Abstract
The cilium is a signaling platform of the vertebrate cell. It has a critical role in polycystic kidney disease and nephronophthisis. Cilia have been detected on endothelial cells, but the function of these organelles in the vasculature remains incompletely defined. In this study, using genetic and chemical genetic tools in the model organism zebrafish, we reveal an essential role of cilia in developmental vascular integrity. Embryos expressing mutant intraflagellar transport genes, which are essential and specific for cilia biogenesis, displayed increased risk of developmental intracranial hemorrhage, whereas the morphology of the vasculature remained normal. Moreover, cilia were present on endothelial cells in the developing zebrafish vasculature. We further show that the involvement of cilia in vascular integrity is endothelial autonomous, because endothelial-specific re-expression of intraflagellar transport genes in respective mutants rescued the intracranial hemorrhage phenotype. Finally, whereas inhibition of Hedgehog signaling increased the risk of intracranial hemorrhage in ciliary mutants, activation of the pathway rescued this phenotype. In contrast, embryos expressing an inactivating mutation in pkd2, one of two autosomal dominant cystic kidney disease genes, did not show increased risk of developmental intracranial hemorrhage. These results suggest that Hedgehog signaling is a major mechanism for this novel role of endothelial cilia in establishing vascular integrity.
Keywords: vascular disease, cystic kidney, signaling, endothelium
Longtime obscure, the cilium has received much belated recognition in the past decade as a cellular antenna. Protruding from the cell surface into its environment, the cilium is well equipped to detect both biochemical signals and mechanical stimuli. Not surprisingly, ciliary defects have been linked to an increasing number of human diseases, including polycystic kidney disease (PKD), retinal degeneration, and obesity, collectively referred to as ciliopathies.1
Previous studies suggest that cilia can be found on endothelial cells and participate in shear stress response, cell migration, and calcium and nitric oxide signaling in cultured endothelial cells.2–7 Most recently, cilia were found on caudal artery and vein in developing zebrafish embryos, and it was shown that the flow-sensing function of cilia is involved in the morphogenesis of caudal plexus.8 However, ciliary function in the developing brain vasculature and any potential involvement in vascular disease have not been reported.
By using zebrafish as an animal model for studying cilia function, we unexpectedly discovered that several intraflagellar transport (IFT) mutants show increased incidence of intracranial hemorrhage (ICH) compared with control siblings, whereas the morphology and junctions of the vasculature are not obviously altered. IFT genes encode core components of IFT particles, which were first discovered in the green alga Chlamydomonas.9 IFT particles move on microtubule tracks in the ciliary axoneme, carrying cargos in and out of the flagellum. They are essential for flagellum/cilium biogenesis and maintenance in nearly all ciliated organisms from protozoans to vertebrates.10–14 They are only conserved in ciliated organisms, suggesting that they are specialized for cilia.10,13,14 Thus, the increased risk of ICH in multiple IFT mutants points to a ciliary involvement. Additional analyses show that cilia are present on endothelial cells in the developing zebrafish brain vasculature. Moreover, targeted re-expression of IFT genes in the endothelium of respective morpholino knockdown animals (morphants) and mutants rescued the ICH phenotype, showing that endothelial cilia are essential for developmental vascular integrity.
To tease out the potential mechanism underlying the role of endothelial cilia in vascular integrity, we investigated the involvement of the Hedgehog pathway, a major signaling pathway mediated by cilia in vertebrates. The Hedgehog receptor Patched is localized on the cilium of vertebrate cells. On binding of Hedgehog, Patched leaves the cilium and relieves repression of Smoothened, thereby triggering additional downstream responses.12,15–19 In the absence of cilia, Hedgehog signaling is dampened.12,17,20 We show that cilia mutants are sensitized to ICH induced by inhibition of Hedgehog signaling, and conversely, activation of the pathway can suppress ICH risk in cilia mutants, revealing a critical role of defective Hedgehog signaling in ICH in cilia mutants.
Because endothelial cilia are exposed to constant blood flow, we additionally investigated whether the cilia-localized mechanosensory channel Polycystin-2 (Pc2) is involved in this novel function of cilia. However, our results show that pkd2 mutants display no increased risk of developmental ICH.
Combining genetic and chemical genetic analyses, we found that the Hedgehog pathway plays a significant role in developmental ICH caused by defective endothelial cilia, whereas the ciliary mechanosensory channel Pc2 seems to not be involved. Together, our results reveal a novel function of cilia-mediated Hedgehog signaling in developmental vascular integrity.
Results
Zebrafish with Defective IFT Show Increased Risk of ICH during Development
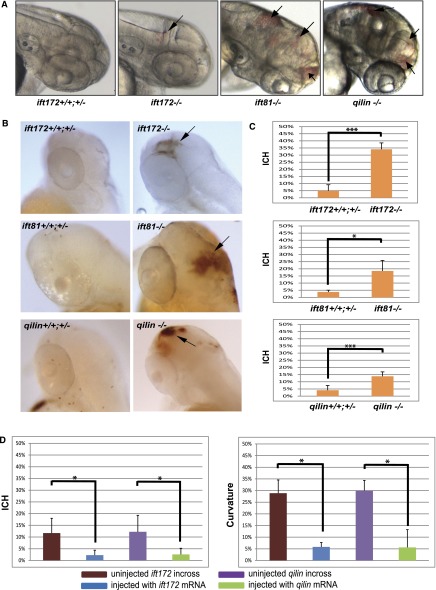
In previous studies, we showed that IFT genes play a conserved role for cilia biogenesis in zebrafish and that IFT mutants show a characteristic collection of cilia-associated phenotypes, including kidney cyst formation and ventral body curvature.11,21,22 Additional inspection of mutant embryos revealed ICH as early as 2 day postfertilization (dpf) in some embryos (Figure 1, A and B). We closely examined three mutants of IFT-B complex genes: ift81hi409, ift172hi2211, and qilinhi3959A. All showed significantly increased incidence of ICH compared with wild-type and heterozygous siblings. Specifically, 13.8% of qilinhi3959A mutants, 34% of ift172hi2211 mutants, and 18.5% of ift81hi409 mutants developed ICH, whereas 4.2%, 4.9%, and 3.8%, respectively, of their wild-type siblings showed spontaneous ICH (Figure 1, A–C). The site of hemorrhage varies and can be seen in both the forebrain and the hindbrain regions, and multiple hemorrhages can be observed in some embryos (Figure 1, A and B). To confirm that this phenotype is caused by IFT defects, we then performed rescue experiment by ubiquitous re-expression of affected genes. Injection of qilin or ift172 mRNA not only rescued the body curvature and cystic kidney phenotypes but also, reduced ICH rates to 2.6% or 2.3%, respectively, compared with 12.3% and 11.7%, respectively, in control embryos, confirming that IFT defects lead to the increased risk of ICH in developing zebrafish embryos (Figure 1D).
Figure 1.
