Abstract
Antibody-mediated rejection is a major complication in renal transplantation. The pathologic manifestations of acute antibody-mediated rejection that has progressed to functional impairment of a renal transplant have been defined in clinical biopsy specimens. However, the initial stages of the process are difficult to resolve with the unavoidable variables of clinical studies. We devised a model of renal transplantation to elucidate the initial stages of humoral rejection. Kidneys were orthotopically allografted to immunodeficient mice. After perioperative inflammation subsided, donor-specific alloantibodies were passively transferred to the recipient. Within 1 hour after a single transfer of antibodies, C4d was deposited diffusely on capillaries, and von Willebrand factor released from endothelial cells coated intravascular platelet aggregates. Platelet-transported inflammatory mediators platelet factor 4 and serotonin accumulated in the graft at 100- to 1000-fold higher concentrations compared with other platelet-transported chemokines. Activated platelets that expressed P-selectin attached to vascular endothelium and macrophages. These intragraft inflammatory changes were accompanied by evidence of acute endothelial injury. Repeated transfers of alloantibodies over 1 week sustained high levels of platelet factor 4 and serotonin. Platelet depletion decreased platelet mediators and altered the accumulation of macrophages. These data indicate that platelets augment early inflammation in response to donor-specific antibodies and that platelet-derived mediators may be markers of evolving alloantibody responses.
Keywords: acute rejection, immunology and pathology, platelets, endothelial cells, transplant pathology, electron microscopy
Antibody-mediated rejection (AMR) of renal transplants eluded diagnosis for many years. Criteria for the diagnosis of acute AMR were added to the Banff classification of renal allograft rejection in 2003.1 These criteria included three cardinal features: serologic evidence of circulating antibodies to donor antigens, immunopathologic evidence for antibody action (most often diffuse deposition of the complement split product C4d on vascular endothelium), and morphologic evidence of acute tissue injury (most often neutrophils or mononuclear cells in peritubular capillaries or glomeruli). Later iterations included considerations of the extent of C4d deposition and presence of microvascular pathology, including formation of microthrombi.2 With the application of molecular diagnostic tests to renal biopsies, a set of endothelial-associated transcripts was identified that correlated with AMR.3 In one study, vWf was the most highly expressed transcript in biopsies with C4d deposits on capillary endothelium and was most strongly associated with graft loss. Not every biopsy suspected of AMR shows all of these pathologic findings. Consequently, schemes delineating the progression of AMR have been proposed to account for incomplete manifestations of AMR.4 However, the progression of AMR is difficult to establish with the unavoidable variables of clinical studies.
Some potential mechanisms involved in the early stages of AMR have been elucidated by experimental models. In vitro experiments revealed that antibodies elicit the rapid exocytosis of preformed adhesion molecules from Weibel–Palade storage granules of endothelial cells. The two major constituents of Weibel–Palade bodies are ultrahigh molecular weight vWf and P-selectin, both of which are exocytosed within minutes after antibodies to MHC class I antigens are added to human endothelial cells in vitro.5,6
Animal models have confirmed that donor-specific antibodies (DSAs) are associated with release of vWf in cardiac allografts.7,8 When vWf is released, the end of the vWf multimer remains attached to endothelial cells, and strings of vWf interact with integrin GPIb-XI-V and GPIIb/IIIa on platelets.9 In models of AMR, release of vWf is associated with adhesion of platelets to vascular endothelium of cardiac allografts.10,11
Before the general recognition of AMR, several groups showed that radiolabeled platelets localized to renal transplants in the early stages of rejection.12–15 More recently, immunohistologically identified platelet aggregates were reported in the majority of biopsies diagnosed with AMR on the basis of C4d deposits.16
The initiation of platelet activation by alloantibodies has been shown in skin allografts. Using a model in which immunodeficient mice received passively transferred antibody, Morrell et al.17 directly visualized alloantibody-induced platelet tethering in capillaries of skin allografts by intravital imaging. Immunohistology verified that platelet adhesion to capillary endothelium was associated with vWf release. These interactions activated the platelets to express P-selectin. The alloantibodies also induced perivascular infiltrates of neutrophils that were partly dependent on platelets, because platelet depletion decreased the accumulation of neutrophils.
The potential for platelets to participate in AMR has been recognized18–20 but not tested in a relevant model of organ transplantation. Platelets are not easily observed with routine stains because of their small size and lack of nucleus. Microarrays do not detect platelet activities effectively, because platelets contain limited amounts of RNA.21 These experiments were designed to investigate the contribution of platelets to AMR through immunohistochemistry and assays of intragraft mediators.
Results
Platelet Localization and Activation Is Induced in Orthotopic Renal Allografts by DSA
To investigate alloantibody-induced localization and activation of platelets in allografts, B10.A (H-2a) kidneys were transplanted to immune-deficient RAG−/− C57BL/6 mice. After postoperative inflammation subsided, a mixture of IgG1, -2a, and -2b mAbs to H-2a (or isotype controls) was transferred (experimental design shown in Supplemental Figure 1).
