Abstract
In CKD, uremic solutes may induce endothelial dysfunction, inflammation, and oxidative stress, leading to increased cardiovascular risk. We investigated whether the uremic solute indole-3 acetic acid (IAA) predicts clinical outcomes in patients with CKD and has prooxidant and proinflammatory effects. We studied 120 patients with CKD. During the median study period of 966 days, 29 patients died and 35 experienced a major cardiovascular event. Kaplan–Meier analysis revealed that mortality and cardiovascular events were significantly higher in the higher IAA group (IAA>3.73 µM) than in the lower IAA group (IAA<3.73 µM). Multivariate Cox regression analysis demonstrated that serum IAA was a significant predictor of mortality and cardiovascular events after adjustments for age and sex; cholesterol, systolic BP, and smoking; C-reactive protein, phosphate, body mass index, and albumin; diastolic BP and history of cardiovascular disease; and uremic toxins p-cresyl sulfate and indoxyl sulfate. Notably, IAA level remained predictive of mortality when adjusted for CKD stage. IAA levels were positively correlated with markers of inflammation and oxidative stress: C-reactive protein and malondialdehyde, respectively. In cultured human endothelial cells, IAA activated an inflammatory nongenomic aryl hydrocarbon receptor (AhR)/p38MAPK/NF-κB pathway that induced the proinflammatory enzyme cyclooxygenase-2. Additionally, IAA increased production of endothelial reactive oxygen species. In conclusion, serum IAA may be an independent predictor of mortality and cardiovascular events in patients with CKD. In vitro, IAA induces endothelial inflammation and oxidative stress and activates an inflammatory AhR/p38MAPK/NF-κB pathway.
Keywords: cardiovascular disease, chronic kidney disease, endothelial cells, uremia
Chronic inflammation and oxidative stress are key mechanisms in endothelial dysfunction and atherosclerosis that lead to cardiovascular disease in patients with CKD.1 The uremic syndrome of CKD is attributed to the progressive retention of uremic solutes, which are normally eliminated by the kidneys.2,3 Serum levels of some uremic solutes are correlated with cardiovascular events and mortality in patients with CKD.4 Uremic solutes have been identified as inducers of oxidative stress, inflammation, and endothelial dysfunction.4–6
Indole-3 acetic acid (IAA) is a protein-bound uremic solute from tryptophan metabolism.7 Its serum level is increased in patients with CKD;3 the mean uremic concentration is about 5 µM,8 and the highest concentration is 50 µM.8 IAA belongs to the family of indolic uremic solutes,3 such as indoxyl sulfate.3,5 IAA is an agonist of the transcription factor aryl hydrocarbon receptor (AhR),9,10 which regulates the cell response to environmental xenobiotics such as 2,3,7,8-tetrachlorodibenzo(p)dioxin.11 AhR activation by exogenous ligands promotes vascular inflammation, oxidative stress,5,12 and atherosclerosis13 and plays a role in cardiovascular diseases.14 However, the vascular toxicity of IAA has not been thoroughly studied. We recently reported that IAA induces endothelial tissue factor expression, leading to a procoagulant effect,9 and increases the mRNA expression of the enzyme cyclooxygenase-2 (COX-2), which is primarily responsible for the synthesis of inflammatory prostanoids.15
In the present work, we test the hypothesis that IAA is associated with mortality and cardiovascular events in patients with CKD. In addition, we demonstrate in vitro that IAA induces endothelial oxidative stress and inflammation by activating an AhR/p38MAPK/NF-κB pathway, which leads to COX-2 upregulation.
Results
We studied 120 patients with CKD. The baseline characteristics, according to the median IAA level (IAA<3.73 µM versus IAA>3.73 µM), are presented in Table 1. The group of patients with higher IAA serum levels had lower eGFR and more patients undergoing dialysis (Table 1). The patients with higher IAA serum levels were older, had higher serum C-reactive protein (CRP) and malondialdehyde levels, and lower triglycerides and diastolic BP (Table 1).
Table 1.
Baseline characteristics of the study population
| Characteristic | All Patients (n=120) | IAA<3.73 µM | IAA>3.73 µM | P Value |
|---|---|---|---|---|
| Age (yr) | 67 (23, 91) | 64 (23, 87) | 71 (32, 91) | <0.05 |
| Women/men (n/n) | 46/74 | 25/35 | 21/39 | 0.5 |
| Body mass index (kg/m2) | 24.1 (14.3, 47) | 24.4 (16.9, 47) | 23.7 (14.3, 39.8) | <0.05 |
| Dialyzed patients (%) | 73 (61) | 43 | 78 | <0.05 |
| eGFRa (ml/min per 1.73 m2) | 26±12 | 28±11 | 19±13 | <0.05 |
| Kidney disease | ||||
| GN | 23 (19) | 11 (18) | 12 (20) | 0.8 |
| ADPKD | 9 (8) | 5 (8) | 4 (7) | 0.7 |
| Vascular nephropathy | 34 (28) | 13 (22) | 21 (35) | 0.2 |
| Interstitial nephropathy | 24 (20) | 14 (23) | 10 (17) | 0.4 |
| Other hereditary | 5 (4) | 4 (7) | 1 (1) | 0.2 |
| Unknown | 25 (21) | 13 (22) | 12 (20) | 0.8 |
| Hypertension | 110 (92) | 55 (92) | 55 (92) | 1 |
| Systolic BP (mmHg) | 142±23 | 145±24 | 140±23 | 0.3 |
| Diastolic BP (mmHg) | 76±14 | 78±14 | 73±14 | <0.05 |
| Current smokers | 48 (40) | 27 (45) | 21 (35) | 0.4 |
| History of cardiovascular diseases | 42 (35) | 16 (27) | 26 (43) | 0.1 |
| Antihypertensive drugs | 87 (73) | 43 (72) | 44 (73) | 0.9 |
| Statins | 38 (32) | 16 (27) | 22 (37) | 0.3 |
| Antiplatelet drugs | 47 (39) | 17 (28) | 30 (50) | <0.06 |
| Anticoagulant drugs | 27 (23) | 12 (20) | 15 (25) | 0.5 |
| Erythropoietin therapy | 67 (56) | 26 (43) | 41 (68) | 0.06 |
| Serum CRP level (mg/L) | 5 (0.1, 78) | 4 (0.1, 30) | 7 (0.1, 78) | <0.01 |
| Hemoglobin (g/dl) | 12±1.4 | 12±1.6 | 11.9±1.2 | 0.7 |
| Serum albumin level (g/L) | 36±4 | 35.9±3.4 | 36±4.6 | 0.8 |
| Serum calcium level (mmol/L) | 2.33 (1.84, 2.66) | 2.34 (2.06, 2.55) | 2.32 (1.84, 2.66) | 0.8 |
| Serum phosphate level (mmol/L) | 1.28 (0.54, 3.17) | 1.22 (0.65, 2.91) | 1.44 (0.54, 3.17) | <0.1 |
| Serum cholesterol level (mmol/L) | 4.79±1.26 | 4.94±1.33 | 4.63±1.17 | 0.2 |
| Serum LDL cholesterol level (mmol/L) | 3.09±1.08 | 3.02±1.11 | 3.18±1.06 | 0.4 |
| Serum triglyceride level (mmol/L) | 1.44 (0.37, 5.96) | 1.65 (0.37, 5.96) | 1.22 (0.37, 3.67) | <0.05 |
| Serum malondialdehyde level b (nmol/L) | 200 (100, 500) | 150 (100, 500) | 215 (120, 430) | <0.01 |
| Serum IAA level (µmol/L) | 3.73 (0.6, 18.58) | 1.86 (0.6, 3.71) | 6.67 (3.75, 18.58) | <0.001 |
Results are given as mean±SD if distribution is Gaussian or as median (minimum, maximum) if not. All other values are expressed as number (percentage) of patients. ADPKD, autosomal dominant polycystic kidney disease.
Calculated by Modification of Diet in Renal Disease formula for patients with CKD who were not undergoing dialysis (n=47).
Measured only in 68 patients (40 patients in the IAA<3.73 µg/ml group and 28 patients in the IAA>3.73 µg/ml group) randomly chosen in the different CKD stage populations.