IFT mutants develop intracranial hemorrhage. (A) Head regions of IFT mutants showing ICH (arrows). (B) Head regions of IFT mutants and their wild-type siblings stained with o-Dianisidine showing hemorrhage (arrows). (C) Percentage of cranial hemorrhage observed in ift172hi2211 mutants, ift81hi409 mutants, and qilinhi3959A mutants compared with their wild-type and heterozygotes siblings (n=3; with an average of 25 embryos in each repeat for each experiment). (D) Percentage of (left panel) cranial hemorrhage and (right) body curvature. Data are compiled from three repeats with >50 embryos in each repeat and presented as means+SDs. *P<0.05; ***P<0.001.
The ICH phenotype is not fully penetrant in IFT mutants. In subsequent experiments using many different families, we observed variable frequencies of ICH. To further confirm that, despite this variation of phenotype penetrance, inactivation of IFT genes leads to ICH, we used morpholino oligos to knock down the expression of ift81 and qilin in the EK strain. Results showed consistent incidence of ICH (Figure 2, A–D). Similar results were also obtained in the TAB strain (not shown).
Figure 2.
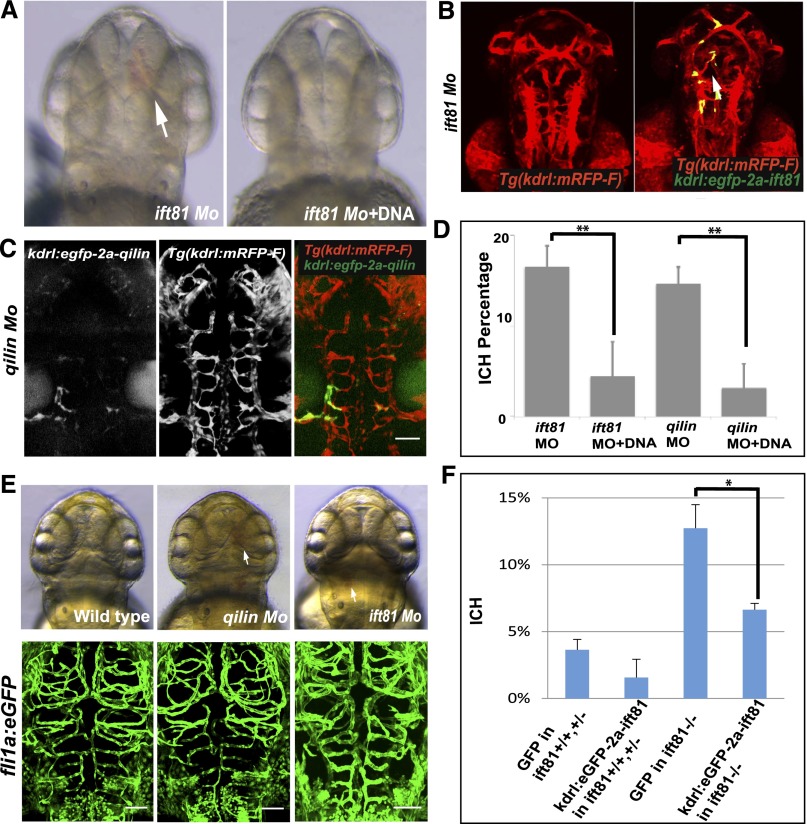
Intracranial hemorrhage phenotype in IFT mutants is endothelial autonomous. (A) Differential interference contrast (DIC) image of Tg(kdrl:mRFP-F) embryos injected with ift81 morpholino (Mo) alone or together with kdrl:eGFP-2a-ift81 DNA. Arrow points to ICH. (B) Fluorescent images of Tg(kdrl:mRFP-F) embryos injected with ift81 Mo alone or together with kdrl:eGFP-2a-ift81 DNA. Arrow points to eGFP signal in the vasculature. (C) Fluorescent images of Tg(kdrl:mRFP-F) embryos injected with qilin Mo alone or together with kdrl:e-GFP-2a-qilin DNA. Scale bar, 100 µm. (D) Percentage of ICH. Data are compiled from three repeats with >23 embryos in each repeat and presented as means+SDs. (E) Cranial vessel morphology in Tg(fli1a:eGFP) embryos (left panel) uninjected (wild type), (center panel) injected with qilin Mo, or (right panel) injected with ift81 Mo. Upper panel shows DIC images at 54 hpf. Lower panel shows confocal projection of cranial vessels in the midbrain and hindbrain regions at 62 hpf. Arrows point to hemorrhage. Scale bar, 50 µm. (F) Percentage of ICH in wild-type siblings and ift81hi409 mutant embryos. Data are compiled from three repeats with >40 embryos in each repeat and presented as means+SDs. *P<0.05; **P<0.001.
Cilia Are Present on the Endothelial Cells in the Developing Zebrafish Vasculature
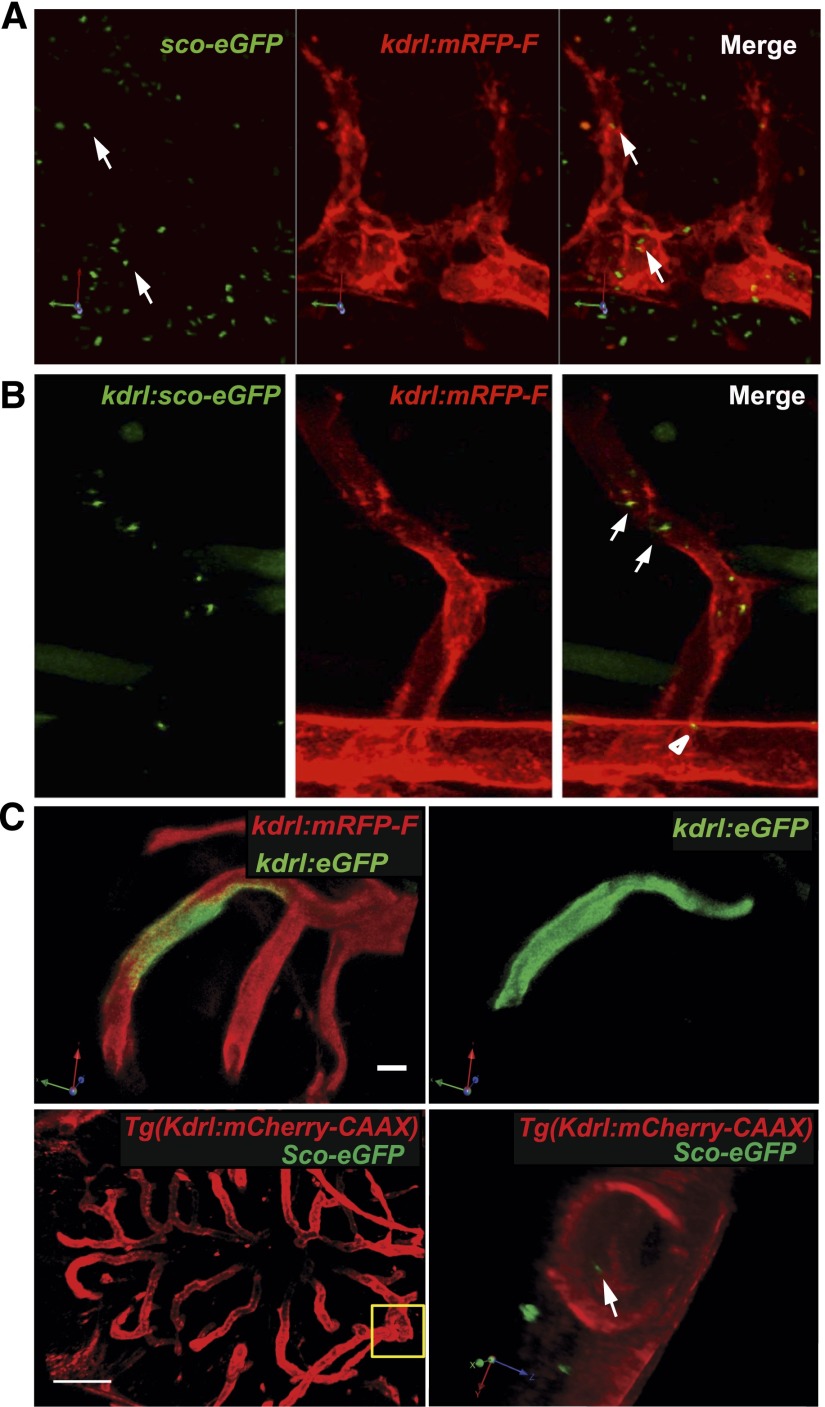
Multiple previous studies suggest that cilia can be found on mammalian endothelial cells at various sites. Prompted by the ICH phenotype observed in IFT mutants, we expressed Sco-eGFP, a highly specific cilia marker,23 ubiquitously through mRNA injection into the Tg(kdrl:mRFP-F) transgenic zebrafish line, in which all endothelial cells are red fluorescent. Confocal imaging of injected embryos at 24 hours postfertilization (hpf) revealed wide distribution of cilia in many tissues, including some apparently localized within mRFP-positive endothelial cells (Figure 3A, Supplemental Movie 1). To verify that the ciliary signal is, indeed, within endothelial cells, we expressed Sco-eGFP in endothelial cells through meganuclease-mediated gene insertion.24,25 Specifically, DNA carrying kdrl:sco-eGFP flanked by the recognition site of the meganuclease I-SceI was injected together with I-SceI into Tg(kdrl:mRFP) transgenic embryos. In injected embryos, the expression of Sco-eGFP is driven by the endothelial-specific kdrl1 promoter. Confocal imaging at 24 hpf clearly showed the presence of cilia in both the dorsal aorta and intersegmental vessels, confirming the presence of cilia on developing zebrafish vascular endothelial cells (Figure 3B, Supplemental Movie 2). By injecting sco-eGFP mRNA into Tg(kdrl:mcherry-CAAX) transgenic embryos, we also detected cilia in cranial vessels at 60 hpf (Figure 3C, Supplemental Movie 3). In contrast, eGFP expressed in cranial vessels showed diffusive signal (Figure 3C).