One hour after a single injection of alloantibodies, complement activation was detected by immunohistochemical staining for C4d. C4d was deposited on arteries, glomerular capillaries, peritubular capillaries, and veins in a strong and diffuse pattern after transfer of alloantibody but not isotype control antibody (Figure 1, A and D). In addition to activation of complement, antibodies to MHC class I antigens can elicit exocytosis of vWf from Weibel–Palade bodies in endothelial cells.5,6 In allografts from recipients treated with control antibodies, vWf was largely confined to intracytoplasmic granules that were most evident in arterial endothelial cells. In contrast, passive transfer of alloantibodies resulted in extensive coating of platelets with vWf (Figure 1, B and E). The intravascular aggregates of platelets were activated and stained strongly for P-selectin (Figure 1, C and F). The fact that the platelets formed small aggregates precluded counting individual platelets. Therefore, the relative area occupied by the P-selectin–stained platelets was measured and found to be about 28-fold higher after transfer of alloantibodies versus control antibodies (Figure 2A).
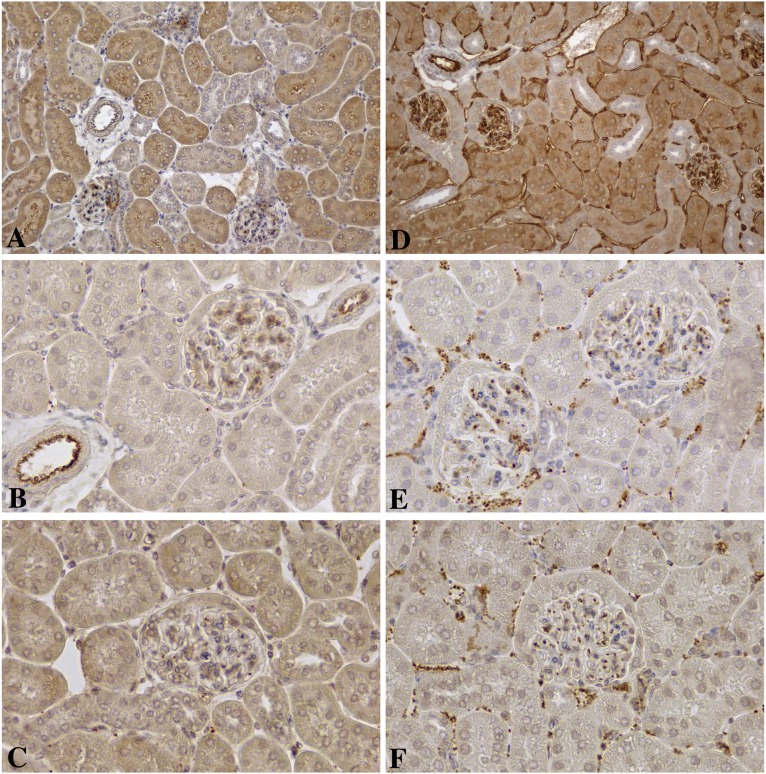
Figure 1.
Immunohistologic findings in renal allografts 1 hour after a single transfer of alloantibodies. Comparisons of immunohistologic findings in renal allografts after transfer of (A–C) isotype control antibodies versus (D–F) DSA. Low magnification (×200) shows that intense deposits of C4d were diffusely distributed on arteries, glomerular capillaries, peritubular capillaries, and veins 1 hour after a single transfer of (D) alloantibodies but not (A) isotype control antibodies. In control allografts, (B) vWf was largely confined to endothelial storage granules most evident in arteries, but (E) vWf coated intravascular aggregates of platelets in alloantibody-treated recipients. (F) Alloantibodies but not (C) isotype control antibodies induced large numbers of intravascular P-selectin–positive platelets. B, C, E, and F were imaged at ×400 to show platelets.
Figure 2.
Quantification of platelet accumulation in renal allografts. (A) Digital planimetric measurements of P-selectin–stained platelets were about 28-fold higher after single transfer of DSA versus isotype control antibodies. After four repeated transfers, the area was about 5.6-fold higher in animals treated with alloantibody versus isotype control antibody. (B) Both PF4 and serotonin were greatly increased in allografts after single or multiple transfers of alloantibody compared with isotype control antibody. Each symbol represents data from an individual animal. AlloAb, alloantibody; IsoAb, isotype control antibody. *P<0.05 versus control; **P<0.01 versus control.
Electron micrographs showed extensive interactions of platelets with endothelial cells. The luminal membranes of capillary endothelial cells formed numerous projections and microvilli. Such microvilli have been reported to serve as docking structures for leukocytes in inflammatory sites.22,23 In our model, platelets were found to interact with these endothelial microvilli (Figure 3A). Electron micrographs also documented signs of acute endothelial cell injury associated with AMR,24 including swelling, blebbing, and expansion of subendothelial space (Figure 3B). In agreement with the immunohistologic findings, electron micrographs showed few platelets in the vessels of the control graft, and there was little contact between endothelial cells and platelets or leukocytes (Figure 3C).