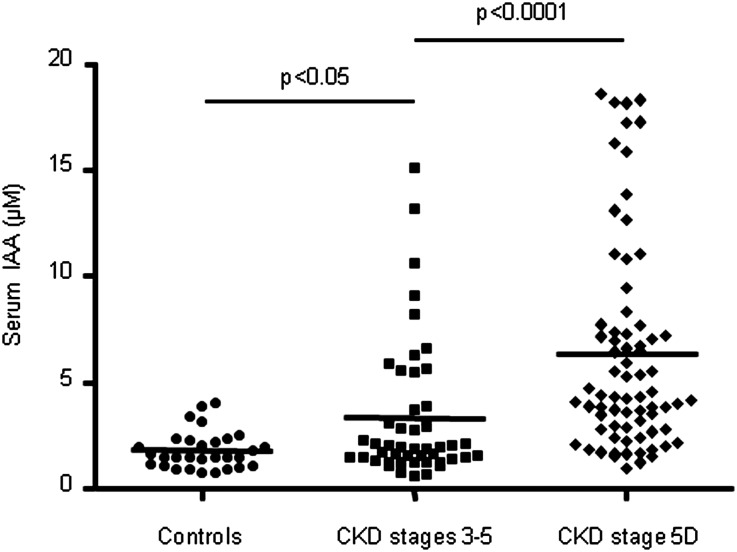
IAA levels were significantly higher in patients with stage 3–5 CKD (P<0.05) and stage 5D CKD (P<0.001) than in controls (1). Mean IAA values were 1.9±0.8 µM (range, 0.7–3.9 µM), 3.3±3.2 µM (range, 0.6–15.1 µM), and 6.3±4.9 µM (range, 0.9–18.5 µM), respectively in controls, in patients with stage 3–5 CKD, and in patients with stage 5D CKD (Figure 1).
Figure 1.
Serum levels of IAA are increased in patients with CKD. Plasma IAA levels are higher in patients with stage 3–5 and stage 5D CKD than in controls .
At baseline, IAA levels positively correlated with age (r=0.25; P<0.01), CRP (r=0.28; P<0.01), malondialdehyde (r=0.32; P<0.01), and history of cardiovascular diseases (r=0.25; P<0.01); IAA levels negatively correlated with diastolic BP (r=−0.16; P<0.01) and triglycerides (r=−0.22; P<0.05) (Table 2). In the 47 patients with stage 3–5 CKD, IAA levels negatively correlated with eGFR estimated by the Modification of Diet in Renal Disease simplified formula (r=−0.31; P<0.05) (Table 2).
Table 2.
Spearman correlations of baseline characteristics with IAA serum concentrations
| Variable | r | P Value |
|---|---|---|
| Age | 0.25 | <0.01 |
| Sex | 0.07 | 0.4 |
| Body mass index | −0.14 | 0.10 |
| eGFRa | −0.31 | <0.1 |
| Systolic BP | −0.01 | 0.8 |
| Diastolic BP | −0.16 | <0.01 |
| Current smoking | −0.09 | 0.3 |
| History of cardiovascular diseases | 0.25 | <0.01 |
| Serum CRP level | 0.28 | <0.01 |
| Hemoglobin | −0.03 | 0.7 |
| Serum albumin level | 0.14 | 0.12 |
| Serum calcium level | −0.03 | 0.7 |
| Serum phosphate level | 0.14 | 0.12 |
| Serum cholesterol level | −0.11 | 0.20 |
| Serum LDL cholesterol level | 0.05 | 0.5 |
| Serum triglyceride level | −0.22 | <0.1 |
| Serum malondialdehydeb level | 0.32 | <0.01 |
Calculated for patients with CKD who were not undergoing dialysis (n=47).
Measured only in 68 patients randomly chosen in the different CKD stage populations.
IAA Predicted the Risk of Death and Cardiovascular Events in Patients with CKD
After a mean follow-up of 804±346 days (median, 966 [range, 9–1100] days), 29 patients died (18 of cardiovascular causes, 4 of infectious causes, and 7 of other causes). Among the patients who died, 27 had been undergoing dialysis. Thirty-five patients displayed a cardiovascular event, which included a new nonfatal cardiovascular event (myocardial infarction, n=11; peripheral vascular event with amputation or need for angioplasty, n=2; and stroke, n=4) or death from cardiovascular cause (n=18). Among the patients who displayed a cardiovascular event, 30 had been undergoing dialysis. During the follow-up, 33 patients dropped out the study: 22 because of kidney transplantation and 11 because of loss to follow-up.
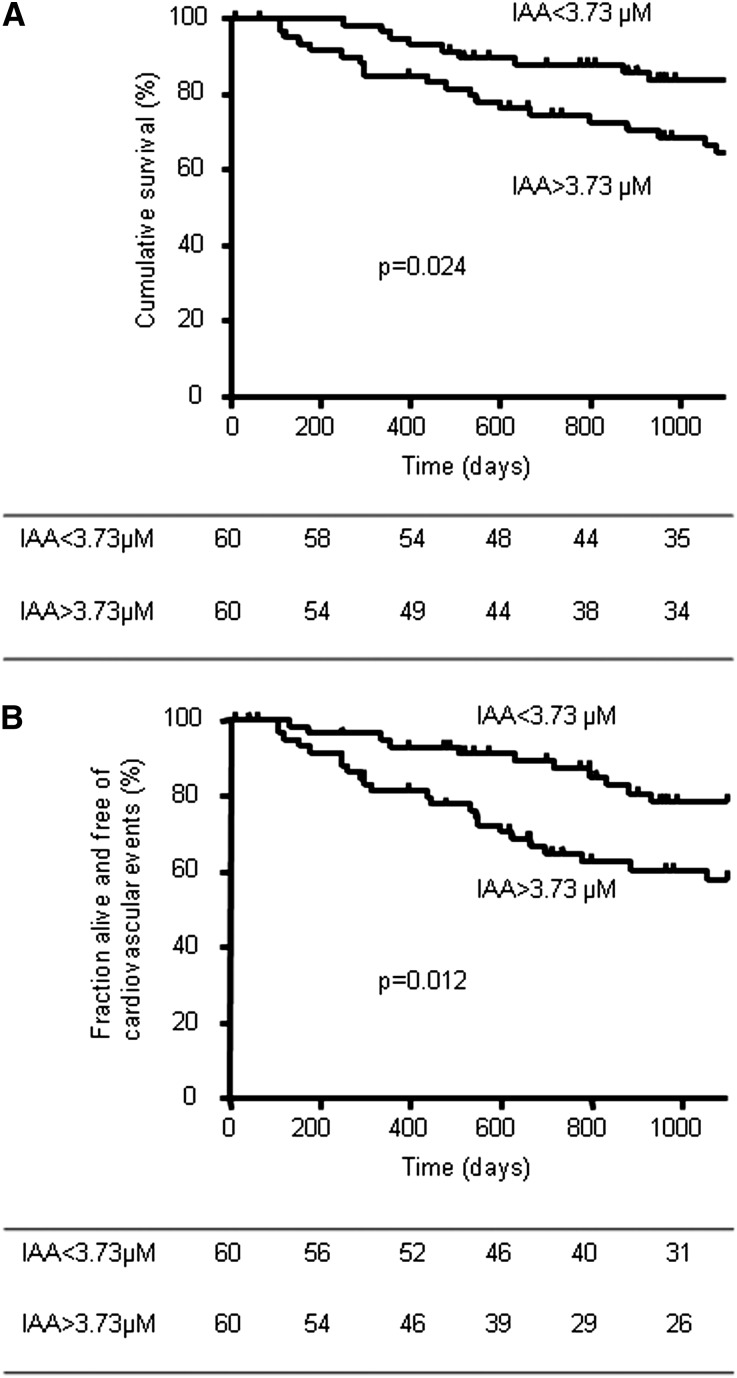
The Kaplan–Meier analysis revealed that all-cause mortality (Figure 2A) and cardiovascular events (Figure 2B) were significantly higher in patients with high IAA levels (IAA>3.73 µM) than in those with low IAA levels (log-rank comparison of the curves: P=0.024 and P=0.01, respectively). Mortality did not differ between patients with high and low IAA levels in the dialysis group. In unadjusted Cox regression analysis, older age, dialysis status, lower diastolic BP, and a history of cardiovascular disease were also significantly associated with an increased risk of death and cardiovascular events (data not shown). In addition, CRP was significantly associated with the risk of death (data not shown).
Figure 2.