Figure 3.
Cilia are present on endothelial cells in the developing zebrafish vasculature. (A) A snapshot of a three-dimensional projection reconstituted from confocal sections of a 24-hpf Tg(kdrl:mRFP-F) embryo injected with sco-eGFP mRNA. A rotating movie of the three-dimensional projection is presented in Supplemental Movie 1. Arrows indicate primary cilia localized in endothelial cells. (B) Confocal image of a 24-hpf Tg(kdrl:mRFP-F) embryo injected with kdrl:sco-eGFP DNA. Mosaic expression of Sco-eGFP is detected in dorsal aorta (arrowhead) and intersegmental vessels (arrow) (Supplemental Movie 2). (C) Cilia in cranial vessels in 60-hpf embryos (Supplemental Movie 3). Upper panel shows mosaic expression of cytosolic eGFP in a Tg(kdrl:mRFP-F) embryo injected with kdrl:eGFP DNA. Scale bar, 20 µm. Lower panel shows a Tg(Kdrl:mCherry-CAAX) embryo injected with sco-eGFP mRNA. The boxed area in the left is zoomed in and rotated in the right. Arrow points to a cilium. Scale bar, 100 µm; 5 µm in three-dimensional images.
The ICH Phenotype in IFT Mutants Is Endothelial Cell Autonomous
Crosstalk between endothelial cells and mural cells is essential for vascular integrity. Considering that cilia are widely distributed, including in smooth muscle cells,26 we investigated whether the observed ICH phenotype is endothelial autonomous. We re-expressed ift81 and qilin in the endothelium of the respective morphants using the same meganuclease-mediated gene insertion approach described above. Specifically, kdrl:eGFP-2A-ift81 or kdrl:eGFP-2A-qilin DNA was coinjected with respective morpholino into Tg(kdrl:mRFP-F) embryos. Embryos were then sorted for eGFP signal. The overlap of the eGFP and the mRFP signal confirmed that the re-expression was specific in the endothelium (Figure 2, B and C). Importantly, the re-expression of ift81 reduced ICH rates from 16.5% to 4.4% in ift81 morphants (Figure 2, A, B, and D). Similarly, the re-expression of qilin reduced ICH from 14.6% to 3.1% in qilin morphants (Figure 2, C and D). Finally, we used the same method in ift81hi409 mutants, and results showed that re-expression of Ift81 reduced ICH incidence from 12.7% to 6.6% (Figure 2F). Combined, these results suggest that defective endothelial cilia are responsible for the ICH phenotype in IFT mutants.
Defective Hedgehog Signaling Is a Major Cause of ICH in IFT Mutants
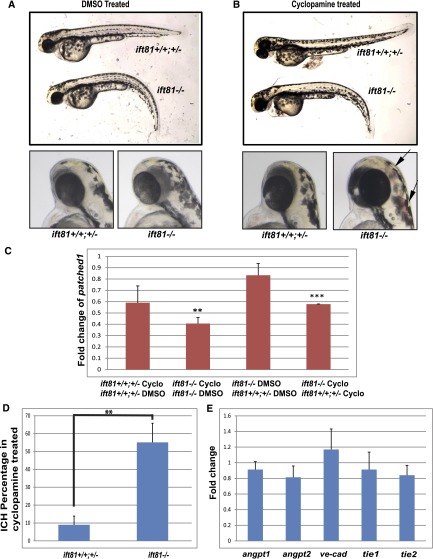
Cilia function as a signaling platform of the cell, particularly Hedgehog signaling.27 Interestingly, previous work showed that treatment with cyclopamine, an antagonist of the Hedgehog pathway, causes ICH in zebrafish embryos reminiscent of the phenotype that we observed in IFT mutants, suggesting that this pathway may be important for developmental vascular integrity.28 To investigate whether the Hedgehog pathway is associated with cilia-associated ICH, we first identified a suboptimal regimen of cyclopamine treatment by titrating the dosage and time window. Treatment with 25–30 µmol/L cyclopamine from 25 hpf did not change the embryo morphology significantly (Figure 4, A and B), which allowed us to identify mutant embryos unequivocally on the basis of their body curvature phenotype. Interestingly, the expression of patched 1, a target of the Hedgehog pathway, is significantly reduced in ift81hi409 mutants by this treatment, whereas it is not significantly altered in wild-type and heterozygous siblings (Figure 4C), suggesting that it is a suboptimal dosage that sensitizes the Hedgehog pathway. We then took advantage of the variable penetrance of ICH in different families and subjected families of ift81hi409 that show no spontaneous ICH phenotype in both the mutants and wild-type siblings to this treatment; 30 µmol/L cyclopamine treatment led to ICH in 55% of ift81hi409 mutant embryos at 52 hpf, significantly higher than 9% in control siblings (Figure 4, A, B, and D). These results suggest that IFT mutants are sensitized to ICH caused by cyclopamine treatment.