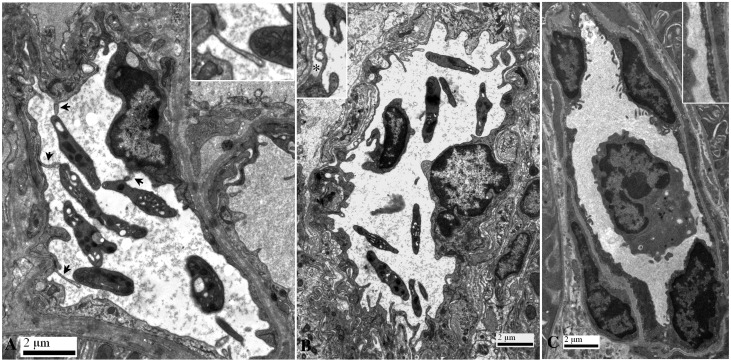
Figure 3.
Ultrastructure of capillary endothelial changes and platelet interactions 1 hour after passive transfer of alloantibodies or control antibodies. (A) After transfer of alloantibodies, microvillous protrusions (arrows) extend from luminal surfaces of endothelial cells, many of which contact platelets (Inset). (B) Transfer of alloantibodies also induced endothelial injury evidenced by swelling, blebbing, and expansion of the subendothelial space (asterisk). Microvillous protrusions extend from luminal surfaces of endothelial cells, some of which are in close proximity to a leukocyte and platelets. (C) After transfer of control antibodies, endothelial cells maintained contact with the basement membrane, and there was no evidence of interactions between leukocytes or platelets with endothelial cells. Insets are enlarged 2-fold.
The α-granules and dense granules of platelets contain highly active mediators for transport and release. We measured two signature mediators contained in platelet granules: PF4, an α-granule constituent, and serotonin, a constituent of dense granules. Both PF4 and serotonin were highly concentrated in allografts after passive transfer of alloantibody (10–30 ng/mg tissue) compared with isotype controls (Figure 2B). In contrast, other cytokines stored in platelet α-granules (IL-1β, MCP1/CCL2, MIP-1α/CCL3, and RANTES/CCL5) were found in only microgram amounts (data not shown).
When the interval between antibody transfer and euthanasia was extended from 1 to 5 hours, C4d deposits were still diffuse and strong, but platelet aggregates had decreased. By this time, intragraft levels of PF4 and serotonin had also decreased but were still in the range of 2–7 ng/mg tissue.
Prolonging the exposure to circulating alloantibodies by delivering four injections of alloantibodies over an interval of 1 week resulted in strong, diffuse C4d deposits accompanied by platelet aggregates 1 hour after the last dose of DSA. Although the platelet aggregates were less extensive on immunohistology than after a single injection of alloantibodies, the fraction of P-selectin–positive area was still 5.6-fold higher in alloantibody-treated animals than in the isotype control group (Figure 2A), and large amounts of both PF4 and serotonin (7–20 ng/mg tissue) were concentrated in allografts (Figure 2B).
Platelet Localization in Human Renal Allografts Diagnosed with AMR
Recent molecular and immunohistologic studies of renal biopsies have correlated vWf deposits and platelet aggregates with AMR.3,16,25 To verify that platelets are components of AMR in clinical samples, we examined five biopsies that had diffuse deposits of C4d within 3 weeks after transplantation and were diagnosed with acute AMR by the Banff criteria.1,2,26,27 Stains for CD41 (GpIIb), a platelet-specific marker, showed platelet aggregates in all of these biopsies. The distribution of the platelet aggregates correlated with the Banff injury scores as assessed at the time of diagnosis. All of the biopsies had glomerular platelet aggregates together with Banff g scores 1–3 (Figure 4, Supplemental Table 1). Focal platelet aggregates were present in peritubular capillaries in four of the biopsies. One biopsy with a Banff ptc score of 2 had more extensive platelets in peritubular capillaries, and some of these platelets were attached to leukocytes. Only scattered isolated platelets were found in control biopsies with no evidence of AMR.