Kaplan–Meier estimates of cumulative survival (A) and major cardiovascular events (B) of all patients according to serum IAA level above and below the median of 3.73 µM. (A) P=0.02 in a log-rank comparison of the curves. (B) P=0.01 in a log-rank comparison of the curves. The number of patients indicated in the tables is the number of patients still followed at the indicated time.
We performed univariate and multivariate Cox analyses with the Box-Cox–transformed IAA entered as a continuous variable. In the unadjusted model, higher serum levels of IAA were associated with an increased risk of death (Table 3) and cardiovascular events (Table 4). After adjustment for the different models for age and male sex (model 1); cholesterol, systolic BP, and smoking (model 2); CRP, phosphate, body mass index (BMI), and albumin (model 3); and diastolic BP and history of cardiovascular disease (model 4), serum level of IAA remained a significant predictor of an increased risk of death (Table 3) and of cardiovascular events (Table 4). Even when adjusted for CKD stage (model 5), level of IAA remained a predictor of death (Table 3) but not of cardiovascular events (Table 4).
Table 3.
Multivariate Cox regression analysis of risk factors for overall mortality with Box-Cox normalized serum IAA entered as continuous variable
| Models of Patient Survival | RR (95% CI) | P Value |
|---|---|---|
| Unadjusted | 2.44 (1.34 to 4.46) | 0.004 |
| Model 1a | 1.95 (1.05 to 3.65) | 0.04 |
| Model 2b | 2.19 (1.15 to 4.14) | 0.02 |
| Model 3c | 2.01 (1.01 to 4.00) | 0.05 |
| Model 4d | 2.09 (1.11 to 3.93) | 0.02 |
| Model 5e | 2.46 (1.35 to 4.50) | 0.003 |
RR, relative risk; 95% CI, 95% confidence interval.
Model 1 was adjusted for age and male sex.
Model 2 was adjusted for cholesterol, systolic BP, and smoking.
Model 3 was adjusted for CRP, phosphate, BMI, and albumin.
Model 4 was adjusted for diastolic BP and history of cardiovascular disease.
Model 5 was adjusted for CKD stage.
Table 4.
Multivariate Cox regression analysis of risk factors for cardiovascular events with Box-Cox normalized serum IAA entered as continuous variable
| Major Cardiovascular Event | RR (95% CI) | P Value |
|---|---|---|
| Unadjusted | 2.16 (1.26 to 3.71) | <0.01 |
| Model 1a | 1.81 (1.04 to 3.14) | 0.04 |
| Model 2b | 1.91 (1.10 to 3.32) | 0.02 |
| Model 3c | 1.89 (1.08 to 3.33) | 0.03 |
| Model 4d | 1.77 (1.02 to 3.09) | 0.04 |
| Model 5e | 1.39 (0.77 to 2.48) | <0.3 |
RR, relative risk; 95% CI, 95% confidence interval.
Model 1 was adjusted for age and male sex.
Model 2 was adjusted for cholesterol, systolic BP, and smoking.
Model 3 was adjusted for CRP, phosphate, BMI, and albumin.
Model 4 was adjusted for diastolic BP and history of cardiovascular disease.
Model 5 was adjusted for CKD stage.
We finally compared the predictive power of IAA with that of uremic toxins p-cresyl sulfate and indoxyl sulfate. In univariate Cox analyses, p-cresyl sulfate predicted overall mortality and cardiovascular events, and indoxyl sulfate predicted mortality (not shown). In multivariate analyses that entered IAA, p-cresyl sulfate, and indoxyl sulfate as continuous variables, IAA remained a significant predictor of mortality and cardiovascular events whereas p-cresyl sulfate and indoxyl sulfate did not (Tables 5 and 6).
Table 5.
Multivariate Cox regression analysis of risk factors for overall mortality in a model with Box-Cox normalized serum IAA, indoxyl sulfate, and p-cresylsulfate entered as continuous variables
| Models of Patient Survival (Event 29/120) | RR (95% CI) | P Value |
|---|---|---|
| IAA | 2.04 (1.05 to 3.95) | 0.03 |
| Indoxyl sulfate | 1.05 (0.95 to 1.16) | <0.4 |
| p-cresylsulfate | 1.05 (0.97 to 1.12) | 0.21 |
RR, relative risk; 95% CI, 95% confidence interval.
Table 6.
Multivariate Cox regression analysis of risk factors for cardiovascular events in a model with Box-Cox normalized serum IAA, indoxyl sulfate, and p-cresylsulfate entered as continuous variables
| Major Cardiovascular Event (Event 35/120) | RR (95% CI) | P Value |
|---|---|---|
| IAA | 1.95 (1.09 to 3.50) | 0.03 |
| Indoxyl sulfate | 0.99 (0.90 to 1.08) | <0.8 |
| p-cresylsulfate | 1.03 (0.96 to 1.10) | <0.4 |
RR, relative risk; 95% CI, 95% confidence interval.
IAA Increased COX-2 Expression and Activity
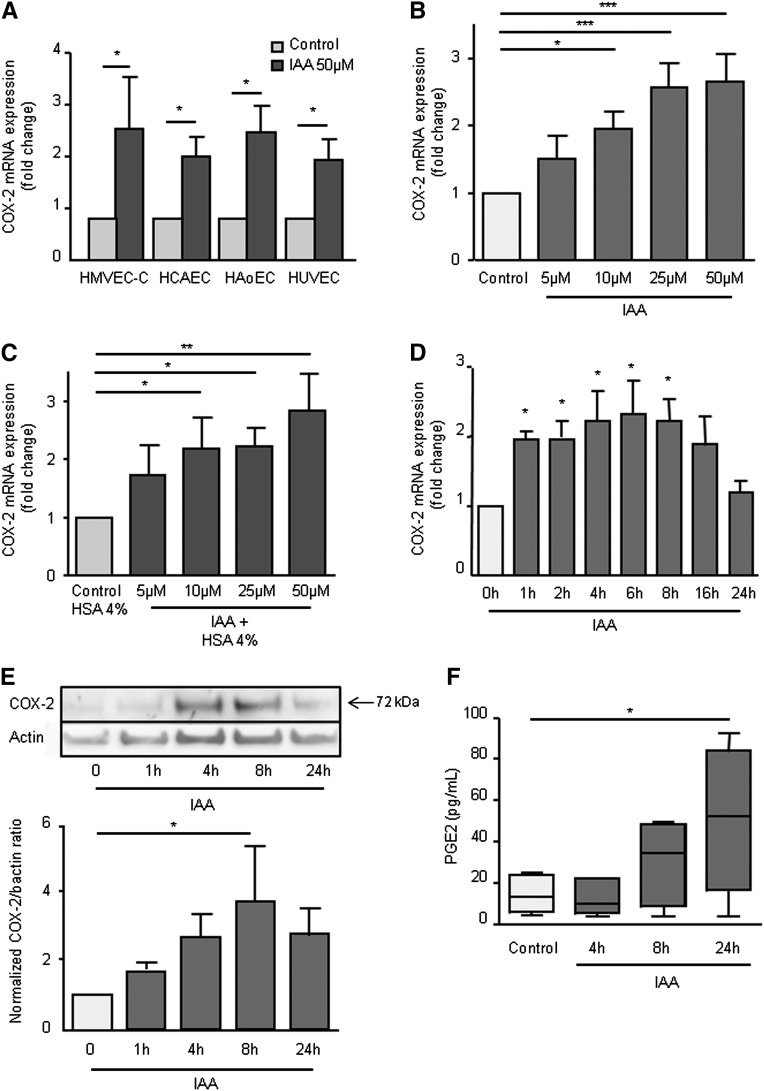
The correlation between IAA level and the inflammatory marker CRP led us to study the proinflammatory effect of IAA in cultured vascular endothelial cells. We first studied, in vitro, the effect of IAA on the upregulation of the inflammatory molecule COX-2 in different types of human endothelial cells. In cardiac-derived microvascular (HMVEC-C), coronary artery (HCAEC), aortic (HAoEC), and human umbilical vein (HUVEC) endothelial cells, IAA increased COX-2 mRNA expression (Figure 3A). In addition, the mRNA expression of inflammatory molecules IL-8, intercellular adhesion molecule-1 (ICAM-1), monocyte chemotactic protein (MCP-1), and, to a lesser extent, IL-6, was significantly increased by IAA in HUVEC (Supplemental Figure 1).
