C, Rfidah E, Rigdon S, Sandford R, Savage M, Scanlan J, Sinha S, Stephens S, Stewart A, Storr J, Taheri S, Taylor CM, Tizard J, Trompeter R, Tullus K, Verber I, Van't Hoff W, Vernon S, Verrier-Jones K, Watson A, Webb N, Wilcox D, Woolf AS.
The 4C Study Group consists of Aksu N, Alpay H, Anarat A, Arbeiter K, Ardissino GL, Balat A, Baskin E, Bayazit A, Büscher R, Cakar N, Caldas Afonso A, Caliskan S, Candan C, Canpolat N, Donmez O, Doyon A, Drozdz D, Dusek J, Duzova A, Emre S, Erdogan H, Feldkötter M, Fischbach M, Galiano G, Haffner D, Harambat J, Jankauskiene A, Jeck N, John U, Jungraithmair T, Kemper M, Kiyak A, Kracht D, Kranz B, Laube G, Litwin M, Matteucci CM, Montini G, Melk A, Mir S, Niemirska A, Peco-Antic A, Ozcelik G, Pelan E, Picca S, Pohl M, Querfeld U, Ranchin B, Schaefer F, Shroff R, Simonetti G, Sözeri B, Soylemezoglu O, Tabel Y, Testa S, Trivelli A, Vidal E, Wigger M, Wühl E, Wygoda S, Yalcinkaya F, Yilmaz E, Zeller R, Zurowska AM.
We thank Josephine Mulligan for curation of samples and family information and Ambrose Gullett and Aisling Stewart for collection of samples.
These studies were supported by research funding from the Children’s Medical & Research Foundation and the EU 7th Framework Programme (EURenOmics, 2012-305608), the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA), the German Federal Ministry of Education and Research (reference number: 01EO0802), the KfH Foundation for Preventive Medicine, Kidneys for Life, Kidney Research UK, the Medical Research Council (G0600040 and MR/L002744/1), and the Wellcome Trust (066647). H.M.S. is a Wellcome Trust Clinical Training Fellow. The work was facilitated by the Manchester Biomedical Research Centre and the National Institute for Health Research Greater Manchester Clinical Research Network.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013090961/-/DCSupplemental.
References
- 1.Online Mendelian Inheritance in Man. Available at: http://www.ncbi.nlm.nih.gov/omim. Accessed April 25, 2014
- 2.Ochoa B: Can a congenital dysfunctional bladder be diagnosed from a smile? The Ochoa syndrome updated. Pediatr Nephrol 19: 6–12, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Newman WG, Woolf AS, Stuart HM: Urofacial syndrome. GeneReviews 1993–2013, 2013. Available at: http://www.ncbi.nlm.nih.gov/books/NBK154138/. Accessed April 24, 2014 [Google Scholar]
- 4.Daly SB, Urquhart JE, Hilton E, McKenzie EA, Kammerer RA, Lewis M, Kerr B, Stuart H, Donnai D, Long DA, Burgu B, Aydogdu O, Derbent M, Garcia-Minaur S, Reardon W, Gener B, Shalev S, Smith R, Woolf AS, Black GC, Newman WG: Mutations in HPSE2 cause urofacial syndrome. Am J Hum Genet 86: 963–969, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang J, Zhang S, Yang P, Hawkins-Lee B, Zhong J, Zhang Y, Ochoa B, Agundez JAG, Voelckel MA, Fisher RB, Gu W, Xiong WC, Mei L, She JX, Wang CY: Loss-of-function mutations in HPSE2 cause the autosomal recessive urofacial syndrome. Am J Hum Genet 86: 957–962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Badr W, Al Bader S, Otto E, Hildebrandt F, Ackley T, Peng W, Xu J, Li J, Owens KM, Bloom D, Innis JW: Exome capture and massively parallel sequencing identifies a novel HPSE2 mutation in a Saudi Arabian child with Ochoa (urofacial) syndrome. J Pediatr Urol 7: 569–573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmood S, Beetz C, Tahir MM, Imran M, Mumtaz R, Bassmann I, Jahic A, Malik M, Nürnberg G, Hassan SA, Rana S, Nürnberg P, Hübner CA: First HPSE2 missense mutation in urofacial syndrome. Clin Genet 81: 88–92, 2012 [DOI] [PubMed] [Google Scholar]
- 8.McKenzie E, Tyson K, Stamps A, Smith P, Turner P, Barry R, Hircock M, Patel S, Barry E, Stubberfield C, Terrett J, Page M: Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochem Biophys Res Commun 276: 1170–1177, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Levy-Adam F, Feld S, Cohen-Kaplan V, Shteingauz A, Gross M, Arvatz G, Naroditsky I, Ilan N, Doweck I, Vlodavsky I: Heparanase 2 interacts with heparan sulfate with high affinity and inhibits heparanase activity. J Biol Chem 285: 28010–28019, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fux L, Ilan N, Sanderson RD, Vlodavsky I: Heparanase: Busy at the cell surface. Trends Biochem Sci 34: 511–519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenzie EA: Heparanase: A target for drug discovery in cancer and inflammation. Br J Pharmacol 151: 1–14, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts NA, Woolf AS, Stuart HM, Thuret R, McKenzie EA, Newman WG, Hilton EN. Heparanase 2, mutated in urofacial syndrome, mediates peripheral neural development in