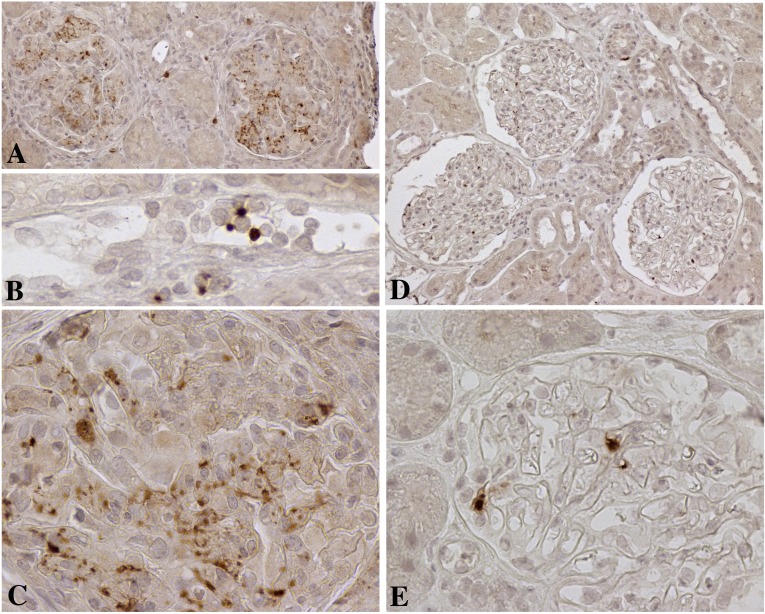
Figure 4.
Immunohistology for CD41 (GpIIb), a platelet-specific marker, on biopsies from clinical renal transplants. (A) Low-power (×200) image of platelet aggregates in two glomeruli in a biopsy with a Banff g score of 3. (B) High-power (×600) image of platelets attached to leukocytes in the peritubular capillary of a biopsy with a Banff ptc score of 2. (C) High-power (×600) image of glomerular platelet aggregates and platelet fragments associated with leukocytes. (D) Low-power (×200) image of isolated platelets in three glomeruli in a normal protocol biopsy. (E) High-power (×600) image of solitary platelets in open glomerular capillary loops of a normal renal transplant.
Macrophage Localization and Formation of Macrophage-Platelet Conjugates Induced in Renal Allografts by DSA
Clinically, AMR has been associated with acute glomerulitis distinguished by infiltrates of macrophages,1,28 and glomerular monocytes predict poorer graft outcomes.29 In our model, passive transfer of alloantibodies also increased macrophage infiltrates in the grafts. In comparison, only minor numbers of neutrophils were found. When the numbers of Mac2-positive macrophages were counted in 100 glomeruli per mouse (n=4), alloantibodies were found to increase the average number of macrophages and percentage of glomeruli with two or more macrophages (Figure 5).
Figure 5.
Quantification of macrophage infiltrates in renal grafts. (A) Mac2-positive macrophages (brown) were localized to the glomeruli (arrow) by immunohistochemistry. (B) P-selectin stains revealed intravascular conjugates between P-selectin–positive (brown) platelets and monocytes (arrows) in allografts after alloantibody transfer. (C) Macrophage counts on >100 glomeruli per allograft tabulated as percentages of glomeruli with two or more or three or more macrophages and average numbers of macrophages per glomerulus (Mφ per Glo.) presented as a bar graph±SEM. Allo Ab, alloantibody; Iso Ab, isotype control antibody. *P<0.05 alloantibody versus isoantibody; **P<0.01 alloantibody versus isoantibody.
Effects of Platelet Depletion on Macrophage Infiltration
Our immunohistologic stains for P-selectin and vWf showed that many intravascular leukocytes had one or more platelets attached to their surface (Figure 5). The formation of platelet–leukocyte conjugates can increase activation and localization of leukocytes in inflammatory sites.30 To determine whether platelets were critical for the localization of macrophages to allografts, platelets were depleted from recipients by administering mAbs to GPIb 1 hour before alloantibody. Control mice received monoclonal rat IgG. In the single passive transfer protocol, antibody to GPIb acutely decreased peripheral platelet counts to about 0.81% of the control group. In mice that received multiple doses of alloantibodies, injections of antibodies to GPIb were given 1 hour before each alloantibody transfer. This treatment protocol was variably effective in sustaining platelet depletion over 1 week, with platelet numbers ranging from 0.78% to 51.9% of the control group. Serotonin and PF4 levels in the grafts were also decreased in the platelet-depleted groups (Figure 6A).
Figure 6.
Effects of platelet depletion on macrophages in glomeruli and platelet mediators in allografts. (A and B) Platelet depletion (Plt deplt) slightly increased acute glomerular infiltrates of both Mac2+ and YM1+ macrophages in response to a single DSA transfer. Multiple doses of antibodies to platelet GPIb decreased glomerular infiltrates of both Mac2+ and YM1+ macrophages. (C) ELISA on tissue homogenates of allografts showed that platelet-depleting treatment decreased levels of platelet-specific molecules, PF4, and serotonin. Each symbol represents data from an individual animal, and brackets represent±SEM. Ctrl, control; Mφ, macrophage. *P<0.05 versus control; **P<0.01 versus control.
Platelet depletion slightly increased the acute localization of activated macrophages in the glomeruli after a single transfer of alloantibody (Figure 5). However, platelet depletion decreased accumulation of macrophages in glomeruli induced by more prolonged alloantibody exposure (Figure 6). The decrease in macrophage accumulation was most pronounced in mice with the greatest depletion of platelets. To determine whether platelet depletion differentially affected alternatively activated macrophages, YM1+ cells were counted. Platelet depletion affected alternatively activated macrophages similarly to Mac 2+ macrophages (Figure 6).