Figure 4.
IFT mutants are sensitized to ICH induced by inhibition of the Hedgehog pathway. (A, B) Morphology of ift81hi409 mutants and control siblings treated with DMSO or cyclopamine. Arrows indicate ICH. (C) Fold change of patched 1 expression comparing wild-type and heterozygous siblings treated with cyclopamine versus DMSO, ift81hi409 mutants treated with cyclopamine versus DMSO, DMSO-treated ift81hi409 mutants versus wild-type and heterozygous siblings, and cyclopmaine-treated ift81hi409 mutants versus wild-type and heterozygous siblings. Cyclo, cyclopamine. **P<0.01; ***P<0.001. (D) Percentage of ICH in ift81hi409 mutant and control siblings treated with cyclopamine (n=3 with an average of 20 embryos in each experiment). Vehicle-treated embryos did not show any hemorrhage (data not shown). **P<0.01. (E) Expression level of multiple genes involved in vascular integrity as shown by quantitative PCR. y Axis shows fold changes in ift81hi409 mutants compared with control siblings. No statistical difference was detected. Results are represented as means+SDs from three replicates.
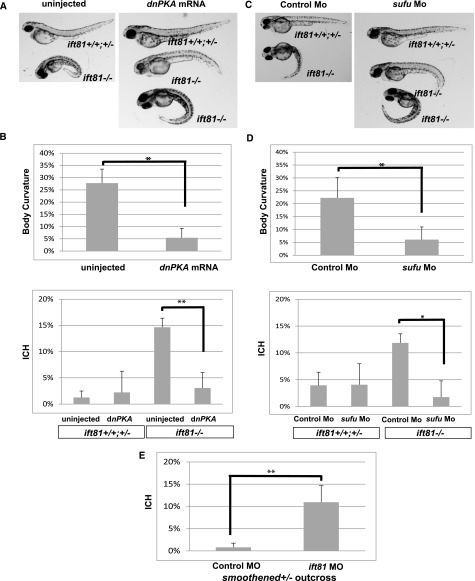
We additionally tested the effect of reduction of Hedgehog signaling on ICH genetically. Because Hedgehog signaling is essential for patterning the vasculature and because the zebrafish smoothenedhi229 mutant never establishes active blood flow,29,30 precluding ICH analysis, we sought to test potential genetic interaction between smoothened and ift81. We outcrossed smoothenedhi229/+ carriers with wild-type fish and partially knocked down ift81 by morpholino injection. At the suboptimal dosage used, the ift81 morpholino did not cause any morphologic defects. However, 10.9% of injected embryos developed ICH (Figure 5E). Genotyping experiments showed that, of 25 embryos that developed ICH, 21 embryos were heterozygous carriers of smoothenedhi229. A chi-squared test suggested that this ratio significantly deviated from the 50%:50% expected ratio of wild-type and carrier siblings (P<0.001), suggesting that decreasing Smoothened function sensitizes ICH risk induced by Ift81 reduction.
Figure 5.
Defective Hedgehog signaling plays a major role in ICH phenotype. (A) Morphology of ift81hi409 mutant and control siblings uninjected or injected with dnPKA mRNA. (B) Percentage of severe body curvature and ICH in uninjected ift81hi409/+ incross embryos versus injected with dnPKA mRNA embryos. (C) Morphology of ift81hi409 mutant and control siblings injected with control Mo or sufu Mo. (D) Percentage of severe body curvature and ICH in ift81hi409/+ incross embryos injected with control Mo or sufu Mo. (E) Percentage of ICH in smoothenedhi229/+ outcross embryos injected with control Mo versus ift81 Mo. Data are compiled from three repeats with >30 embryos in each repeat and presented as means+SDs. Mo, morpholino. *P<0.05; **P<0.01.
We further analyzed the effect of activation of Hedgehog signaling. We first expressed a dominant negative form of protein kinase A (dnPKA) through mRNA injection. PKA is a negative regulator of Hedgehog signaling that functions at the basal body.31 The expression of dnPKA can activate the Hedgehog pathway in zebrafish.32–34 Interestingly, expression of dnPKA at the dosage that we used (300 pg) did not cause any obvious developmental defects; however, it partially rescued the body curvature phenotype of ift81hi409 mutants (full curvature from 27.7% to 5.43%) (Figure 5, A and B). More importantly, expression of dnPKA also reduced ICH phenotype in ift81hi409 mutant embryos from 14.7% to 3.1% (Figure 5B). We also activated Hedgehog signaling through an alternative approach, which is to knockdown sufu, a negative regulator of the Hedgehog pathway.35,36 Consistent with a previous report, reduction of sufu by itself did not cause overt body axis defect in zebrafish.37 However, it partially rescued the body curvature (full curvature from 22.2% to 6%) and ICH phenotype (from 11.8% to 1.7%) of ift81hi409 mutant embryos (Figure 5, C and D).
Combined, these results suggest that defective Hedgehog signaling plays a major role in the ICH phenotype in cilia mutants.
Cilia Mutants Show Normal Vascular Morphology and Junctions
To search for the cellular basis of ICH in cilia mutants, we first analyzed the morphology of the vasculature. We used morpholino to knock down qilin and ift81 in Tg(fli1:eGFP) fish and examined the morphology of the vasculature of morphants that displayed ICH. Results show that the morphology of cranial vessels is not significantly altered in either morphant (Figure 2E).