Xenopus. Hum Mol Genet 23: 4302–4314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz F, Aebi M: Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol 21: 576–582, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Stuart HM, Roberts NA, Burgu B, Daly SB, Urquhart JE, Bhaskar S, Dickerson JE, Mermerkaya M, Silay MS, Lewis MA, Olondriz MB, Gener B, Beetz C, Varga RE, Gülpınar O, Süer E, Soygür T, Ozçakar ZB, Yalçınkaya F, Kavaz A, Bulum B, Gücük A, Yue WW, Erdogan F, Berry A, Hanley NA, McKenzie EA, Hilton EN, Woolf AS, Newman WG: LRIG2 mutations cause urofacial syndrome. Am J Hum Genet 92: 259–264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silay MS, Tanriverdi O, Karatag T, Ozcelik G, Horasanli K, Miroglu C: Twelve-year experience with Hinman-Allen syndrome at a single center. Urology 78: 1397–1401, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Cordell HJ, Darlay R, Charoen P, Stewart A, Gullett AM, Lambert HJ, The UK, VUR Study Group : Malcolm S, Feather SA, Goodship THJ, Woolf AS, Kenda RB, Goodship JA. Whole-genome linkage and association scan in primary, non-syndromic vesicoureteric reflux. J Am Soc Nephrol 21: 113–123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darlow JM, Dobson MG, Darlay R, Molony CM, Hunziker M, Green AJ, Cordell HJ, Puri P, Barton DE: A new genome scan for primary nonsyndromic vesicoureteric reflux emphasizes high genetic heterogeneity and shows linkage and association with various genes already implicated in urinary tract development. Mol Genet Genomic Med 2: 7–29, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesan I, Thomas T: More than meets the smile: facial muscle expression in children with Ochoa syndrome. Med J Malaysia 66: 507–509, 2011 [PubMed] [Google Scholar]
- 19.Wiese CB, Ireland S, Fleming NL, Yu J, Valerius MT, Georgas K, Chiu HS, Brennan J, Armstrong J, Little MH, McMahon AP, Southard-Smith EM: A genome-wide screen to identify transcription factors expressed in pelvic ganglia of the lower urinary tract. Front Neurosci 6: 130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benarroch EE: Neural control of the bladder: Recent advances and neurologic implications. Neurology 75: 1839–1846, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Smeulders N, Woolf AS, Wilcox DT: Smooth muscle differentiation and cell turnover in mouse detrusor development. J Urol 167: 385–390, 2002 [PubMed] [Google Scholar]
- 22.GenitoUrinary Molecular Anatomy Project. Available at: http://www.gudmap.org/gudmap/pages/database_homepage.html. Accessed April 23, 2014
- 23.Cui H, Shao C, Liu Q, Yu W, Fang J, Yu W, Ali A, Ding K: Heparanase enhances nerve-growth-factor-induced PC12 cell neuritogenesis via the p38 MAPK pathway. Biochem J 440: 273–282, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Rondahl V, Holmlund C, Karlsson T, Wang B, Faraz M, Henriksson R, Hedman H: Lrig2-deficient mice are protected against PDGFB-induced glioma. PLoS ONE 8: e73635, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber S, Thiele H, Mir S, Toliat MR, Sozeri B, Reutter H, Draaken M, Ludwig M, Altmüller J, Frommolt P, Stuart HM, Ranjzad P, Hanley NA, Jennings R, Newman WG, Wilcox DT, Thiel U, Schlingmann K-P, Beetz R, Hoyer PF, Konrad M, Schaefer F, Nürnberg P, Woolf AS: Muscarinic acetylcholine receptor M3 mutation causes urinary bladder disease and a prune -belly-like syndrome. Am J Hum Genet 89: 668–674, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danzer E, Kiddoo DA, Redden RA, Robinson L, Radu A, Zderic SA, Doolin EJ, Adzick NS, Flake AW: Structural and functional characterization of bladder smooth muscle in fetal rats with retinoic acid-induced myelomeningocele. Am J Physiol Renal Physiol 292: F197–F206, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Singh S, Robinson M, Ismail I, Saha M, Auer H, Kornacker K, Robinson ML, Bates CM, McHugh KM: Transcriptional profiling of the megabladder mouse: A unique model of bladder dysmorphogenesis. Dev Dyn 237: 170–186, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Baralle M, Baralle D, De Conti L, Mattocks C, Whittaker J, Knezevich A, Ffrench-Constant C, Baralle FE: Identification of a mutation that perturbs NF1 agene splicing using genomic DNA samples and a minigene assay. J Med Genet 40: 220–222, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeRossi C, Bode L, Eklund EA, Zhang F, Davis JA, Westphal V, Wang L, Borowsky AD, Freeze HH: Ablation of mouse phosphomannose isomerase (Mpi) causes mannose 6-phosphate accumulation, toxicity, and embryonic lethality. J Biol Chem 281: 5916–5927, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.