Discussion
We have used a model of orthotopic renal transplants to elucidate the effects of DSA in the absence of T cells. Passive transfer of DSA activated complement and caused diffuse deposition of C4d on capillary endothelium.1 This was accompanied by increased macrophage infiltrates in the same range as reported for AMR in clinical studies.28,29,31 In addition, electron micrographs showed evidence of endothelial cell injury, including swelling, blebbing, and expansion of subendothelial space, that have been associated with AMR clinically.24 Immunohistology of five clinical biopsies diagnosed with AMR by Banff criteria1,2,26,27 showed aggregates of CD41-positive platelets, and the distribution of the platelet aggregates correlated with the Banff injury scores.
This model provided new mechanistic insights concerning the interaction of antibodies, endothelial cells, platelets, and macrophages. These interactions progressed rapidly. Within 1 hour, alloantibodies caused extensive release of vWf from the Weibel–Palade storage granules of endothelial cells. This rapid response parallels the kinetics of vWf release from human endothelial cells in culture triggered by antibodies.6 Concurrently, small aggregates of platelets that expressed P-selectin localized in renal capillaries.
The consequences of platelet localization to the transplants were examined at several levels. Homogenates of the allografts were prepared to quantify critical mediators transported by platelets. PF4 was detected in much greater quantities (10–30 ng/mg tissue) than other cytokines (IL-1β, MCP-1/CCL2, MIP-1α/CCL3, and RANTES/CCL5) stored in platelet α-granules (5–150 pg/mg tissue). This large quantitative difference parallels recent in vitro findings for mediators released by human platelets to various agonists.32
The localization of large quantities of PF4 in the allograft has multiple consequences. Although the independent chemotactic effects of PF4 are modest, PF4 associates with the glycosaminoglycans of endothelial cells and modulates the effects of other chemokines.33 By forming heteromers with RANTES and IL-8, PF4 enhances RANTES but decreases IL-8 chemotactic functions.34–36 In addition to chemotaxis, PF4 promotes monocyte survival and macrophage differentiation.37 This may account for the greater influx of monocytes than neutrophils observed in our model of AMR. Macrophages also dominate the infiltrate in human biopsies.25 In vitro studies have shown that PF4 can stimulate monocytes to cause apoptosis of endothelial cells.38 More recently, PF4 has been found to stimulate release of inflammatory mediators from parenchymal cells, such as vascular smooth muscle cells.39
Serotonin was also detected in large quantities in the allografts. Platelets express serotonin reuptake transporter proteins and store serotonin in their dense granules in quantities that make platelets the major source of serotonin in the circulation. Release of serotonin from platelets causes endothelial cells to exocytose vWf and P-selectin and promotes recruitment of leukocytes.40 More recently, the increased vascular permeability caused by platelet-derived serotonin has been found to be a critical step in the inflammatory lesions of rheumatoid arthritis and lupus.41,42 More prolonged effects of serotonin include the induction of fibrotic responses.43
In conjunction with the release of chemokines, the expression of P-selectin on activated platelets promotes interactions with macrophages.44 Immunohistology of both mouse and human renal allografts showed P-selectin expressing platelets attached to macrophages in capillaries and venules. The formation of platelet–leukocyte conjugates is known to result in increased activation and localization of leukocytes in inflammatory sites.30,45,46
Platelet responses to DSA were very dynamic. Within 5 hours, intravascular platelet aggregates detected by immunohistology had decreased. Simultaneously, the amount of PF4 and serotonin decreased about 5-fold in the allografts. However, platelet functions require only transient interactions. Even platelets that roll along capillaries and then return to the circulation have been shown to deposit trails of RANTES on inflamed vascular endothelium.47 Platelets can also fragment into microparticles that deliver mediators to neighboring cells.42,48 Finally, platelets are phagocytized by macrophages. In all of these mechanisms, intact platelets disappear but are instrumental in the process of perpetuating inflammation. For example, macrophages remove platelets by phagocytosis, but this process results in decreased macrophage apoptosis and increased macrophage function.49–51
The same mechanisms probably account for the smaller numbers of platelets observed after repeated transfers of alloantibodies. After 1 week of repeated alloantibody transfers, platelet aggregates were decreased by about 7-fold, but concentrations of PF4 and serotonin in the allografts were still substantial (5–15 ng/mg tissue). PF4 and serotonin were decreased when platelet depletion was maintained effectively for 1 week. More prolonged platelet depletion also decreased the accumulation of macrophages.
The transfer of alloantibodies to RAG-deficient mice precludes interaction of platelets with T cells. It is likely that the dynamic interactions of platelets in allografts would be enhanced by T cells. Platelets are known to stimulate T-cell function, including the release of proinflammatory cytokines.52,53 In skin allografts, Morrell and coworkers54 have found that platelets sustain T-cell recruitment and IFN-γ production over a 5-day period.