Figure 3.
IAA induces endothelial COX-2. (A) The induction of COX-2 mRNA by IAA in different types of endothelial cells: HMVEC-C (cardiac-derived microvascular), HCAEC (coronary artery), HAoEC (aortic), and HUVEC (umbilical vein) was studied by comparative RT-qPCR and expressed in mRNA fold change versus control. Data represent the mean±SEM of four independent experiments. *P<0.05. (B) Dose effect of COX-2 mRNA induction by IAA in HUVEC after 4-hour incubation was studied by comparative RT-qPCR and expressed in mRNA fold change versus control. Data represent the mean±SEM of five independent experiments. *P<0.05, ***P<0.001. (C) Dose effect of COX-2 mRNA induction by IAA in HUVEC in medium containing 4% human serum albumin. Data represent the mean±SEM of five independent experiments. *P<0.05. (D) The kinetics of COX-2 mRNA expression induced by 50 µM IAA in HUVECs were studied by comparative RT-qPCR and expressed in mRNA fold change versus control. Data represent the mean±SEM of five independent experiments. *P<0.05. (E) COX-2 protein induction by IAA in HUVECs was studied by Western blot. Data, expressed as normalized COX-2/βactin ratio versus control, represent the mean±SEM of three independent experiments. *P<0.05. (F) PGE2 was measured by ELISA in the supernatants of HUVECs stimulated with 50 µM IAA. Data are expressed as minimum-to-maximum box and whiskers plot indicating the median, the first quartile and the third quartile. *P<0.05.
We verified that incubation with IAA was not associated with cellular cytotoxicity. IAA did not decrease HUVEC viability at the concentrations studied (5, 10, 25, and 50 µM) (Supplemental Figure 2) and did not increase lactate dehydrogenase release at 50 µM (Supplemental Figure 3).
We then studied how IAA affected COX-2 expression. Dose-response experiments showed that IAA at concentrations of 10, 25, and 50 µM significantly increased COX-2 mRNA expression after 4-hour incubation (Figure 3B). A similar increase in COX-2 mRNA was observed when human serum albumin at 4 g/dl was added in the culture medium (Figure 3C). Kinetic study showed that COX-2 mRNA expression increased significantly after 1 hour of incubation with IAA, reached a plateau between 4 and 8 hours, and returned to basal levels after 24 hours (Figure 3D). By Western blot, we showed that IAA at 50 µM increased endothelial COX-2 protein level (Figure 3E). Kinetic study showed that COX-2 protein level increased after 4 hours of incubation with IAA, was maximal at 8 hours, and decreased at 24 hours (Figure 3E).
To study COX-2 activity, we measured prostaglandin E2 (PGE2) production after IAA stimulation. An increased PGE2 concentration could be detected in the supernatants after 8 hours of incubation, and the increase reached significance after 24 hours of incubation (Figure 3F).
We finally studied whether IAA affected the expression of the cyclooxygenase COX-1. COX-1 mRNA was not modified after 4-hour incubation with IAA at 50 µM (Supplemental Figure 4).
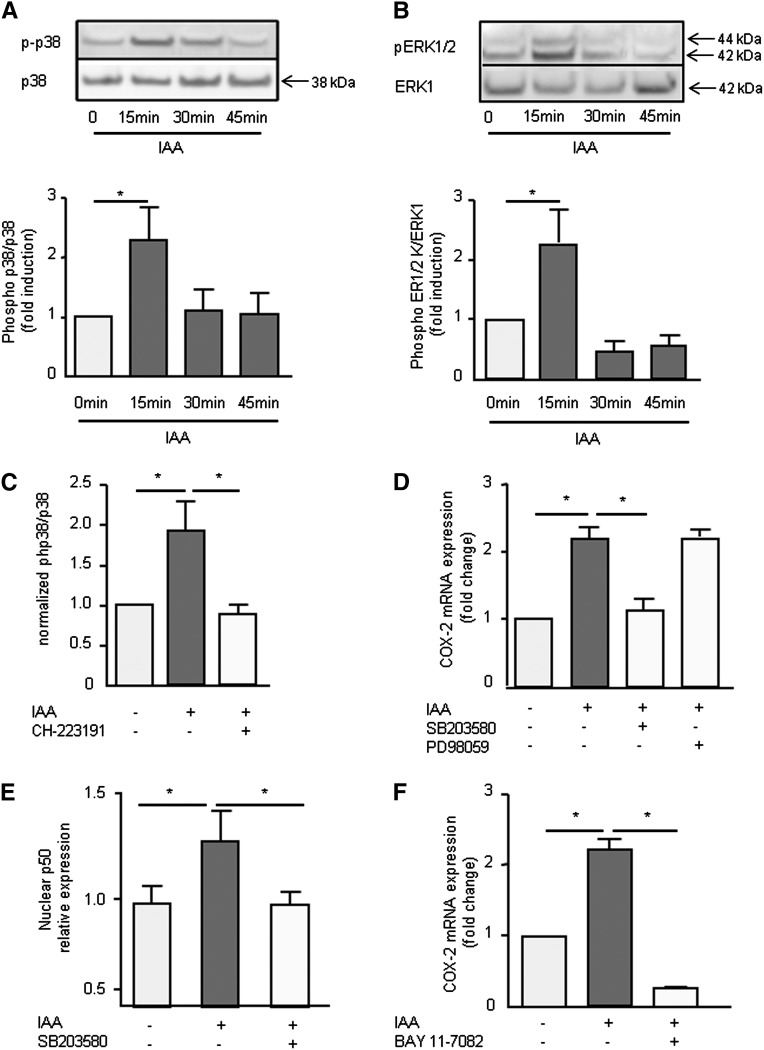
IAA Activated an AhR/p38 MAPK/NF-κB Signaling Pathway Involved in COX-2 Upregulation
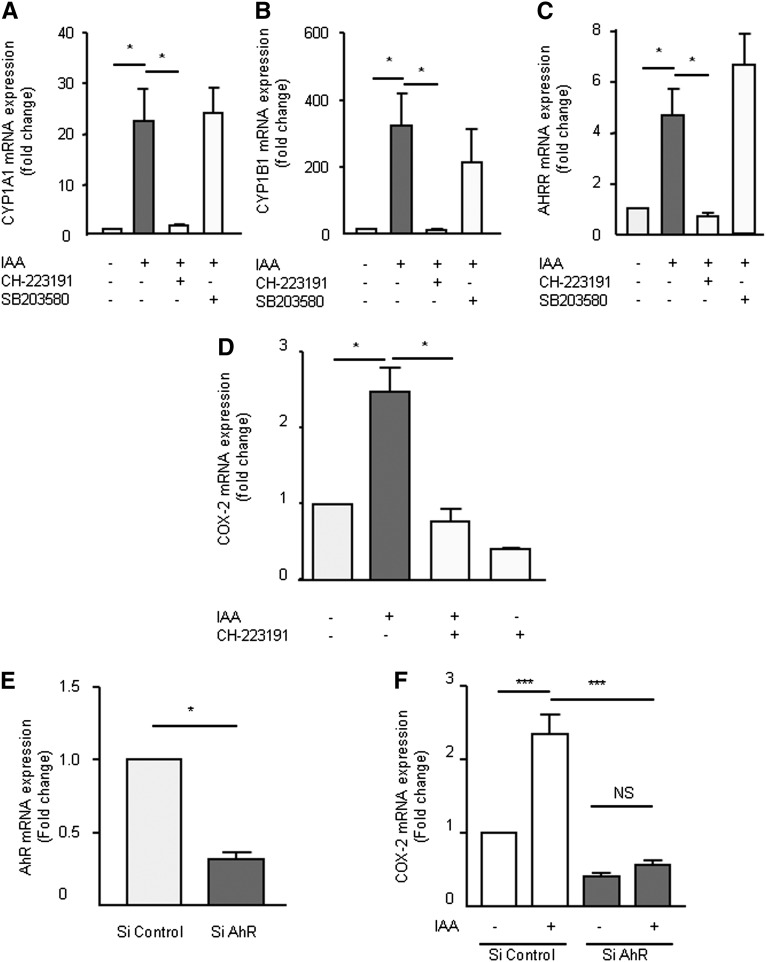
Because IAA is an AhR agonist, we verified that IAA induced the upregulation of AhR target genes in HUVEC. IAA markedly increased the mRNA expression of CYP1A1 (Figure 4A), CYP1B1 (Figure 4B), and AHRR (Figure 4C). This induction was completely abolished by the AhR inhibitor CH-223191 (Figure 4, A–C). The p38 MAPK inhibitor, SB203580, did not decrease IAA-induced CYP1A1, CYP1B1, and AHRR mRNA expression (Figure 4, A–C).