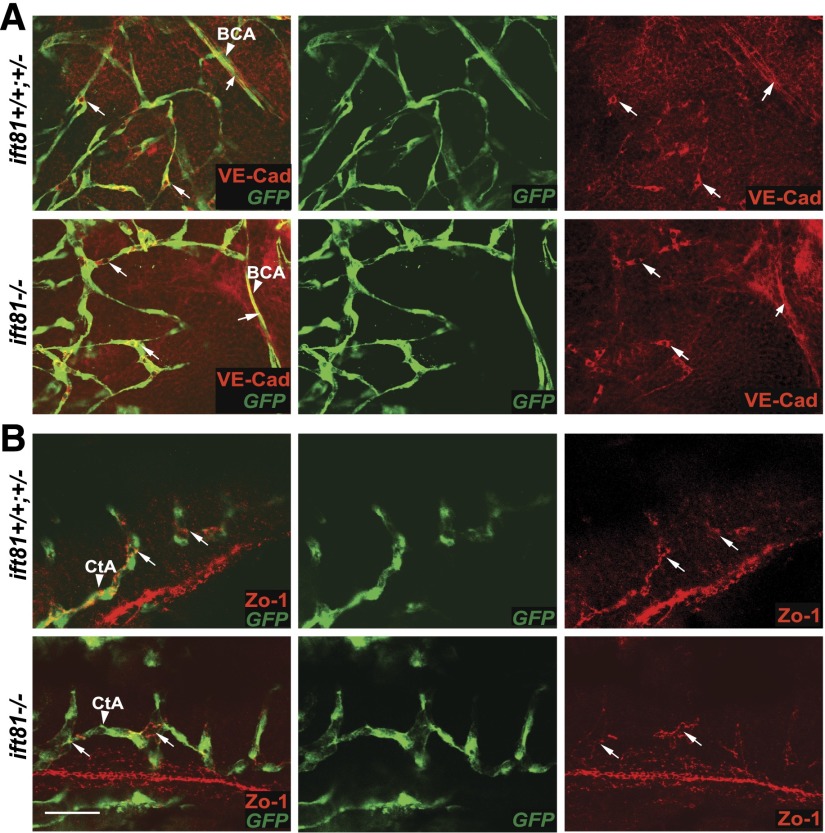
We then examined adherens junctions between endothelial cells in the brain. We stained ift81hi409 mutant embryos and siblings carrying the Tg(kdrl:eGFP) transgene with anti-vascular endothelial cadherin (anti–VE-Cadherin)38 at 2 dpf. Results showed no difference in the mutants compared with siblings (Figure 6A). We additionally stained embryos with anti-Zo1, which labels tight junctions and potentially some adherent junctions.39 Again, no defect was detected in ift81hi409 mutants (Figure 6B). Combined, these results suggest that there are no obvious defects in adherens and tight junctions between brain endothelial cells in IFT mutants.
Figure 6.
Cilia mutants show normal endothelial junctions. (A) An ift81hi409 mutant and a control sibling carrying Tg(fli1:eGFP) stained with anti–VE-Cad (red; arrows) at 2 dpf. BCA, basal communicating artery (arrowhead). (B) An ift81hi409 mutant and a control sibling carrying Tg(kdrl:eGFP) stained with anti–Zo-1 (red; arrows) at 2 dpf. Anti-GFP marks the blood vessels in green. CtA, central arteries (arrowhead). Scale bar, 30 µm.
Because it has been shown that the Hedgehog pathway target angiopoietin 1 is required for developmental vascular integrity in zebrafish,28 we performed quantitative PCR to analyze its expression level. However, no significant change of angiopoietin 1 was detected in ift81hi409 mutants compared with their siblings (Figure 4E). The expression level of angiopoietin 2 was also not changed (Figure 4E). We additionally analyzed the expression levels of ve-cad, tie1 and tie2, genes previously shown to be involved in developmental vascular integrity in zebrafish,40,41 and found no significant change between ift81hi409 mutants and control siblings (Figure 4E).
Inactivation of Pc2 Does Not Lead to Developmental ICH in Zebrafish
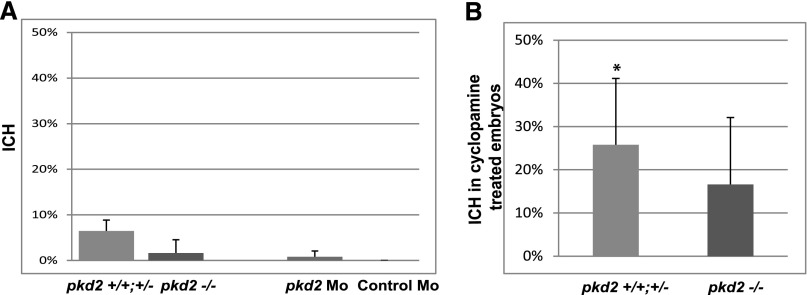
To investigate the potential role of cilia as a flow sensor in developmental ICH in IFT mutants, we first analyzed the incidence of ICH in pkd2hi4166 mutant embryos, an inactivating mutant of the ciliary mechanosensory channel Pc2,22 which together with its binding partner Pc1, is required for flow-sensing response in cultured endothelial cells.2,6 However, close inspection revealed that, on average, 1.6% of mutant embryos showed ICH, not significantly different from the 6.5% observed in wild-type and heterozygous carries (Figure 7A). Considering the variable penetrance of ICH phenotype in IFT mutants, to provide additional support for this result, we generated pkd2 morphants using a previously validated morpholino. Again, no increase of ICH was observed in pkd2 morphants compared with control embryos (1.8% ICH in pkd2 morphants versus 0% in control) (Figure 7A). We additionally tested whether pkd2hi4166 mutants are sensitized to ICH induced by Hedgehog inhibition. When subjected to the same cyclopamine treatment as used on IFT mutants, 18.18% of treated mutant embryos showed ICH, not significantly different from 23.6% in control siblings (Figure 7B). In control siblings, incidence of ICH is increased in the cyclopamine-treated group compared with the vehicle-only group, which is 0% (Figure 7B), validating the effectiveness of cyclopamine treatment. These results suggest that Pc2 is not a major player in the developmental ICH phenotype that we observed in cilia mutants.
Figure 7.
pkd2 Mutant embryos do not show increased risk of developmental ICH. (A) Percentage of ICH observed in pkd2hi4166 mutants, morphants (Mos), and their control siblings (n=3 with an average of 100 embryos in each repeat). No significant difference was detected. (B) Percentage of ICH in pkd2hi4166 mutants and control siblings treated with cyclopamine (n=3 with an average of 20 embryos in each repeat). Vehicle-treated embryos did not show any hemorrhage. No significant difference is detected between mutants and control siblings. *P<0.05 between cyclopamine- and vehicle-treated groups.
Discussion
Through this study, we show that defective IFT leads to ICH during zebrafish development. Increased ICH was observed in the zebrafish iguana mutant,28 which harbors an inactivation mutation in a Hedgehog pathway component called the daz-interacting protein 1 (dZip1).42,43 Intriguingly, dZip1 was later shown to be involved in cilia biogenesis.44–46 Another link between cilia and vascular integrity is provided by the zebrafish talpid3 mutant47; talpid3 is required for cilia biogenesis, and maternal and zygotic mutant embryos show ICH. Compared with dzip1 and talpid3, IFT genes are specifically required for cilia biogenesis. Interestingly, mouse loss-of-function mutants of Ift172 and Ift122 show cranial neural tube defect and hemorrhages.48,49 However, the cause of the hemorrhage phenotype is unclear. Our results, for the first time, formally establish a role of endothelial cilia in developmental vascular integrity.