In summary, we have examined the effects of alloantibodies on platelets in a clinically relevant model of renal transplantation. Alloantibodies rapidly activate platelets in renal allografts. This results in the localized concentration of platelet-derived mediators and the interaction of platelets with endothelial cells and macrophages.
Concise Methods
Mice
Male B10.A (H-2a) and B6.129S7-Rag1tm1Mom/J (SCID; H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used at 8–12 weeks of age. All mice were treated in accordance with procedures approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Orthotopic Renal Transplantation
Both kidneys of an SCID recipient were removed, and a kidney from a B10.A donor was transplanted by previously described techniques.55 Surgical inflammation was allowed to subside for 1 week before antibody transfer. Only mice with normal creatinine values were used for experiments. Each experimental group included four to six mice.
Passive Transfer of Alloantibody
A mixture of IgG1 (AF3–12.1.3), IgG2a (16.1.2N), and IgG2b (15.1.5P) antibodies to H-2a (BioXCell, West Lebanon, NH) was transferred intraperitoneally at a dose of 100 μg each antibody. In control groups, animals received the same dose of isotype control antibodies (MOPC-21, C1.18, and MPC-11; BioXCell). Mice were euthanized 1 hour after the final transfer of antibodies.
In Vivo Platelet Depletion
One hour before alloantibody transfer, animals received 25 μg rat mAbs intraperitoneally (Emfret Analytics, Eibelstadt, Germany). Numbers of platelets in the circulation were determined by automated counts on an ADVIA Hematology System (Siemens, Hoffman Estates, IL) at the time of euthanasia.
Histology
Samples containing full cross-sections through the renal grafts were immediately fixed in acid methanol (60% methanol and 10% acetic acid). Paraffin-embedded sections (5 μm) underwent high-temperature antigen retrieval and paraffin removal in Trilogy (Cell Marque, Hot Springs, AR) in a pressure cooker. Endogenous peroxidase activity was blocked by incubation with 0.3% H2O2 in 80% methanol, and nonspecific protein interactions were blocked by incubation with a serum-free protein block (DAKO, Carpinteria, CA). Slides were incubated with one of the following primary antibodies: monoclonal rat antibody to mouse neutrophils (RB6–8C5; BioXCell), monoclonal rat antibody to mouse Mac2, a marker of inflammatory macrophages (Cedarlane Laboratories, Burlington, NC), rabbit polyclonal antibody to YM-1, a marker of alternative macrophages (Stemcell Technologies, Vancouver, Canada), rabbit polyclonal antibody to human vWf (DAKO), or mouse C4d8 for 60 minutes at room temperature. Antibodies were visualized using the Vectastain Elite ABC Kit (Vector, Burlingame, CA) or the SuperPicture Kit (Invitrogen, Carlsbad, CA) followed by diaminobenzidine and counterstained with hematoxylin.
Excess sections of human renal biopsies were stained with rabbit monoclonal to human CD41 (Epitomics, Burlingham, CA) under Institutional Review Board approval.
Tissues were fixed by immersion in 2.5% gluteraldehyde and 4% paraformaldehyde for transmission electron microscopy. After routine processing, embedding, and thin sectioning, the grids were evaluated with an FEI Tecnai G2 Spirit BioTWIN transmission electron microscope equipped with a Gatan SC1000W, Peltier-cooled, 14-bit, 11-megapixel camera.
Digital Planimetry
Five digital pictures were captured at high power of randomly selected fields of paraffin-embedded tissue sections that were stained for P-selectin. The fraction of P-selectin–positive area was measured by NIS-Elements BR analysis software (Nikon, Melville, NY).
Total Protein Extraction from Samples of Allografts
Tissue samples were frozen in liquid nitrogen immediately after harvest from animals and stored in −80°C. Tissue samples were minced with a razor blade in 500 μl reconstituted protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Minced tissues were transferred to Eppendorf tubes, and 1.5% Triton X in PBS was added. After 30 minutes of incubation at 4°C on a shaker, the homogenates were centrifuged at 12,000×g for 10 minutes, and supernatants were collected. The total protein content of each sample was quantified with the BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL).
ELISA
Tissue homogenates were tested following the protocols of the vender: PF4, IL-1β, MCP-1, and MIP-1α from R&D Systems (Minneapolis, MN); serotonin from IBL-America (Minneapolis, MN); and mouse IgG1, IgG2a, and IgG2b from Affymetrix (Santa Clara, CA).
Statistical Analyses
All statistical analyses were performed using GraphPad Prism 5.02 (GraphPad Software). Levels of P-selectin–positive area on immunohistology-stained tissue sections, macrophage infiltrates, or proteins measured with ELISA assays were compared between treated and control groups by one-tailed or two-tailed unpaired t test with Welch correction. P values<0.05 were considered significant. Data are expressed as means±SEMs.
Disclosures
W.M.B. has a licensing agreement with Hycult Biotech, Abcam, Inc., and Thermo Fisher Scientific for the polyclonal rabbit antibody to mouse C4d used in these studies.