Figure 4.
The AhR pathway is involved in COX-2 mRNA upregulation by IAA. mRNA expression of AhR-regulated genes CYP1A1 (A), CYP1B1 (B), and AHRR (C) after 4-hour incubation with IAA was studied by comparative RT-qPCR in presence of an AhR inhibitor (CH-223191) or a p38 inhibitor (SB203580). (D) mRNA expression of COX-2 after 4-hour incubation with IAA was studied by comparative RT-qPCR in the presence of CH-223191. (E) mRNA expression of AhR studied by comparative RT-qPCR in HUVEC transfected with control siRNA or AhR siRNA. (F) mRNA expression of COX-2 induced by IAA in HUVECs transfected with control siRNA or AhR siRNA. Data, expressed in mRNA fold change versus control, represent the mean ± SEM of three (A–C), four (D), or five (E and F) independent experiments. *P<0.05, ***P<0.001.
We studied the involvement of AhR activation in IAA-induced COX-2 upregulation. COX-2 mRNA upregulation by IAA was abolished by the AhR inhibitor CH-223191 (Figure 4D). We then depleted AhR levels in HUVEC by transfection of small interfering RNA (siRNA) targeting AhR mRNA, which dramatically decreased AhR levels after 72 hours (Figure 4E). CYP1A1, CYP1B1, and AHRR induction by IAA was also significantly reduced (Supplemental Figure 5, E–G). Of importance, lowering the amount of AhR strongly inhibited IAA-induced COX-2 mRNA expression (Figure 4F).
To study the signaling pathways activated by IAA, we first determined whether IAA could trigger mitogen-activated protein kinase (MAPK) activation. IAA induced p38 MAPK (Figure 5A) and ERK1/2 (Figure 5B) phosphorylations in HUVEC that were transient and robust, peaking at 15 minutes. IAA-induced p38 phosphorylation was abolished by the AhR inhibitor CH-223191 (Figure 5C), suggesting IAA induced p38 MAPK phosphorylation through AhR activation.
Figure 5.
MAPK and NF-κB are involved in COX-2 upregulation by IAA. The phosphorylations of p38 (A) and ERK1/2 (B) were measured in HUVECs by Western blot after incubation with IAA during 15, 30, and 45 minutes. (C) IAA-induced p38 phosphorylation was measured by ELISA after 15 minutes in the presence of the AhR inhibitor CH-223191. (D) mRNA expression of COX-2 after 4-hour incubation with IAA in presence of a p38 inhibitor (SB203580) and an ERK inhibitor (PD98059) was studied by comparative RT-qPCR. Data are expressed in mRNA fold change versus control. (E) Endothelial p50 expression in nuclear extracts was measured after cell incubation with IAA 50 µM, with and without the p38 inhibitor SB203580. (F) mRNA expression of COX-2 after 4-hour incubation with IAA in presence of the NF-κB inhibitor BAY 11–7082 was studied by comparative RT-qPCR and expressed in mRNA fold change versus control. Data represent the mean±SEM of three (A and B), four (C, D, and F), and six (E) independent experiments. *P<0.05.
We analyzed the involvement of MAPK in IAA-induced COX-2 mRNA upregulation. COX-2 mRNA upregulation was decreased by the p38 inhibitor SB203580 but not by the ERK1/2 inhibitor PD98059 (Figure 5D), suggesting that the p38, but not the ERK, pathway is involved in COX-2 upregulation.
We finally evaluated NF-κB activation. IAA caused an increase in nuclear p50 (Figure 5E) and p65 level (not shown). The p38 inhibitor blocked IAA-induced p50 nuclear translocation (Figure 5E), suggesting that IAA activated NF-κB via p38 phosphorylation. In addition, IAA-induced COX-2 mRNA expression was completely abolished by the NF-κB inhibitor BAY 11–7082 (Figure 5F).
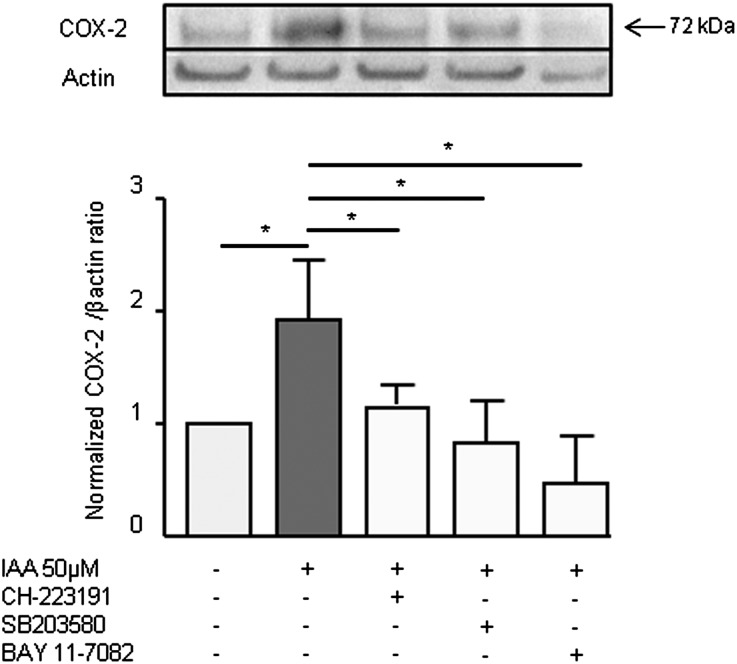
To confirm the involvement of the AhR/p38 MAPK/NF-κB pathway in COX-2 upregulation, we studied the increase in COX-2 protein level induced by IAA in presence of the AhR inhibitor CH-223191, the p38 inhibitor SB203580, and the NF-κB inhibitor BAY 11–7082. All inhibitors decreased COX-2 protein induction by IAA (Figure 6).
Figure 6.
The AhR/p38MAPK/NF-κB pathway is involved in COX-2 protein induction. COX-2 protein level was studied by Western blot after 8-hour incubation with 50 µM IAA, in presence of the AhR inhibitor CH-223191, the p38 inhibitor SB203580, and the NF-κB inhibitor BAY 11–7082. Data are expressed as normalized COX-2/βactin ratio versus control and represent the mean±SEM of three independent experiments. *P<0.05.
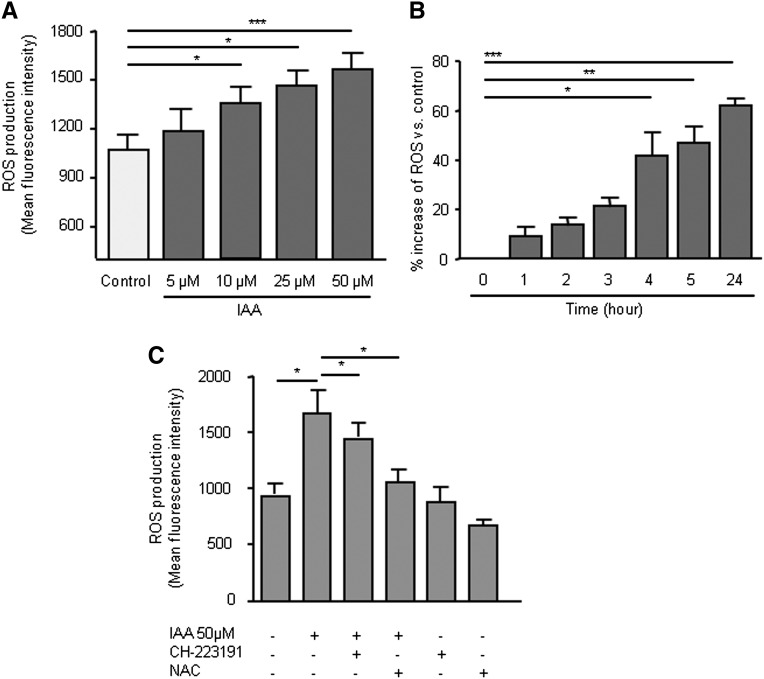
IAA Increased Endothelial Reactive Oxygen Species Production
To determine whether IAA had a prooxidant effect, we studied the ability of IAA to increase endothelial reactive oxygen species (ROS) production. Treatment of HUVEC with IAA at uremic concentrations of 10, 25, and 50 µM significantly enhanced ROS production. No increase was observed with the IAA concentration of 5 µM, which is the highest concentration found in control participants (Figure 7A). The increase in ROS production induced by IAA started to be significant after 4 hours and up to 24 hours (Figure 7B). ROS production induced by IAA was moderately inhibited by the AhR inhibitor CH-223191 (Figure 7C) and was markedly decreased by the ROS scavenger N-acetylcysteine (Figure 7C).