The ICH phenotype that we observed in IFT mutants is not fully penetrant, and the frequency varies between different families. However, statistical analysis between IFT mutants and their siblings and rescue experiments strongly support the association between IFT mutations and developmental ICH risk. Interestingly, similar variable penetrance of ICH has been described in both zebrafish and human.28,50–52 In contrast to mouse strains, zebrafish lines are generally of very mixed genetic background.53 Clearly, there are additional risk factors that remain to be identified. Using zebrafish to screen for genetic modifiers is an attractive future direction.
Exposed to pulsating blood flow, endothelial cilia could be involved in regulating vascular integrity through mechanosensation or signaling pathways, particularly the Hedgehog pathway. Using chemical genetic and genetic approaches, we show that, inhibition of the Hedgehog signaling sensitizes IFT mutants to ICH risk, and additionally activation of Hedgehog signaling suppresses ICH in IFT mutants, revealing a critical role of the Hedgehog pathway in cilia-mediated developmental vascular integrity. It is known that cilia regulate the Hedgehog pathway and that the Hedgehog pathway is required for arterial differentiation.12,29,30 However, arterial differentiation is unlikely to be involved in the ICH phenotype observed in ciliary mutants here. Arterial differentiation defect in Hedgehog pathway mutants is apparent at 24 hpf,29 before cilia formation in the vasculature at 24–28 hpf.8 In addition, we detected no morphologic defects in the vasculature in these mutants. Consistent with this idea, the Hedgehog pathway is not restricted to the cilium. For example, long-range transduction of Hedgehog signaling is detected between limb mesenchymal filopodia.54 Most of the cells in Drosophila are devoid of cilia, but they are capable of Hedgehog signaling. Therefore, it is plausible that vascular patterning mediated by Hedgehog signaling, which is established before circulation starts, is independent of endothelial cilia.
The downstream targets of cilia-mediated Hedgehog signaling in developmental vascular integrity remain unidentified. It was reported that, in iguana (dzip1) mutants, the downregulation of the Hedgehog target angiopoietin 1 is responsible for a similar ICH phenotype.28 However, we failed to detect such a transcriptional downregulation of the Angiopoietin-Tie system as well as the adherens junction marker VE-Cad in IFT mutants. Moreover, we also did not detect any abnormalities in VE-Cad or Zo-1 staining in the brain vasculature of IFT mutants, suggesting that endothelial adherens and tight junctions could not account for the ICH phenotype. Interestingly, in zebrafish, interference with the pathway involved in cerebral cavernous malformation (CCM), an autosomal dominant human ICH disease, leads to very similar ICH phenotypes.50,52,55 It will be interesting to test the relationship between cilia, Hedgehog signaling, and the CCM signaling pathway in developmental vascular integrity.
We also investigated potential involvement of the ciliary mechanosensory channel Pc2 in this novel function of cilia. Pc2 is encoded by Pkd2, one of two responsible genes for autosomal dominant PKD. In cultured kidney epithelial cells and at mouse node, physical bending of cilia leads to a calcium influx into the cell.56–61 Pc2 is localized to the cilium and required for calcium signaling in response to flow in cultured renal epithelial cells, although not for cilia biogenesis.57,58 Intriguingly, in cultured endothelial cells, Pc2 and its binding partner Pc1 are also required for sensing fluid flow.2,6 However, neither mutants nor morphants of pkd2 showed any increased risk of developmental ICH. Moreover, pkd2 mutants are not sensitized to ICH induced by Hedgehog inhibition, which is in contrast to IFT mutants. It is worth noting that patients with autosomal dominant PKD show a significantly increased risk of intracranial aneurysm,62,63 although the defect is thought to originate from poor coverage of smooth muscle cells.64–66 Indeed, the fact that mural cells are not recruited to the blood vessel during early zebrafish development67 suggests that other mechanism(s) could lead to ICH in early-stage IFT mutants.
Despite the appreciation that genetic factors contribute significantly to ICH, very few risk genes have been identified. Whether developmental vascular integrity in zebrafish is relevant to human ICH is an open question. So far, the best supporting evidence comes from the genetics of CCM.50,52,55 Because cilia have also been detected in mature vasculature, it will be interesting to test the physiologic role of cilia in adult vascular integrity. Moreover, because ciliary defects are thought to be at the center of a wide spectrum of human diseases collectively referred to as ciliopathies, our results also suggest the importance of monitoring hemorrhaging incidence in patients with ciliopathy and investigating the possibility of ciliopathy genes as modifiers of ICH.
Concise Methods
Zebrafish Husbandry
Zebrafish were maintained according to standard protocols.68 TAB and EK strains were obtained through natural spawning. All IFT mutant lines were maintained in the TAB background, and transgenic Tg(kdrl:mRFP-F) and Tg(kdrl:mcherry-CAAX) lines were maintained in the EK background. About 100 fish were used for this study.
All zebrafish works have been conducted according to protocols approved by Institutional Animal Care and Use Committee of Yale University.
Whole-Mount o-Dianisidine Staining
Embryos at 52 hpf were fixed in 1:2.7 dilution of formalin in PBS containing 0.1% Tween-20 (PBST) for 1 hour followed by 7 minutes incubation at −20°C in acetone. The embryos were then stained for hemoglobin using o-Dianisidine (OD) dihydrochloride, a sensitive marker of hemoglobin, in the dark for 15–25 minutes at room temperature. The OD solution was comprised of OD (0.6 mg/ml; Sigma-Aldrich), 0.01 M sodium acetate (pH 5.5), 0.65% hydrogen peroxide, and 40% ethanol. Finally, the embryos were washed in PBST three times for 5 minutes each.
Morpholino Knockdown
Morpholino oligos were purchased from Gene Tools and stored at −20°C; 5′- CGATAAATTTAAGCTGTTCGCTCAT -3′ was used to block the translation of ift81. For knockdown experiment, 0.5 pmol was injected. For suboptimal knockdown, 0.25 pmol was injected. Previously established morpholino oligonucleotides were used to block the translation of pkd2 and qilin21,22 and the splicing of sufu.37 Before use, aliquots were heated at 65°C for 5 minutes, snap cooled, and injected into zebrafish embryos at one- to four-cell stages according to a previously published protocol.69
ift81 rescue Construct
Because the ift81 morpholino targets the 5′ coding region of ift81, in the kdrl:eGFP-2a-ift81 rescue construct, the third base of every codon in the target region was replaced with a synonymous nucleotide. Specifically, 5′-ATGAGCGAACAGCTTAAATTTATC-3′ was changed to 5′-ATGAGtGAgCAatTaAAgTTcATt-3′.
Live Imaging
Live embryos were mounted in 0.7% low-melting agarose gel for imaging. Confocal images were acquired using a FluoView 1000 Confocal Microscope (Olympus, Center Valley, PA) or a Leica TCS SP5II Confocal Microscope (Leica Microsystems, Wetzlar, Germany). Z-series stacks were captured every 250 nm for three-dimensional reconstruction using the software Volocity 6.0 (PerkinElmer, Waltham, MA). Three-dimensional rotation movies were made using ImageJ (National Institutes of Health).