Supplementary Material
Acknowledgments
We thank Mei Yin for preparing the electron micrographs and Dr. E. Rene Rodriguez for his help in interpreting the ultrastructural changes.
This work was supported by National Institutes of Health Grant P01-AI087586.
Part of this work was presented at the American Transplant Congress in Seattle, WA, May 18–22, 2013.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121289/-/DCSupplemental.
References
- 1.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 3: 708–714, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF: Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant 9: 2312–2323, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Colvin RB, Smith RN: Antibody-mediated organ-allograft rejection. Nat Rev Immunol 5: 807–817, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Valenzuela NM, Mulder A, Reed EF: HLA class I antibodies trigger increased adherence of monocytes to endothelial cells by eliciting an increase in endothelial P-selectin and, depending on subclass, by engaging FcγRs. J Immunol 190: 6635–6650, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, Wasowska BA, Baldwin WM, 3rd, Pober JS, Lowenstein CJ: Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci U S A 104: 1301–1306, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minami K, Murata K, Lee C-Y, Fox-Talbot K, Wasowska BA, Pescovitz MD, Baldwin WM, 3rd: C4d deposition and clearance in cardiac transplants correlates with alloantibody levels and rejection in rats. Am J Transplant 6: 923–932, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Murata K, Fox-Talbot K, Qian Z, Takahashi K, Stahl GL, Baldwin WM, 3rd, Wasowska BA: Synergistic deposition of C4d by complement-activating and non-activating antibodies in cardiac transplants. Am J Transplant 7: 2605–2614, 2007 [DOI] [PubMed] [Google Scholar]
- 9.De Ceunynck K, De Meyer SF, Vanhoorelbeke K: Unwinding the von Willebrand factor strings puzzle. Blood 121: 270–277, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Rahimi S, Qian Z, Layton J, Fox-Talbot K, Baldwin WM, 3rd, Wasowska BA: Non-complement- and complement-activating antibodies synergize to cause rejection of cardiac allografts. Am J Transplant 4: 326–334, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Wasowska BA, Qian Z, Cangello DL, Behrens E, Van Tran K, Layton J, Sanfilippo F, Baldwin WM, 3rd: Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation 71: 727–736, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Smith N, Chandler S, Hawker RJ, Hawker LM, Barnes AD: Indium-labelled autologous platelets as diagnostic aid after renal transplantation. Lancet 2: 1241–1242, 1979 [DOI] [PubMed] [Google Scholar]
- 13.Fenech A, Smith FW, Power DA, MacLeod AM, Nicholls AJ, Edward N, Catto GR, Bennett B: The value of autologous indium (111In)-labelled platelets in the diagnosis of renal transplant rejection. Clin Nephrol 21: 220–222, 1984 [PubMed] [Google Scholar]
- 14.Collier BD, Adams MB, Kauffman HM, Trembath L, Hoffmann RG, Tisdale PL, Rao SA, Hellman RS, Isitman AT: Accurate diagnosis of renal transplant rejection by indium-111 platelet imaging despite postoperative cyclosporin therapy. Clin Nucl Med 13: 606–610, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Lederer ED, Gillum DM, Jhingran S, Matula C, Truong L: Correlation between percutaneous renal biopsy and the indium-111-labeled platelet scan in diagnosing acute renal allograft rejection. Transplantation 53: 945–947, 1992 [PubMed] [Google Scholar]
- 16.Meehan SM, Limsrichamrern S, Manaligod JR, Junsanto T, Josephson MA, Thistlethwaite JR, Haas M: Platelets and capillary injury in acute humoral rejection of renal allografts. Hum Pathol 34: 533–540, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Morrell CN, Murata K, Swaim AM, Mason E, Martin TV, Thompson LE, Ballard M, Fox-Talbot K, Wasowska B, Baldwin WM, 3rd: In vivo platelet-endothelial cell interactions in response to major histocompatibility complex alloantibody. Circ Res 102: 777–785, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Morrell CN, Sun H, Swaim AM, Baldwin WM, 3rd: Platelets an inflammatory force in transplantation. Am J Transplant 7: 2447–2454, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kirk AD, Morrell CN, Baldwin WM, 3rd: Platelets influence vascularized organ transplants from start to finish. Am J Transplant 9: 14–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo HH, Morrell CN, Baldwin WM, 3rd: Alloantibody induced platelet responses in transplants: Potent mediators in small packages. Hum Immunol 73: 1233–1238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS: Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell 122: 379–391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA: Leukocyte extravasation: Chemokine transport and presentation by the endothelium. Blood 100: 3853–3860, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Whittall C, Kehoe O, King S, Rot A, Patterson A, Middleton J: A chemokine self-presentation mechanism involving formation of endothelial surface microstructures. J Immunol 190: 1725–1736, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drachenberg CB, Papadimitriou JC: Endothelial injury in renal antibody-mediated allograft rejection: A schematic view based on pathogenesis. Transplantation 95: 1073–1083, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Batal I, Azzi J, El-Haddad N, Riella LV, Lunz JG, 3rd, Zeevi A, Sasatomi E, Basu A, Tan H, Shapiro R, Randhawa P: Immunohistochemical markers of tissue injury in biopsies with transplant glomerulitis. Hum Pathol 43: 