Figure 7.
IAA induces ROS production in HUVEC. (A) ROS production induced by IAA was measured by cytofluorometry after 5-hour incubation with 5, 10, 25, and 50 µM IAA. (B). Kinetic study of endothelial ROS production induced by IAA. (C) IAA-induced endothelial ROS production was measured after 5-hour incubation in presence of N-acetylcysteine (NAC 10 mM) and of the AhR inhibitor CH-223191 (10 µM). Data represent the mean±SEM of five (A), four (B), and six (C) independent experiments. *P<0.05, **P<0.01, ***P<0.001.
Discussion
In this study, we show that the uremic solute IAA is a predictor of mortality and major cardiovascular events in CKD. In addition, we found that serum IAA positively correlates with CRP and malondialdehyde, used to evaluate inflammation and oxidative status. Finally, we provide experimental data demonstrating the proinflammatory and the prooxidant effects of IAA on cultured endothelial cells.
The extremely high prevalence of cardiovascular disease in patients with CKD suggests the involvement of uremia-specific factors, such as uremic solutes.16–19 Here, we showed that patients with higher IAA display higher rates of mortality and cardiovascular events. More important, we demonstrated that serum IAA is a significant predictor of cardiovascular events and mortality. IAA is therefore a new member within the group of uremic solutes associated with mortality and cardiovascular events in CKD patients.
Little is known about the factors influencing IAA serum levels in patients with CKD. IAA is produced by intestinal bacteria from tryptophan, but it is also produced by cells. One can suppose that diet plays a role in IAA levels. However, in patients undergoing hemodialysis, IAA levels are not related to protein equivalent of total nitrogen appearance, a reliable estimate of dietary protein intake.20 IAA is bound to albumin with a high affinity,21 and the protein binding of IAA is about 80%.22 IAA is party removed during hemodialysis, with a reduction rate of about 45% during the dialysis session.22 Dialysis therapy, efficiency, and method may also influence IAA levels. However, predialysis concentrations of IAA seem to depend on residual renal function rather than on dialysis adequacy, assessed by Kt/Vurea.20
This observational clinical study has some limits. First, the relation to outcomes is only observational. Second, clinical endpoints were correlated with a single serum IAA measurement at the inclusion, without information about IAA intra-individual variability. In the literature, no study is specifically focused on intra-individual variability of IAA. Only data retrieved from an interventional study on sevelamer revealed no significant change in IAA level in patients with CKD over 16 weeks.23 Third, our data obtained in a small group of patients have to be confirmed in larger CKD populations. Notably, the absence of difference in mortality between dialysis patients with high and low IAA levels has to be confirmed in larger cohorts of dialysis patients. Fourth, it was difficult to dissociate the predictive value of IAA from that of low eGFR and CKD severity, because eGFR and percentage of dialysis patients were different between the high and the low IAA groups. Note that serum IAA level increases with CKD stage but is poorly associated with eGFR in patients with CKD not on dialysis.24 In our study, when adjusted with eGFR, IAA was not predictive of mortality and cardiovascular events. This was probably because analysis was limited to the 47 patients with CKD who were not undergoing dialysis, who were the only patients with laboratory values available for eGFR calculations. To perform the statistical analysis on the whole population of 120 patients, we used CKD stage rather than eGFR to correct for renal function. In the model adjusted for CKD stage, serum level of IAA was still a significant and independent predictor of increased risk of death. In addition, IAA level remained predictive of mortality and cardiovascular events when adjusted for other protein-bound uremic toxins, p-cresyl sulfate and indoxyl sulfate. This suggests that IAA was a predictor on its own rather than just a reflection of the group of protein-bound toxins.
To demonstrate that IAA is a real mediator of vasculotoxicity and not merely a marker of impaired renal function, we studied the IAA effects on cultured endothelial cells, focusing on its prooxidant and proinflammatory effects. In vitro, IAA mediated endothelial oxidative stress by increasing ROS production. IAA also increased the expression of endothelial inflammatory genes: IL-6, IL-8, ICAM-1, and MCP-1. Finally, IAA increased endothelial COX-2 expression and function.
A mechanism to explain IAA cellular effects could be AhR activation. Indeed, IAA is a ligand of the transcription factor AhR, whose activation is involved in atherogenesis,13 vascular inflammation,13 and oxidative stress.25 Upon ligand binding, AhR translocates into the nucleus and binds to xenobiotic-responsive elements within the promoters of target genes11 such as CYP1A1, CYP1B1, AHRR, and COX-2.26 AhR activation also initiates alternative signaling pathways called “inflammatory nongenomic pathways” without direct DNA binding of AhR. We previously reported that IAA induces AhR nuclear translocation in HUVEC.9 Here, we showed IAA upregulates CYP1A1, CYP1B1, and AHRR. IAA induced AhR activation, which subsequently activated an inflammatory signaling pathway involving p38 MAPK and NF-κB activation.
IAA induced inflammation notably through COX-2 upregulation, without modification of COX-1 expression. Although COX-1 and COX-2 share a high level of homology, their expressions and activities are differentially regulated, and they can function independently within the same cell type.27 Because COX-2 leads to the synthesis of prostanoids involved in inflammatory states,15 the IAA-induced overexpression of COX-2 may cause an imbalance in prostanoid synthesis. Studies support the notion that the COX-2/PGE2 axis has an important role in atherosclerosis.28–30 In animal models of CKD, COX-2 is upregulated31,32 and associated with a proinflammatory and prooxidant profile.31,32 Serum from patients with CKD increases COX-2 expression in smooth muscle cells33 and induces endothelial p38 MAPK and NF-κB activation.34 In our cultured endothelial cells, IAA increased COX-2 level and PGE2 production, induced NF-κB activation, and increased oxidative stress, mimicking the inflammatory and prooxidative profile found in CKD.
Endothelial COX-2 is regulated by several signaling pathways, including ERK1/2 and p38 MAPK,35 with subsequent activation of transcription factors such as AP-136 and NF-κB.35 COX-2 production is also regulated by AhR via a genomic and a nongenomic pathway.37,38 Here, we demonstrated the crucial involvement of AhR and subsequent p38 MAPK/NF-κB activation in IAA-induced COX-2 upregulation. Further studies on COX-2 promoter activation would be of interest to better understand COX-2 regulation by IAA.
AhR activation by exogenous ligands results in induction of endothelial oxidative stress, notably through CYP1.12,14 We report that IAA increases ROS production, which was moderately decreased by the AhR inhibitor, suggesting the involvement of other pathways. In addition to CYP1, several sources of ROS exist, notably the mitochondrial electron transport chain, nicotinamide adenine dinucleotide phosphate-oxidase, and cyclooxygenases.39–41 Note that IAA also has a prooxidant effect in renal tubular cells.42 Via prooxidant, proinflammatory, and prothrombotic9 mechanisms, IAA could act as a mediator of endotheliotoxicity of uremia.
In conclusion, IAA has proinflammatory and prooxidant effects in endothelial cells and activates a signaling pathway related to AhR that leads to COX-2 upregulation. These biologic effects are clues to explain the association between IAA concentration and the increased risk of mortality and cardiovascular events in patients with CKD.
Concise Methods
Patients
We performed a single-center, prospective study in 120 patients with CKD, enrolled between April 2007 and July 2010, selected according to the following criteria: age>18 years; no diabetes mellitus; no cardiovascular event, infection, or surgical intervention (except for vascular access angioplasty) in the last 3 months; no pregnancy; no recent history of malignancy; and no intake of corticosteroids or immunosuppressive agents. Patients with CKD who were undergoing dialysis had a dialysis session at least three times a week for a minimum of 6 months. Standard laboratory procedures were used for blood chemistry evaluations.