Immunohistochemistry
Zebrafish embryos at 2 dpf were fixed with Dent’s fixative (for Zo-1 staining) or formalin (VE-Cad). The embryos were rehydrated through a sequential dilution of methanol with PBST followed by incubation in 0.125% Trypsin in PBS for 7 minutes. Embryos were washed three times for 5 minutes each in blocking solution (0.8% Triton X-100, 10% normal goat serum, 1% BSA, and 0.01% sodium azide in PBST) and then blocked at room temperature for 2 hours in blocking solution and incubated with primary antibodies at 4°C overnight. Embryos were washed in wash buffer (0.8% Triton X-100, 1% BSA, and 0.01% sodium azide in PBST) for a minimum of 4 hours, incubated in secondary antibodies overnight at 4°C, washed for 4 hours at room temperature, and mounted onto slides.
Primary antibodies used were rabbit polyclonal anti–VE-Cad70 (1:100), rabbit polyclonal anti–Zo-1 (1:300; Zymed), and mouse monoclonal anti-GFP (1:300; Invitrogen).
Secondary antibodies from Jackson ImmunoResearch Laboratories were used at 1:500 dilutions.
Slides were mounted using Vectashield Hard Set mounting medium (Vector Laboratories) and analyzed with a Leica SP5 microscope.
Genotyping
Embryos (2 dpf) were incubated in 50 μL SDS lysis buffer (100 mM Tris, pH 8.3, 200 mM NaCl, 0.4% SDS, and 5 mM EDTA) with freshly added proteinase K (final concentration=200 μg/mL) at 55°C on a rocker overnight. They were then diluted 20-fold and incubated at 94°C for 15 minutes for proteinase K inactivation. A microliter of the diluted embryo DNA was used to set up a standard 20-μL PCR reaction for genotyping. Primers used were 5′-CTCAGAAAACTCAAGAGGAAGCCTTC-3′ and 5′-GCTAGCTTGCCAAACCTACAGGT-3′.
Quantitative PCR
Quantitative PCR was performed as previously described.71
Cyclopamine Treatment
Cyclopamine was purchased from Cayman Chemicals (catalog no. 11321) and stored at −20°C. Dilutions were made to 25–30 µM using embryo medium containing 1% DMSO. Embryos from incrosses of heterozygous carries were collected. At 25 hpf, embryo medium was replaced with embryo medium containing 30 µM freshly diluted cyclopamine and 1% DMSO; 1% DMSO was used as vehicle alone control. At 2 dpf, embryos were sorted into mutant and wild-type groups on the basis of the presence or absence of the body curvature phenotype and then inspected for ICH.
Statistical Analyses
A t test was performed using Microsoft Excel. Chi square was calculated using the Pearson chi-squared test.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank N. Semanchik for superb technical support and Suk-Won Jin for helpful discussions. This work was supported by National Institutes of Health Grant R01-DK092808 (to Z.S.), National Institutes of Health Grant 1P30-DK090744 Animal Core, National Institute of Child Health and Development Intramural Program Grant ZO1-001011 (to B.M.W.), National Heart, Lung, and Blood Institute Grant ROO-HL105791-04 (to S.N.), and American Cancer Society Research Grant RSG-10-247-01-DDC (to Z.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121314/-/DCSupplemental.
References
- 1.Hildebrandt F, Benzing T, Katsanis N: Ciliopathies. N Engl J Med 364: 1533–1543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM: Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res 104: 860–869, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collin SP, Barry Collin H: Primary cilia in vertebrate corneal endothelial cells. Cell Biol Int 28: 125–130, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Egorova AD, van der Heiden K, Poelmann RE, Hierck BP: Primary cilia as biomechanical sensors in regulating endothelial function. Differentiation 83: S56–S61, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Jones TJ, Adapala RK, Geldenhuys WJ, Bursley C, AbouAlaiwi WA, Nauli SM, Thodeti CK: Primary cilia regulates the directional migration and barrier integrity of endothelial cells through the modulation of hsp27 dependent actin cytoskeletal organization. J Cell Physiol 227: 70–76, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J: Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117: 1161–1171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Heiden K, Hierck BP, Krams R, de Crom R, Cheng C, Baiker M, Pourquie MJ, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE: Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis 196: 542–550, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Goetz JG, Steed E, Ferreira RR, Roth S, Ramspacher C, Boselli F, Charvin G, Liebling M, Wyart C, Schwab Y, Vermot J: Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Reports 6: 799–808, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL: A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A 90: 5519–5523, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS: Decoding cilia function: Defining specialized genes required for compartmentalized cilia biogenesis. Cell 117: 527–539, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Park A, Sun Z: Intraflagellar transport proteins are essential for cilia formation and for planar cell polarity. J Am Soc Nephrol 21: 1326–1333, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang P, Schier AF: Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136: 3089–3098, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK: Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117: 541–552, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum JL, Witman GB: Intraflagellar transport. Nat Rev Mol Cell Biol 3: 813–825, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Anderson KV: Cilia and Hedgehog signaling in the mouse embryo. Harvey Lect 102: 103–115, 2006-2007 [DOI] [PubMed] [Google Scholar]
- 16.Goetz SC, Ocbina PJ, Anderson KV: The primary cilium as a Hedgehog signal transduction machine. Methods Cell Biol 94: 199–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK: Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1: e53, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huangfu D, Anderson KV: Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A 102: 11325–11330, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohatgi R, Milenkovic L, Scott MP: Patched1 regulates hedgehog signaling at the primary cilium. Science 317: 372–376, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV: Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426: 83–87, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Li J, Sun Z: Qilin is essential for cilia assembly and normal kidney development in zebrafish. PLoS ONE 6: e27365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N: A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Duldulao NA, Lee S, Sun Z: Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development 136: 4033–4042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grabher C, Joly JS, Wittbrodt J: Highly efficient zebrafish transgenesis mediated by the meganuclease I-SceI. Methods Cell Biol 77: 381–401, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS: I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev 118: 91–98, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Poole CA, Jensen CG, Snyder JA, Gray CG, Hermanutz VL, Wheatley DN: Confocal analysis of primary cilia structure and colocalization with the Golgi apparatus in chondrocytes and aortic smooth muscle cells. Cell Biol Int 21: 483–494, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Goetz SC, Anderson KV: The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet 11: 331–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamont RE, Vu W, Carter AD, Serluca FC, MacRae CA, Childs SJ: Hedgehog signaling via angiopoietin1 is required for developmental vascular stability. Mech Dev 127: 159–168, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Lawson ND, Vogel AM, Weinstein BM: Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 3: 127–136, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Williams C, Kim SH, Ni TT, Mitchell L, Ro H, Penn JS, Baldwin SH, Solnica-Krezel L, Zhong TP: Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Dev Biol 341: 196–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuson M, He M, Anderson KV: Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development 