69–80, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Ozdemir BH, Demirhan B, Güngen Y: The presence and prognostic importance of glomerular macrophage infiltration in renal allografts. Nephron 90: 442–446, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Tinckam KJ, Djurdjev O, Magil AB: Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int 68: 1866–1874, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Neumann FJ, Marx N, Gawaz M, Brand K, Ott I, Rokitta C, Sticherling C, Meinl C, May A, Schömig A: Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation 95: 2387–2394, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Magil AB, Tinckam K: Monocytes and peritubular capillary C4d deposition in acute renal allograft rejection. Kidney Int 63: 1888–1893, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Jonnalagadda D, Izu LT, Whiteheart SW: Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood 120: 5209–5216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber C, Koenen RR: Fine-tuning leukocyte responses: Towards a chemokine ‘interactome’. Trends Immunol 27: 268–273, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Baltus T, von Hundelshausen P, Mause SF, Buhre W, Rossaint R, Weber C: Differential and additive effects of platelet-derived chemokines on monocyte arrest on inflamed endothelium under flow conditions. J Leukoc Biol 78: 435–441, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Nesmelova IV, Sham Y, Dudek AZ, van Eijk LI, Wu G, Slungaard A, Mortari F, Griffioen AW, Mayo KH: Platelet factor 4 and interleukin-8 CXC chemokine heterodimer formation modulates function at the quaternary structural level. J Biol Chem 280: 4948–4958, 2005 [DOI] [PubMed] [Google Scholar]
- 36.von Hundelshausen P, Koenen RR, Sack M, Mause SF, Adriaens W, Proudfoot AE, Hackeng TM, Weber C: Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 105: 924–930, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Scheuerer B, Ernst M, Dürrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, Flad HD, Petersen F: The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 95: 1158–1166, 2000 [PubMed] [Google Scholar]
- 38.Woller G, Brandt E, Mittelstädt J, Rybakowski C, Petersen F: Platelet factor 4/CXCL4-stimulated human monocytes induce apoptosis in endothelial cells by the release of oxygen radicals. J Leukoc Biol 83: 936–945, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Shi G, Field DJ, Long X, Mickelsen D, Ko KA, Ture S, Korshunov VA, Miano JM, Morrell CN: Platelet factor 4 mediates vascular smooth muscle cell injury responses. Blood 121: 4417–4427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M, Idzko M, Bode C, Wagner DD: Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 121: 1008–1015, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boilard E, Blanco P, Nigrovic PA: Platelets: Active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol 8: 534–542, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Cloutier N, Paré A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S, Boilard E: Platelets can enhance vascular permeability. Blood 120: 1334–1343, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Grewal JS, Mukhin YV, Garnovskaya MN, Raymond JR, Greene EL: Serotonin 5-HT2A receptor induces TGF-beta1 expression in mesangial cells via ERK: Proliferative and fibrotic signals. Am J Physiol 276: F922–F930, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA: Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest 95: 2297–2303, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, Kroczek RA: CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391: 591–594, 1998 [DOI] [PubMed] [Google Scholar]
- 46.van Gils JM, Zwaginga JJ, Hordijk PL: Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol 85: 195–204, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C: Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 106: 1523–1529, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C: Platelet microparticles: A transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol 25: 1512–1518, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Lang D, Dohle F, Terstesse M, Bangen P, August C, Pauels HG, Heidenreich S: Down-regulation of monocyte apoptosis by phagocytosis of platelets: Involvement of a caspase-9, caspase-3, and heat shock protein 70-dependent pathway. J Immunol 168: 6152–6158, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Brown GC, Neher JJ: Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem Sci 37: 325–332, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Scull CM, Hays WD, Fischer TH: Macrophage pro-inflammatory cytokine secretion is enhanced following interaction with autologous platelets. J Inflamm (Lond) 7: 53–62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H, Hansson GK, Li N: Platelets regulate CD4⁺ T-cell differentiation via multiple chemokines in humans. Thromb Haemost 106: 353–362, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Zamora C, Cantó E, Nieto JC, Ortiz MA, Diaz-Torné C, Diaz-Lopez C, Llobet JM, Juarez C, Vidal S: Functional consequences of platelet binding to T lymphocytes in inflammation. J Leukoc Biol 94: 521–529, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Swaim AF, Field DJ, Fox-Talbot K, Baldwin WMIII, 3rd, Morrell CN: Platelets contribute to allograft rejection through glutamate receptor signaling. J Immunol 185: 6999–7006, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R: Improved techniques for kidney transplantation in mice. Microsurgery 16: 103–109, 1995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.