CKD stages 3, 4, and 5 were defined according to eGFR. CKD stage 3 (n=16) was defined as 30 ml/min per 1.73 m2<eGFR<60 ml/min per 1.73 m2; stage 4 (n=17) as 15 ml/min per 1.73 m2<eGFR<30 ml/min per 1.73 m2; and stage 5 (n=14) as eGFR<15 ml/min per 1.73 m2. Stage 5D (n=73) was defined as patients receiving dialysis.
During the study period, clinical events, including overall mortality and cardiovascular events, were recorded. The causes of death were categorized as cardiovascular, infectious, or other. Cardiovascular events included death from cardiac cause, nonfatal myocardial infarction, nonfatal stroke, and nonfatal peripheral vascular disease with amputation or need for angioplasty. Patients without clinical event might have subclinical cardiovascular disease because this disease was not systematically explored by imaging.
Patients were compared with 51 controls (25 men and 26 women) with normal renal function, no diabetes, no cardiovascular event, and no chronic medication. They were recruited by the Centre d’Investigation Clinique of the Assistance Publique Hôpitaux de Marseille. Their median age was 64 years (range, 49–85 years).
All participants gave their written informed consent. The study was approved by the local ethics committee and conforms to the principles outlined in the Declaration of Helsinki.
Statistical Analyses
Data are expressed as mean±SD for values with normal distribution or median (minimum, maximum) for non-normally distributed values. All numeric variables were tested for normality by the Kolmogorov-Smirnov test. Statistical analyses were performed with the Prism (GraphPad Software Inc., La Jolla, CA) and MedCalc (Ostend, Belgium) software. Differences were considered significant at P<0.05. Baseline variables were compared by chi-squared tests for categorical variables, t tests for continuous variables with Gaussian distributions, and Mann–Whitney tests for continuous variables with non-Gaussian distributions. Correlations between IAA levels and continuous variables were obtained using Spearman correlation coefficients. The Kaplan–Meier method was used to estimate the cumulative event-free rate for the time to overall mortality and the first cardiovascular event in patients with IAA level above and below the median (3.73 µM). The log-rank test was used to compare the Kaplan–Meier curves. Univariate and multivariate analyses of overall mortality and cardiovascular events were performed using a Cox proportional hazard model as a function of serum IAA as continuous variable. Variables with non-Gaussian distribution were normalized by Box-Cox transformation when used in tests that assume normally distributed variables.
In multivariate analyses, models were adjusted for demographic variables (age and male sex; model 1), Framingham cardiovascular risk factors (cholesterol, systolic BP, and smoking; model 2), factors associated with mortality in patients with CKD (CRP, phosphate, BMI, and albumin; model 3), confounding factors associated with mortality in univariate analysis (diastolic BP and history of cardiovascular disease; model 4), and CKD stage (model 5). Univariate and multivariate analyses were also performed to compare IAA, p-cresyl sulfate, and indoxyl sulfate.
For in vitro experiments, significant differences were revealed by the Wilcoxon signed-rank test or by the Mann–Whitney test. Data are expressed as mean±SEM or mean to maximum box and whiskers plot of independent experiments performed on different cell preparations. Statistical analyses were performed with Prism software. A P value <0.05 was considered to represent a statistically significant difference.
Measurement of IAA, p-Cresylsulfate, and Indoxyl Sulfate
IAA, p-cresylsulfate, and indoxyl sulfate were measured in serum by HPLC using a reversed phase column, an ion-pairing mobile phase, and isocratic flow, as described.43
Malondialdehyde Assay
Malondialdehyde was analyzed in serum by HPLC using the Chromsystems reagent kit (Chromsystems, Gräfelfing, Germany) according to the manufacturer’s instructions.
Endothelial Cell Culture
HUVECs were obtained from umbilical cord vein by collagenase digestion as described elsewhere44 and grown to the fourth passage in EGM-2 medium (Lonza, France) (containing 2% FBS), under standard cell culture conditions (humidified atmosphere at 37°C, 5% CO2). The endothelial nature of HUVECs was confirmed by flow cytometry using the endothelial-specific markers CD31, CD144, Von Willebrand factor, and CD146 and by negative staining of the leukocyte marker CD45.
HAoECs, HCAECs, and HMVEC-Cs were obtained from Lonza. Routine endothelial characterization and analysis of purity of HAoECs, HCAECs, and HMVEC-Cs were performed by immunofluorescent staining by the supplier. HCAECs and HMVEC-Cs were grown, respectively, to the fourth and the fifth passages in EGM-2 MV medium (Lonza) (containing 5% FBS) under standard cell culture conditions. HAoECs were grown to the fifth passage in EGM-2 medium under standard cell culture conditions.
Treatment with the Uremic Solute IAA
Cells were treated with IAA (Sigma-Aldrich, France) at concentrations of 5, 10, 25, and 50 µM, with and without different inhibitors (Supplemental Table 1). An IAA value of 5 µM corresponds to the highest concentration of IAA found in controls; 10 and 25 µM are in the range of IAA concentrations found in uremic patients; and 50 µM is the highest IAA concentration described in uremic patients.8 Because IAA was diluted in ethanol, ethanol 1/1000 was used as a vehicle control. In some experiments, human serum albumin (LFB, France) was added in the culture medium, at the concentration found in human serum (4 g/dl).
siRNA Knockdown of AhR
HUVECs were transfected with siRNA control (Negative Universal Control, Stealth RNAi; Life Technologies, France) or a pool containing three Silencer Select siRNA directed against AhR (Life Technologies) by magnetofection using SilenceMag beads (OZ Biosciences, France), according to the manufacturer’s instructions. The knockdown of AhR was confirmed by reverse transcription (RT) and quantitative PCR (qPCR) 72 hours after transfection. Dose responses and transfection efficiency of AhR siRNA are described in the Supplemental Methods and displayed in Supplemental Figure 5. The induction of COX-2, Cyp1A1, Cyp1B1, and AHRR mRNA by IAA was determined 72 hours after transfection.
RNA Extraction and Quantitative RT-PCR Analysis of mRNA Expression
Total RNA was extracted by an RNeasy mini-kit (Qiagen, France). RT was performed on 1 µg of total RNA using the AffinityScript multiple-temperature cDNA synthesis (Agilent, France) followed by qPCR on 10 ng of cDNA using the Brilliant II SYBR Green QPCR Master Mix (Agilent). We quantified the following target genes: COX-2, Cyp1A1, Cyp1B1, AHRR, AHR, IL-6, IL-8, ICAM-1, VCAM-1, and MCP-1. The housekeeping gene HPRT was used for normalization of the target gene values. The sequences of primers are displayed in Supplemental Table 2. All PCR reaction efficiencies were determined with MxPro software (Agilent) and were always between 90% and 110%. The fusion curves were analyzed to assess the specificity of detected fluorescence.
Western Blotting and Densitometry Analysis of Western Blots
HUVEC were lysed in lysis buffer containing protease inhibitors. Protein concentrations were measured with the Bicinchoninic Acid Kit (BCA1, Sigma-Aldrich). After saturation, the membrane was incubated with antibodies directed against COX-2, phospho-p38, p38, phospho-ERK1/2, ERK1, or actin (all from Cell Signaling Technology, Ozyme, France), and then with the secondary peroxidase-conjugated antibody (Beckman Coulter, France). Revelations were done by chemiluminescence (ECL Western blotting substrate; Pierce). Gel images were captured using the Syngene GBox (Ozyme) and analyzed with the software geneSys (Ozyme). Results were expressed as a ratio between values obtained with IAA and control normalized with values obtained with actin. The effect of AhR inhibition on p38 phosphorylation was studied with the RayBio Cell-based p38 MAPK ELISA kit (Tebu-bio, France).
PGE2 Measurement
PGE2 was measured in the supernatants of HUVEC using the Prostaglandin E2 assay kit (R&D Systems, France).
Measurement of ROS Production
Intracellular ROS production was detected at baseline and after 5 hours of incubation by measuring the fluorescence of 6-carboxy-H2DCF-DA-di-AM (Invitrogen, France) by the Cytofluor Series 4000 (Applied Biosystems), with the excitation filter at 485 nm and the emission filter at 530 nm. Fluorescence intensity was expressed in arbitrary units.