138: 4921–4930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammerschmidt M, Bitgood MJ, McMahon AP: Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev 10: 647–658, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Hammerschmidt M, McMahon AP: The effect of pertussis toxin on zebrafish development: A possible role for inhibitory G-proteins in hedgehog signaling. Dev Biol 194: 166–171, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Ungar AR, Moon RT: Inhibition of protein kinase A phenocopies ectopic expression of hedgehog in the CNS of wild-type and cyclops mutant embryos. Dev Biol 178: 186–191, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Yue S, Xie L, Pu XH, Jin T, Cheng SY: Dual Phosphorylation of suppressor of fused (Sufu) by PKA and GSK3beta regulates its stability and localization in the primary cilium. J Biol Chem 286: 13502–13511, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tukachinsky H, Lopez LV, Salic A: A mechanism for vertebrate Hedgehog signaling: Recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol 191: 415–428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koudijs MJ, den Broeder MJ, Keijser A, Wienholds E, Houwing S, van Rooijen EM, Geisler R, van Eeden FJ: The zebrafish mutants dre, uki, and lep encode negative regulators of the hedgehog signaling pathway. PLoS Genet 1: e19, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blum Y, Belting HG, Ellertsdottir E, Herwig L, Lüders F, Affolter M: Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol 316: 312–322, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Kaiser MS, Larson JD, Nasevicius A, Clark KJ, Wadman SA, Roberg-Perez SE, Ekker SC, Hackett PB, McGrail M, Essner JJ: Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development 137: 3119–3128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gjini E, Hekking LH, Küchler A, Saharinen P, Wienholds E, Post JA, Alitalo K, Schulte-Merker S: Zebrafish Tie-2 shares a redundant role with Tie-1 in heart development and regulates vessel integrity. Dis Model Mech 4: 57–66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montero-Balaguer M, Swirsding K, Orsenigo F, Cotelli F, Mione M, Dejana E: Stable vascular connections and remodeling require full expression of VE-cadherin in zebrafish embryos. PLoS ONE 4: e5772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekimizu K, Nishioka N, Sasaki H, Takeda H, Karlstrom RO, Kawakami A: The zebrafish iguana locus encodes Dzip1, a novel zinc-finger protein required for proper regulation of Hedgehog signaling. Development 131: 2521–2532, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wolff C, Roy S, Lewis KE, Schauerte H, Joerg-Rauch G, Kirn A, Weiler C, Geisler R, Haffter P, Ingham PW: Iguana encodes a novel zinc-finger protein with coiled-coil domains essential for Hedgehog signal transduction in the zebrafish embryo. Genes Dev 18: 1565–1576, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glazer AM, Wilkinson AW, Backer CB, Lapan SW, Gutzman JH, Cheeseman IM, Reddien PW: The Zn finger protein Iguana impacts Hedgehog signaling by promoting ciliogenesis. Dev Biol 337: 148–156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HR, Richardson J, van Eeden F, Ingham PW: Gli2a protein localization reveals a role for Iguana/DZIP1 in primary ciliogenesis and a dependence of Hedgehog signal transduction on primary cilia in the zebrafish. BMC Biol 8: 65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tay SY, Yu X, Wong KN, Panse P, Ng CP, Roy S: The iguana/DZIP1 protein is a novel component of the ciliogenic pathway essential for axonemal biogenesis. Dev Dyn 239: 527–534, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Ben J, Elworthy S, Ng AS, van Eeden F, Ingham PW: Targeted mutation of the talpid3 gene in zebrafish reveals its conserved requirement for ciliogenesis and Hedgehog signalling across the vertebrates. Development 138: 4969–4978, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, Phillips M, Teufel A, Kim C, Malik N, Huttner W, Westphal H: Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev Biol 325: 24–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortellino S, Wang C, Wang B, Bassi MR, Caretti E, Champeval D, Calmont A, Jarnik M, Burch J, Zaret KS, Larue L, Bellacosa A: Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev Biol 325: 225–237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gore AV, Lampugnani MG, Dye L, Dejana E, Weinstein BM: Combinatorial interaction between CCM pathway genes precipitates hemorrhagic stroke. Dis Model Mech 1: 275–281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucas M, Costa AF, García-Moreno JM, Solano F, Gamero MA, Izquierdo G: Variable expression of cerebral cavernous malformations in carriers of a premature termination codon in exon 17 of the Krit1 gene. BMC Neurol 3: 5, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchner DA, Su F, Yamaoka JS, Kamei M, Shavit JA, Barthel LK, McGee B, Amigo JD, Kim S, Hanosh AW, Jagadeeswaran P, Goldman D, Lawson ND, Raymond PA, Weinstein BM, Ginsburg D, Lyons SE: pak2a mutations cause cerebral hemorrhage in redhead zebrafish. Proc Natl Acad Sci U S A 104: 13996–14001, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guryev V, Koudijs MJ, Berezikov E, Johnson SL, Plasterk RH, van Eeden FJ, Cuppen E: Genetic variation in the zebrafish. Genome Res 16: 491–497, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanders TA, Llagostera E, Barna M: Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature 497: 628–632, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Fraser SD, Faloon PW, Rollins EL, Vom Berg J, Starovic-Subota O, Laliberte AL, Chen JN, Serluca FC, Childs SJ: A betaPix Pak2a signaling pathway regulates cerebral vascular stability in zebrafish. Proc Natl Acad Sci U S A 104: 13990–13995, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGrath J, Somlo S, Makova S, Tian X, Brueckner M: Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114: 61–73, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB: Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Praetorius HA, Spring KR: Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Praetorius HA, Spring KR: Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, Nakai J, Dworniczak B, Ehrlich BE, Pennekamp P, Hamada H: Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 338: 226–231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chester AC, Harris JP, Schreiner GE: Polycystic kidney disease. Am Fam Physician 16: 94–101, 1977 [PubMed] [Google Scholar]
- 63.Fehlings MG, Gentili F: The association between polycystic kidney disease and cerebral aneurysms. Can J Neurol Sci 18: 505–509, 1991 [DOI] [PubMed] [Google Scholar]
- 64.Griffin MD, Torres VE, Grande JP, Kumar R: Vascular expression of polycystin. J Am Soc Nephrol 8: 616–626, 1997 [DOI] [PubMed] [Google Scholar]
- 65.Lu CJ, Du H, Wu J, Jansen DA, Jordan KL, Xu N, Sieck GC, Qian Q: Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res 31: 171–184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torres VE, Cai Y, Chen X, Wu GQ, Geng L, Cleghorn KA, Johnson CM, Somlo S: Vascular expression of polycystin-2. J Am Soc Nephrol 12: 1–9, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Santoro MM, Pesce G, Stainier DY: Characterization of vascular mural cells during zebrafish development. Mech Dev 126: 638–649, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westerfield M: The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), Eugene, OR, University of Oregon Press, 2000 [Google Scholar]
- 69.Yuan S, Sun Z: Microinjection of mRNA and morpholino antisense oligonucleotides in zebrafish embryos. J Vis Exp 27: 1113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting HG, Affolter M: Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol 21: 1942–1948, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Zhao L, Yuan S, Cao Y, Kallakuri S, Li Y, Kishimoto N, DiBella L, Sun Z: Reptin/Ruvbl2 is a Lrrc6/Seahorse interactor essential for cilia motility. Proc Natl Acad Sci U S A 110: 12697–12702, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.