Study of NF-κB Activation
Nuclear extracts were prepared using Cayman Chemical’s Nuclear Extraction Kit (interchim, France). NF-κB p50 and p65 were detected in nuclear extracts by Cayman Chemical’s NF-κB (human p50/p65) combo transcription factor assay kit (interchim).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank C. Scagliarini for technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121283/-/DCSupplemental.
References
- 1.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J: Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl 111(Suppl): S4–S9, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Raff AC, Meyer TW, Hostetter TH: New insights into uremic toxicity. Curr Opin Nephrol Hypertens 17: 560–565, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, European Uremic Toxin Work Group : Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P: Vascular incompetence in dialysis patients—protein-bound uremic toxins and endothelial dysfunction. Semin Dial 24: 327–337, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, Brunet P: The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 5: 1302–1308, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Glorieux G, Cohen G, Jankowski J, Vanholder R: Platelet/leukocyte activation, inflammation, and uremia. Semin Dial 22: 423–427, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Vanholder R, Brunet P: Protein-bound toxins—update 2009. Semin Dial 22: 334–339, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, Calaf R, Lacroix R, Jourde-Chiche N, Poitevin S, Arnaud L, Vanholder R, Brunet P, Dignat-George F, Burtey S: Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int 84: 733–744, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS: Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 37: 11508–11515, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Haarmann-Stemmann T, Bothe H, Abel J: Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem Pharmacol 77: 508–520, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Kopf PG, Walker MK: 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol Appl Pharmacol 245: 91–99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu D, Nishimura N, Kuo V, Fiehn O, Shahbaz S, Van Winkle L, Matsumura F, Vogel CF: Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol 31: 1260–1267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korashy HM, El-Kadi AO: The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev 38: 411–450, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Cipollone F, Cicolini G, Bucci M: Cyclooxygenase and prostaglandin synthases in atherosclerosis: Recent insights and future perspectives. Pharmacol Ther 118: 161–180, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liabeuf S, Glorieux G, Lenglet A, Diouf M, Schepers E, Desjardins L, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin (EUTox) Work Group : Does p-cresylglucuronide have the same impact on mortality as other protein-bound uremic toxins? PLoS ONE 8: e67168, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Wu IW, Hsu KH, Hsu HJ, Lee CC, Sun CY, Tsai CJ, Wu MS: Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—a prospective cohort study. Nephrol Dial Transplant 27: 1169–1175, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Eloot S, Van Biesen W, Glorieux G, Neirynck N, Dhondt A, Vanholder R: Does the adequacy parameter Kt/V(urea) reflect uremic toxin concentrations in hemodialysis patients? PLoS ONE 8: e76838, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe H, Miyamoto Y, Otagiri M, Maruyama T: Update on the pharmacokinetics and redox properties of protein-bound uremic toxins. J Pharm Sci 100: 3682–3695, 2011 [DOI] [PubMed] [Google Scholar]
- 22.De Smet R, Dhondt A, Eloot S, Galli F, Waterloos MA, Vanholder R: Effect of the super-flux cellulose triacetate dialyser membrane on the removal of non-protein-bound and protein-bound uraemic solutes. Nephrol Dial Transplant 22: 2006–2012, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Brandenburg VM, Schlieper G, Heussen N, Holzmann S, Busch B, Evenepoel P, Vanholder R, Meijers B, Meert N, Fassbender WJ, Floege J, Jahnen-Dechent W, Ketteler M: Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: a prospective study. Nephrol Dial Transplant 25: 2672–2679, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Eloot S, Schepers E, Barreto DV, Barreto FC, Liabeuf S, Van Biesen W, Verbeke F, Glorieux G, Choukroun G, Massy Z, Vanholder R: Estimated glomerular filtration rate is a poor predictor of concentration for a broad range of uremic toxins. Clin J Am Soc Nephrol 6: 1266–1273, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalton TP, Puga A, Shertzer HG: Induction of cellular oxidative stress by aryl hydrocarbon receptor activation. Chem Biol Interact 141: 77–95, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Degner SC, Kemp MQ, Hockings JK, Romagnolo DF: Cyclooxygenase-2 promoter activation by the aromatic hydrocarbon receptor in breast cancer mcf-7 cells: Repressive effects of conjugated linoleic acid. Nutr Cancer 59: 248–257, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Félétou M, Huang Y, Vanhoutte PM: Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol 164: 894–912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipollone F, Prontera C, Pini B, Marini M, Fazia M, De Cesare D, Iezzi A, Ucchino S, Boccoli G, Saba V, Chiarelli F, Cuccurullo F, Mezzetti A: Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation 104: 921–927, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Beloqui O, Páramo JA, Orbe J, Benito A, Colina I, Monasterio A, Díez J: Monocyte cyclooxygenase-2 overactivity: A new marker of subclinical atherosclerosis in asymptomatic subjects with cardiovascular risk factors? Eur Heart J 26: 153–158, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Burleigh ME, Babaev VR, Oates JA, Harris RC, Gautam S, Riendeau D, Marnett LJ, Morrow JD, Fazio S, Linton MF: Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation 105: 1816–1823, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Bai Y, Sigala W, Adams GR, Vaziri ND: Effect of exercise on cardiac tissue oxidative and inflammatory mediators in chronic kidney disease. Am J Nephrol 29: 213–221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaziri ND, Bai Y, Yuan J, Said HL, Sigala W, Ni Z: ApoA-1 mimetic peptide reverses uremia-induced upregulation of pro-atherogenic pathways in the aorta. Am J Nephrol 32: 201–211, 2010 [DOI] [PubMed] [Google Scholar]
- 33.van der Giet M, Tölle M, Pratico D, Lufft V, Schuchardt M, Hörl MP, Zidek W, Tietge UJ: Increased type IIA secretory phospholipase A(2) expression contributes to oxidative stress in end-stage renal disease. J Mol Med (Berl) 88: 75–83, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Caballo C, Palomo M, Cases A, Galán AM, Molina P, Vera M, Bosch X, Escolar G, Diaz-Ricart M: NFκB in the development of endothelial activation and damage in uremia: An in vitro approach. PLoS ONE 7: e43374, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin CC, Hsieh HL, Shih RH, Chi PL, Cheng SE, Yang CM: Up-regulation of COX-2/PGE2 by endothelin-1 via MAPK-dependent NF-κB pathway in mouse brain microvascular endothelial cells. Cell Commun Signal 11: 8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh HL, Lin CC, Chan HJ, Yang CM, Yang CM: c-Src-dependent EGF receptor transactivation contributes to ET-1-induced COX-2 expression in brain microvascular endothelial cells. J Neuroinflammation 9: 152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong B, Nishimura N, Vogel CF, Tohyama C, Matsumura F: TCDD-induced cyclooxygenase-2 expression is mediated by the nongenomic pathway in mouse MMDD1 macula densa cells and kidneys. Biochem Pharmacol 79: 487–497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W, Vogel CF, Wu D, Matsumura F: Non-genomic action of TCDD to induce inflammatory responses in HepG2 human hepatoma cells and in liver of C57BL/6J mice. Biol Chem 391: 1205–1219, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Tardieu D, Jaeg JP, Deloly A, Corpet DE, Cadet J, Petit CR: The COX-2 inhibitor nimesulide suppresses superoxide and 8-hydroxy-deoxyguanosine formation, and stimulates apoptosis in mucosa during early colonic inflammation in rats. Carcinogenesis 21: 973–976, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama T, Yoshimoto T, Sato R, Fukai N, Ozawa N, Shichiri M, Hirata Y: Endothelin-1 induces cyclooxygenase-2 expression and generation of reactive oxygen species in endothelial cells. J Cardiovasc Pharmacol 44[Suppl 1]: S332–S335, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Zhang DX, Gutterman DD: Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292: H2023–H2031, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T: Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney Int 63: 1671–1680, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Calaf R, Cerini C, Génovésio C, Verhaeghe P, Jourde-Chiche N, Bergé-Lefranc D, Gondouin B, Dou L, Morange S, Argilés A, Rathelot P, Dignat-George F, Brunet P, Charpiot P: Determination of uremic solutes in biological fluids of chronic kidney disease patients by HPLC assay. J Chromatogr B Analyt Technol Biomed Life Sci 879: 2281–2286, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Jaffe EA, Nachman RL, Becker CG, Minick CR: Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52: 2745–2756, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.